Naturally Occurring Cinnamic Acid Sugar Ester Derivatives
Abstract
:1. Introduction
2. Chemical Constituents
2.1. Monosaccharide Esters
2.2. Disaccharide Esters
2.3. Trisaccharide Esters
2.4. Tetrasaccharide Esters
2.5. Other Sugar Esters
3. Biological Activities
3.1. Anti-Depression Activity and Neuroprotective Activity
3.2. Anticancer Activity
3.3. Antioxidant Activity
3.4. Antiinflammatory Activity
3.5. Antiviral Activity
3.6. Other Activities
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, P.; Hu, Y.; Guo, D.H.; Wang, D.X.; Tu, H.H.; Ma, L.; Xie, T.T.; Kong, L.Y. Potential antidepressant properties of Radix polygalae (Yuan Zhi). Phytomedicine 2010, 17, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, M.; Konoshima, T.; Kuroki, S.; Tokuda, H.; Nishino, H. Cancer chemopreventive activity of phenylpropanoid esters of sucrose, vanicoside B and lapathoside A, from Polygonum lapathifolium. Cancer Lett. 2001, 173, 133–138. [Google Scholar] [CrossRef]
- Fan, P.; Terrier, L.; Hay, A.E.; Marston, A.; Hostettmann, K. Antioxidant and enzyme inhibition activities and chemical profiles of Polygonum sachalinensis F. Schmidt ex Maxim (Polygonaceae). Fitoterapia 2010, 81, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.L.; Zhang, L.J.; Chen, R.Y.; Kuo, L.M.Y.; Huang, J.P.; Huang, H.C.; Lee, K.H.; Wu, Y.C.; Kuo, Y.H. Antioxidant and Anti-inflammatory Phenylpropanoid Derivatives from Calamus quiquesetiner vius. J. Nat. Prod. 2010, 73, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Kernan, M.R.; Amarquaye, A.; Chen, J.L.; Chan, J.; Sesin, D.F.; Parkinson, N.; Ye, Z.J.; Barrett, M.; Bales, C.; Stoddart, C.A.; et al. Antiviral phenylpropanoidglycosides from the medicinal plant Markhamia lutea. J. Nat. Prod. 1998, 61, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Birkhofer, L.; Kaiser, C.; Thomas, U. Sugar esters. IV. Acteoside and neoacteoside sugar esters from Syringis vulgaris. Z. Naturforsch. B 1968, 23, 1051–1058. [Google Scholar]
- Hu, Y.; Liao, H.B.; Guo, D.H.; Liu, P.; Wang, Y.Y.; Rahman, K. Antidepressant-like effects of 3, 6′-disinapoyl sucrose on hippocampal neuronal plasticity and neurotrophic signal pathway in chronically mild stressed rats. Neurochem. Int. 2010, 56, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.Z.; Huang, C.L.; Yu, B.Y.; Hu, Y.; Mu, L.H.; Liu, P. Effect of Tenuifoliside A isolated from Polygala tenuifolia on the ERK and PI3K pathways in C6 glioma cells. Phytomedicine 2014, 21, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.H.; Liu, P.; Ma, L.; Liao, H.B.; Xie, T.T.; Mu, L.H.; Liu, Y.M. Study on antidepressant components of sucrose ester from Polygala tenuifolia. Chin. J. Chin. Mater. Med. 2008, 33, 1278–1280. [Google Scholar]
- Ikeya, Y.; Sugama, K.; Okada, M.; Mitsuhashi, H. Four new phenolic glycosides from Polygala tenuifolia. Chem. Pharm. Bull. 1991, 39, 2600–2605. [Google Scholar] [CrossRef]
- Miyase, T.; Iwata, Y.; Ueno, A. Tenuifolioses G-P, Oligosaccharide Multi-Esters from the Roots of Polygala tenuifolia WILLD. Chem. Pharm. Bull. 1992, 40, 2741–2748. [Google Scholar] [CrossRef]
