Original Synthesis of Fluorenyl Alcohol Derivatives by Reductive Dehalogenation Initiated by TDAE
Abstract
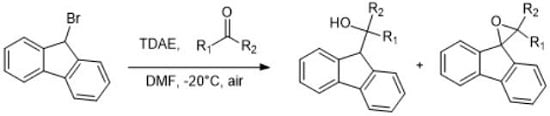
:1. Introduction
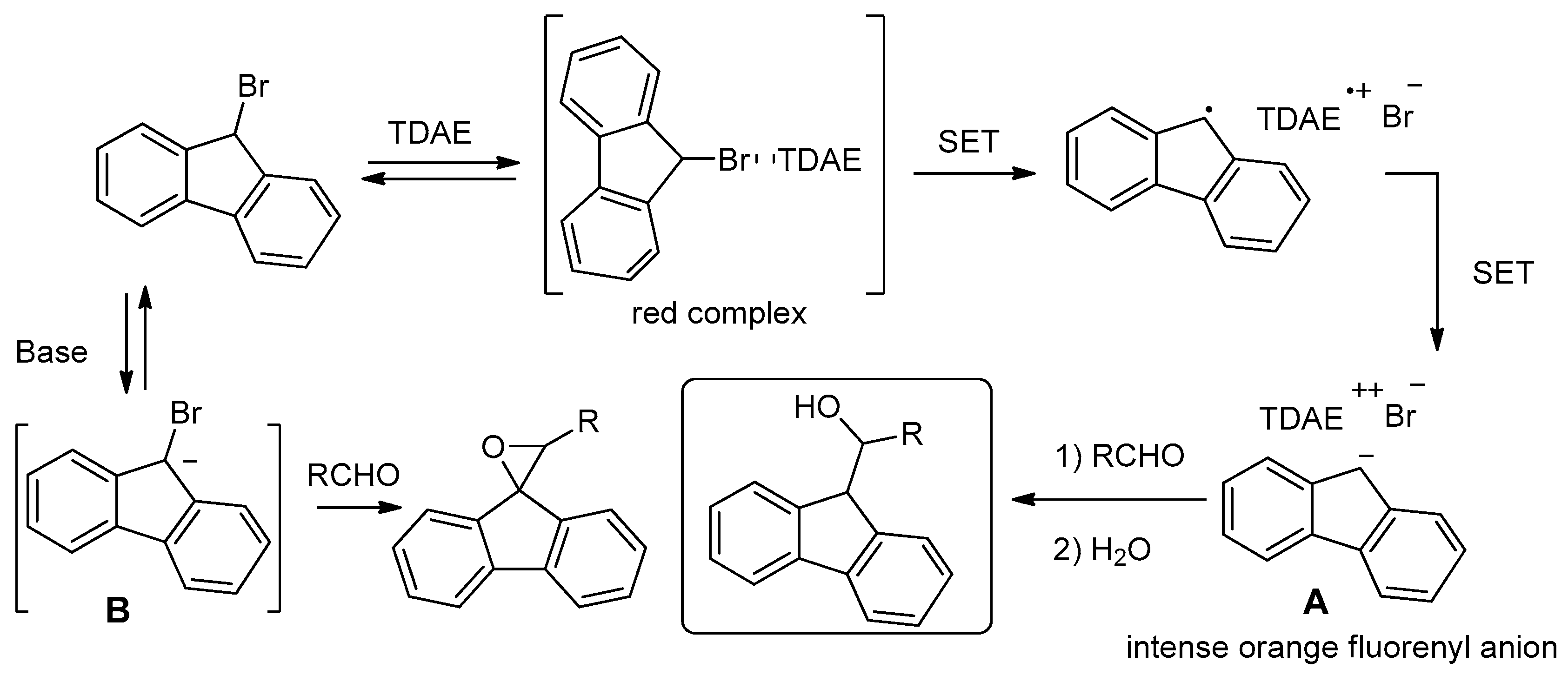
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Typical Procedure
3.3. Compound Characterizations
3.4. Reactivity of 9-Bromofluorene and 4-Cyanobenzaldehyde in the Presence of Triethylamine
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- El-Shekeil, A.; El-Sonbati, A.Z. Fluorenone thiosemicabazone complexes of bivalent metalions. Transit. Met. Chem. 1992, 17, 420–422. [Google Scholar] [CrossRef]
- Ahmad, T.; Kandil, F.; Moustapha, C. Synthesis, Characterization, Biological Evaluation and Antibacterial Activity of some Heterocyclic Fluorene Compounds Derived from Schiff Base. Int. J. ChemTech Res. 2015, 8, 447–458. [Google Scholar]
- Zhang, X.; Xu, J.-K.; Wang, J.; Wang, N.-L.; Kurihara, H.; Kitanaka, S.; Yao, X.-S. Bioactive Bibenzyl Derivatives and Fluorenones from Dendrobium nobile. J. Nat. Prod. 2007, 70, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.-F.; Zhou, B.; Huang, J.-M.; Gao, X.-M.; Shu, L.-D.; Yang, G.-Y.; Che, C.-T. Antiviral Phenolic Compounds from Arundina gramnifolia. J. Nat. Prod. 2013, 76, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.; Herzog, B.; Quass, K. Photostable Cosmetic or Dermatological Compositions Containing Sunscreens. PCT International Application WO 2006034968, 6 April 2006. [Google Scholar]
- Anonymous. 9-Aminoalkylfluorenes and Their Salts. Austrian Patent AT 368125, 10 September 1982. [Google Scholar]
- Britten, N.J.; Burns, J.W.; Hallberg, J.W.; Waldron, N.A.; Watts, J.L. Dispersible Formulations of an Anti-inflammatory Agent. PCT International Application WO 2004082588, 30 September 2004. [Google Scholar]
- Laine, L.; Connors, L.G.; Reicin, A.; Hawkey, C.J.; Burgos-Vargas, R.; Schnitzer, T.J.; Yu, Q.; Bombardier, C. Serious lower gastrointestinal clinical events with nonselective NSAID or coxib use. Gastroenterology 2003, 124, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Giuglio-Tonolo, G.; Terme, T.; Médebielle, M.; Vanelle, P. Original reaction of p-nitrobenzyl chloride with aldehydes using tetrakis(dimethylamino)ethylene (TDAE). Tetrahedron Lett. 2003, 44, 6433–6435. [Google Scholar] [CrossRef]