- Miyase, T.; Iwata, Y.; Ueno, A. Tenuifolioses A-F, Oligosaccharide Multi-Esters from the Roots of Polygala tenuifolia WILLD. Chem. Pharm. Bull. 1991, 39, 3082–3084. [Google Scholar] [CrossRef]
- Miyase, T.; Noguchi, H.; Chen, X.M. Sucrose esters and xanthone C-glycosides from the roots of Polygala sibirica. J. Nat. Prod. 1999, 62, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.H.; Hsu, Y.W.; Liaw, C.C.; Lee, J.K.; Huang, H.; Kuo, L.M.Y. Cytotoxic Phenylpropanoid Glycosides from the Stems of Smilax china. J. Nat. Prod. 2005, 68, 1475–1478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liao, C.C.; Huang, H.C.; Shen, Y.C.; Yang, L.M.; Kuo, Y.H. Antioxidant phenylpropanoid glycosides from Smilax bracteata. Phytochemistry 2008, 69, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Fontaine, J.; Malonne, H.; Claeys, M.; Luhmer, M.; Duez, P. A sugar ester and an iridoid glycoside from Scrophularia ningpoensis. Phytochemistry 2005, 66, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wang, N.L.; Huang, J.H.; Yao, X. Iridoid and phenylpropanoid glycosides from Scrophularia ningpoensis Hemsl. Asian J. Tradit. Med. 2007, 2, 118–123. [Google Scholar]
- Kim, S.R.; Kim, Y.C. Neuroprotective phenylpropanoid esters of rhamnose isolated from roots of Scrophularia buergeriana. Phytochemistry 2000, 54, 503–509. [Google Scholar] [CrossRef]
- Sasaki, H.; Nishimura, H.; Mitsuhashi, H. Hydroxycinnamic acid esters of phenethylalcohol glycosides from Rehmannia glutinosa var. Purpurea. Phytochemistry 1989, 28, 875–879. [Google Scholar] [CrossRef]
- Shimomura, H.; Sashida, Y.; Adachi, T. Phenylpropanoidglucose esters from Prunus buergeriana. Phytochemistry 1988, 27, 641–644. [Google Scholar] [CrossRef]
- Hamerski, L.; Bomm, M.D.; Silva, D.H.S.; Young, M.C.M.; Furlan, M.; Eberlin, M.N.; Castro-Gamboa, I.; Jose Cavalheiro, A.; da Silva Bolzani, V. Phenylpropanoid glucosides from leaves of Coussarea hydrangeifolia (Rubiaceae). Phytochemistry 2005, 66, 1927–1932. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, M.; Kikuchi, M. Studies on the constituents of Osmanthus species. VI. Structures of phenylpropanoid glycosides from the leaves of Osmanthus asiaticus Nakai. Chem. Pharm. Bull. 1990, 38, 2953–2955. [Google Scholar] [CrossRef]
- Xia, P.F.; Feng, Z.M.; Yang, Y.N.; Zhang, P.C. Two flavonoid glycosides and a phenylpropanoid glucose ester from the leaves of Sterculia foetida. J. Asian Nat. Prod. Res. 2009, 11, 766–771. [Google Scholar] [CrossRef] [PubMed]
- She, G.M.; Wang, D.; Zeng, S.F.; Yang, C.R.; Zhang, Y.J. New Phenylethanoid Glycosides and Sugar Esters from Ku-Ding-Cha, a Herbal Tea Produced from Ligustrum purpurascens. J. Food Sci. 2008, 73, C476–C481. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, M.; Galeffi, C.; Messana, I.; Marini-Bettolo, G.B.; Garbarino, J.A.; Gambaro, V. Phenylpropanoid glycosides from Calceolaria hypericina. Phytochemistry 1988, 27, 639–641. [Google Scholar] [CrossRef]
- Wang, X.; Li, L.; Bai, Z.; Peng, Y.; Xiao, P.; Liu, Y. Five new phenylpropanoid glycosides from Paraboea glutinosa (Gesneriaceae). J. Nat. Med. 2011, 65, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Hiroshi, W.; Yasufumi, S.; Nobutoshi, T.; Cambie, R.C.; Braggins, J.E. Chemical and chemotaxonomical studies of ferns. LXXXVII. Constituents of Trichomanes reniforme. Chem. Pharm. Bull. 1995, 43, 461–465. [Google Scholar]
- Taoubi, K.; Fauvel, M.T.; Gleye, J.; Moulis, C.; Fouraste, I. Phenylpropanoid glycosides from Lantana camara and Lippia multiflora. Planta Med. 1997, 63, 192–193. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, O.M.; Kamel, M.S.; Mohamed, M.H. Phenylpropanoid glycosides of Prunus ssiori. Phytochemistry 1994, 37, 1689–1692. [Google Scholar] [CrossRef]
- Lou, H.; Li, X.; Zhu, T.; Li, W. Sinapic acid esters and a phenolic glycoside from Cynanchumhancockianum. Phytochemistry 1993, 32, 1283–1286. [Google Scholar] [CrossRef]
- Hussein, S.A.M.; Ayoub, N.A.; Nawwar, M.A.M. Caffeoyl sugar esters and an ellagitannin from Rubus sanctus. Phytochemistry 2003, 63, 905–911. [Google Scholar] [CrossRef]
- Calisa, I.; Kirmizibekmeza, H.; Tasdemira, D.; Sticherb, O.; Irelandc, C.M. Sugar esters from Globularia orientalis. Z. Naturforsch. 2002, 57c, 591–596. [Google Scholar]
- Kim, I.H.; Kaneko, N.; Uchiyama, N.; Lee, J.E.; Takeya, K.; Kawahara, N.; Goda, Y. Two phenylpropanoid glycosides from Neopicrorhiza scrophulariiflora. Chem. Pharm. Bull. 2006, 54, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.W.; Yoon, K.D.; Ahn, M.J.; Kim, J. Two new phenylpropanoid glycosides from the aerial parts of Paederia scandens. Notes 2010, 31, 1071. [Google Scholar] [CrossRef]
- Wang, P.; Li, S.; Ownby, S.; Zhang, Z.; Yuan, W.; Zhang, W.; Scott Beasley, R. Ecdysteroids and a sucrose phenylpropanoid ester from Froelichia floridana. Phytochemistry 2009, 70, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Takamura, C.; Sugita, F.; Masuoka, C.; Yoshimitsu, H.; Ikeda, T.; Nohara, T. Two new steroid glycosides and a new sesquiterpenoid glycoside from the underground parts of Trillium amtschaticum. Chem. Pharm. Bull. 2007, 55, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, Y.; Tu, P.F. Tricornoses A-L, Oligosaccharide Multi-esters from the Roots of Polygalat ricornis. J. Nat. Prod. 2005, 68, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Miyase, T.; Kuroyanagi, M.; Umehara, K.; Noguchi, H. Oligosaccharide polyesters from roots of Polygala glomerata. Phytochemistry 1998, 47, 45–52. [Google Scholar] [CrossRef]
- Takasaki, M.; Kuroki, S.; Kozuka, M.; Konoshima, T. New phenylpropanoid esters of sucrose from Polygonum lapathifolium. J. Nat. Prod. 2001, 64, 1305–1308. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, J.X.; Xu, Q. Phenylpropanoid glycosides from Smilax glabra. Phytochemistry 2000, 53, 1051–1055. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, W.Y.; Zhang, T.J.; Guo, Y.Q. A novel phenylpropanoid glycosides and a new derivation of phenolic glycoside from Paris Polyphylla var. yunnanensis. Chin. Chem. Lett. 2007, 18, 548–550. [Google Scholar] [CrossRef]
- Hamburger, M.; Hostettmann, K. Hydroxycinnamic acid esters from Polygala chamaebuxus. Phytochemistry 1985, 24, 1793–1797. [Google Scholar] [CrossRef]