- Giuglio-Tonolo, G.; Terme, T.; Médebielle, M.; Vanelle, P. Nitrobenzylation of α-carbonyl ester derivatives using TDAE approach. Tetrahedron Lett. 2004, 45, 5121–5124. [Google Scholar] [CrossRef]
- Amiri-Attou, O.; Terme, T.; Médebielle, M.; Vanelle, P. Original formation of benzyl benzoates by TDAE strategy. Tetrahedron Lett. 2008, 49, 1016–1020. [Google Scholar] [CrossRef]
- Juspin, T.; Terme, T.; Vanelle, P. TDAE Strategy Using α-Diketones: Rapid Access to 2,3-Diphenylquinoline and Acenaphtho[1,2-b]quinoline Derivatives. Synlett 2009, 1485–1489. [Google Scholar] [CrossRef]
- Khoumeri, O.; Montana, M.; Terme, T.; Vanelle, P. Rapid and efficient synthesis of 2-substituted-tetrahydropyrido[3,4-b]quinoxalines using TDAE strategy. Tetrahedron Lett. 2012, 53, 2410–2413. [Google Scholar] [CrossRef]
- Giuglio-Tonolo, A.G.; Spitz, C.; Terme, T.; Vanelle, P. An expeditious method for the selective cyclotrimerization of isocyanates initiated by TDAE. Tetrahedron Lett. 2014, 55, 2700–2702. [Google Scholar] [CrossRef]
- Juspin, T.; Laget, M.; Terme, T.; Azas, N.; Vanelle, P. TDAE-assisted synthesis of new imidazo[2,1-b]thiazole derivatives as anti-infectious agents. Eur. J. Med. Chem. 2010, 45, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Montana, M.; Terme, T.; Vanelle, P. Original synthesis of oxiranes via TDAE methodology: Reaction of 2,2-dibromomethylquinoxaline with aromatic aldehydes. Tetrahedron Lett. 2005, 46, 8373–8376. [Google Scholar] [CrossRef]
- Roche, M.; Lacroix, C.; Khoumeri, O.; Franco, D.; Neyts, J.; Terme, T.; Leyssen, P.; Vanelle, P. Synthesis, biological activity and structure-activity relationship of 4,5-dimethoxybenzene derivatives inhibitor of rhinovirus. Eur. J. Med. Chem. 2014, 76, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.A.; Zhou, S.; Thomson, D.W.; Schoenebeck, F.; Mahesh, M.; Park, S.R.; Tutle, T.; Berlouis, L.E.A. Highly Efficient Reduction of Unactivated Aryl and Alkyl Iodides by a Ground-State Neutral Organic Electron Donor. Angew. Chem. Int. Ed. 2005, 44, 1356–1360. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.A.; Khan, T.A.; Zhou, S.; Thomson, D.W.; Schoenebeck, F.; Mahesh, M.; Park, S.R.; Tuttle, T.; Berlouis, L.E.A. The Generation of Aryl Anions by Double Electron Transfer to Aryl Iodides from a Neutral Ground-State Organic Super-Electron Donor. Angew. Chem. Int. Ed. 2007, 46, 5178–5183. [Google Scholar] [CrossRef] [PubMed]
- Broggi, J.; Terme, T.; Vanelle, P. Organic Electron Donors as Powerful Single-Electron Reducing Agents in Organic Synthesis. Angew. Chem. Int. Ed. 2014, 53, 384–413. [Google Scholar] [CrossRef] [PubMed]
- Kuroboshi, M.; Waki, Y.; Tanaka, H. Palladium-Catalyzed Tetrakis(dimethylamino)ethylene-Promoted Reductive Coupling of Aryl Halides. J. Org. Chem. 2003, 68, 3938–3942. [Google Scholar] [CrossRef] [PubMed]
- Pawelke, G. Tetrakis(dimethylamino)ethylene/trifluoroiodomethane. A specific novel trifluorome-thylating agent. J. Fluor. Chem. 1989, 42, 429–433. [Google Scholar] [CrossRef]
- Takechi, N.; Aït-Mohand, S.; Médebielle, M.; Dolbier, W.R.J. Nucleophilic trifluoromethylation of acyl chlorides using the trifluoromethyl iodide/TDAE reagent. Tetrahedron Lett. 2002, 43, 4317–4319. [Google Scholar] [CrossRef]
- Pawelke, G. Reaction of tetrakis(dimethylamino)ethylene with CF2Br2 in the presence of secondary amines, formation of N-trifluoromethyl-dialkylamines. J. Fluor. Chem. 1991, 52, 229–234. [Google Scholar] [CrossRef]
- Since, M.; Terme, T.; Vanelle, P. Original TDAE strategy using α-halocarbonyl derivatives. Tetrahedron 2009, 65, 6128–6134. [Google Scholar] [CrossRef]
- Juspin, T.; Giuglio-Tonolo, G.; Terme, T.; Vanelle, P. First TDAE-Mediated Double Addition of Nitrobenzylic Anions to Aromatic Dialdehydes. Synthesis 2010, 5, 844–848. [Google Scholar]
- Pooput, C.; Dolbier, W.R., Jr.; Médebielle, M. Nucleophilic Perfluoroalkylation of Aldehydes, Ketones, Imines, Disulfides, and Diselenides. J. Org. Chem. 2006, 71, 3564–3568. [Google Scholar] [CrossRef] [PubMed]
- Pooput, C.; Medebielle, M.; Dolbier, W.R.J. A New and Efficient Method for the Synthesis of Trifluoromethylthio- and Selenoethers. Org. Lett. 2004, 6, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Médebielle, M.; Kato, K.; Dolbier, W.R., Jr. Fluorinated hydrazones. Part 1: Reductive coupling reactions of chlorodifluoroacetylated dialkylhydrazones using tetrakis(dimethylamino)ethylene (TDAE). Tetrahedron Lett. 2003, 44, 7871–7873. [Google Scholar] [CrossRef]
- Montana, M.; Terme, T.; Vanelle, P. Original synthesis of α-chloroketones in azaheterocyclic series using TDAE approach. Tetrahedron Lett. 2006, 47, 6573–6576. [Google Scholar] [CrossRef]
- Bergmann, E.; Hervey, J. Über das Auftreten von freien substituierten Methylenen bei chemischen Reaktionen. Chem. Ber. 1929, 62, 803–916. [Google Scholar] [CrossRef]
- Stösser, R.; Thurne, J.-U.; Tomaschewski, U.; Ewert, P.; Eblik, P.; Sneide, W.; Hanke, T. EPR-Investigations on Radicals and Triplet States of Fluorence Derivatives. Z. Naturforsh. A Phys. Sci. 1982, 37, 1241–1246. [Google Scholar]
- Goodman, C.C.; Roof, A.C.; Tillman, E.S.; Ludwig, B.; Chon, D.; Weigley, M.I. Synthesis and characterization of fluorene end-labeled polystyrene with atom transfer radical polymerization. J. Polym. Sci. Pol. Chem. 2005, 43, 2657–2665. [Google Scholar] [CrossRef]
- Eisch, J.J.; Fregene, P.O. Vanadium(I) Chloride and Lithium Vanadium(I) Dihydride as Selective Epimetallating Reagents for π- and σ- Bonded Organic Substrates. Eur. J. Org. Chem. 2008, 26, 4482–4492. [Google Scholar] [CrossRef]
- Ghera, E.; Sprinzak, Y. Reactions of active methylene compounds in pyridine soln. II. Aldol-type reactions of indene and fluorene. J. Am. Chem. Soc. 1960, 82, 4945–4952. [Google Scholar] [CrossRef]
- Mladenova, G.; Singh, G.; Acton, A.; Chen, L.; Rinco, L.J.; Johnston, E.L.-R. Photochemical Pinacol Rearrangements of Unsymmetrical Diols. J. Org. Chem. 2004, 69, 2017–2023. [Google Scholar] [CrossRef] [PubMed]
- Zhiqin, J.; Shuping, W.; Hansen, S. Studies on photooxidations. II. Electron transfer photooxidation of 9-benzylidenefluorene. Hua Hsüeh Hsüeh Pao 1988, 46, 294–297. [Google Scholar]
- Allen, R.E.; Schumann, E.L.; Day, W.C.; Van Campen, M.G.J. Amidines of certain substituted triphenylethylenes. J. Am. Chem. Soc. 1958, 80, 591–598. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 4a–4g; 4i–4p and 5a–5c; 5f–5k; 6f are available from the authors.

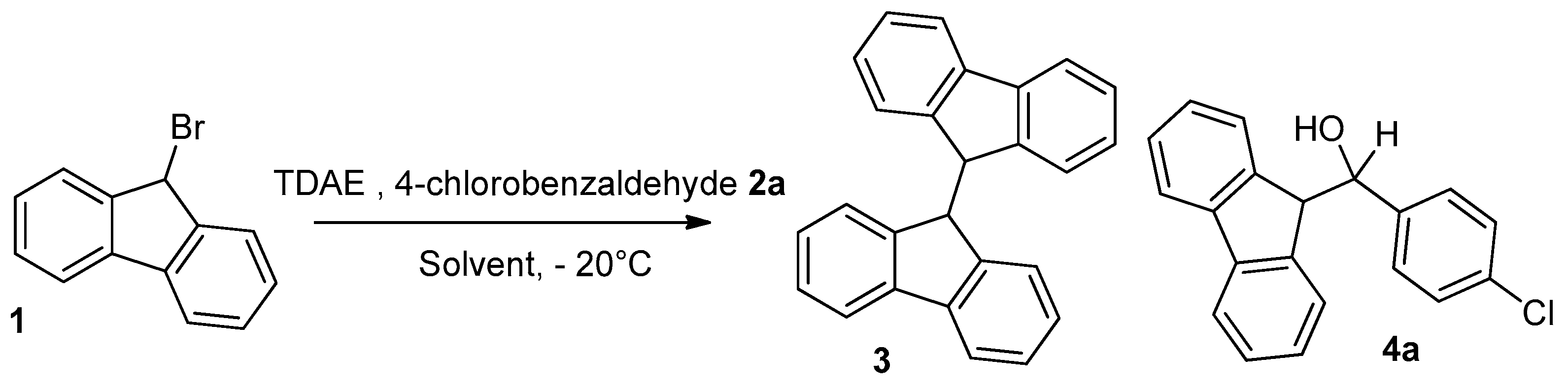

| Entry | Aldehyde 2a (eq.) | TDAE (eq.) | Solvent | Yield 1,2 of Dimer 3 | Yield 1,2 of 4a |
|---|---|---|---|---|---|
| 1 | 2 | 1 | DMF | 44% | 29% |
| 2 | 3 | 1 | DMF | 8% | 50% |
| 3 | 3 | 2 | DMF | 10% | 48% |
| 4 | 4 | 1 | DMF | 8% | 46% |
| 5 | 3 | 1 | THF | 12% | 48% |
| 6 | 3 | 1 | DMF 3 | 9% | 56% |
| 7 | 3 | 1 | CH3CN | 16% | 30% |
| 8 | 3 | 1 | DMSO | 20% | 12% |
| 9 | 3 | 1 | pyridine | 11% | 34% |
| Entry | Electrophiles | Yield 1,2 of 4 | Yield 1,2 of 5 | ||
|---|---|---|---|---|---|
| R1 | R2 | ||||
| 1 | 2a | 4-ClC6H4 | H | 56% (4a) | 14% (5a) |
| 2 | 2b | C6H5 | H | 57% (4b) | 17% (5b) |
| 3 | 2c | 4-BrC6H4 | H | 75% (4c) | 10% (5c) |
| 4 | 2d | 4-OMeC6H4 | H | 34% (4d) | |
| 5 | 2e | 4-FC6H4 | H | 54% (4e) | |
| 6 | 2f | 4-CNC6H4 | H | 41% (4f) | 30% (5f) |
| 7 | 2g | 4-MeC6H4 | H | 24% (4g) | |
| 8 | 2h | 4-(NO2)C6H4 | H | 17% (5h) | |
| 9 | 2i | 4-CF3 C6H4 | H | 49% (4i) | 6% (5i) |
| 10 | 2j | 2-furyl | H | 28% (4j) | 19% (5j) |
| 11 | 2k | 4-pyridinyl | H | 42% (4k) | 11% (5k) |
| 12 | 2l | 3-pyridinyl | H | 19% (4l) | |
| 13 | 2m | Me | CO2Et | 47% (4m) | |
| 14 | 2n | CF3 | CO2Me | 41% (4n) | |
| 15 | 2o | H | CO2Et | 25% (4o) | |
| 16 | 2p | CO2Et | CO2Et | 70% (4p) | |
| 17 | 2q | C6H5 | Et | ||
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuglio-Tonolo, A.G.; Terme, T.; Vanelle, P. Original Synthesis of Fluorenyl Alcohol Derivatives by Reductive Dehalogenation Initiated by TDAE. Molecules 2016, 21, 1408. https://doi.org/10.3390/molecules21101408
Giuglio-Tonolo AG, Terme T, Vanelle P. Original Synthesis of Fluorenyl Alcohol Derivatives by Reductive Dehalogenation Initiated by TDAE. Molecules. 2016; 21(10):1408. https://doi.org/10.3390/molecules21101408
Chicago/Turabian StyleGiuglio-Tonolo, Alain Gamal, Thierry Terme, and Patrice Vanelle. 2016. "Original Synthesis of Fluorenyl Alcohol Derivatives by Reductive Dehalogenation Initiated by TDAE" Molecules 21, no. 10: 1408. https://doi.org/10.3390/molecules21101408