- Fukuyama, Y.; Sato, T.; Miura, I.; Asakawa, Y.; Takemoto, T. Hydropiperoside, a novel coumaryl glycoside from the root of Polygonum hydropiper. Phytochemistry 1983, 22, 549–552. [Google Scholar] [CrossRef]
- Sun, X.; Zimmermann, M.L.; Campagne, J.M.; Sneden, A.T. New sucrose phenylpropanoid esters from Polygonum perfoliatum. J. Nat. Prod. 2000, 63, 1094–1097. [Google Scholar] [CrossRef] [PubMed]
- Nhiem, N.X.; VanKiem, P.; Van Minh, C.; Ban, N.K.; Cuong, N.X.; Tai, B.H.; Kim, Y.H. Phenylpropanoid glycosides from Heterosmilax erythrantha and their antioxidant activity. Arch. Pharm. Res. 2009, 32, 1373–1377. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, H.; Miyase, T.; Ueno, A. Reinioses A-J, oligosaccharide multi-esters from the roots of Polygala reinii Fr. et Sav. Chem. Pharm. Bull. 1994, 42, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.T.; Tu, P.F. New Oligosaccharide Esters and Xanthone C-Glucosides from Polygala telephioides. Helv. Chim. Acta 2007, 90, 944–950. [Google Scholar] [CrossRef]
- Lepore, L.; Malafronte, N.; Condero, F.B.; Gualtieri, M.J.; Abdo, S.; Piaz, F.D.; De Tommasi, N. Isolation and structural characterization of glycosides from an anti-angiogenic extract of Monnina obtusifolia H.B.K. Fitoterapia 2011, 82, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.V.L.; Larson, S.R.; Sneden, A.T. Vanicosides C-F, new phenylpropanoid glycosides from Polygonum pensylvanicum. J. Nat. Prod. 1998, 61, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.J.; Zhang, Y.J.; Yang, C.R. Antioxidant phenolic constituents from Fagopyrum dibotrys. J. Ethnopharmacol. 2005, 99, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, W.; Miyase, T.; Suzuki, S.; Noguchi, H.; Chen, X.M. Oligosaccharide Esters from the Roots of Polygala arillata. J. Nat. Prod. 2000, 63, 1066–1069. [Google Scholar] [CrossRef] [PubMed]
- Van Kiem, P.; Nhiem, N.X.; Cuong, N.X.; Hoa, T.Q.; Huong, H.T.; van Minh, C.; Kim, Y.H. New phenylpropanoid esters of sucrose from Polygonum hydropiper and their antioxidant activity. Arch. Pharm. Res. 2008, 31, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.L.; Sneden, A.T. Vanicosides A and B, protein kinase C inhibitors from Polygonum pensylvanicum. J. Nat. Prod. 1994, 57, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, H.; Sashida, Y.; Mimaki, Y. Bitter phenylpropanoid glycosides from Lilium speciosum var. rubrum. Phytochemistry 1986, 25, 2897–2899. [Google Scholar] [CrossRef]
- Shoyama, Y.; Hatano, K.; Nishioka, I.; Yamagishi, T. Phenolic glycosides from Lilium longiflorum. Phytochemistry 1987, 26, 2965–2968. [Google Scholar] [CrossRef]
- Zhang, D.; Miyase, T.; Kuroyanagi, M.; Umehara, K.; Ueno, A. Five new triterpene saponins, polygalasaponins XXVIII-XXXII from the root of Polygala japonica Houtt. Chem. Pharm. Bull. 1996, 44, 810–815. [Google Scholar] [CrossRef] [PubMed]
- De Tommasi, N.; Piacente, S.; De Simone, F.; Pizza, C. New sucrose derivatives from the bark of Securidaca longipedunculata. J. Nat. Prod. 1993, 56, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, K.; Sashida, Y.; Mimaki, Y.; Shimomura, H. New polyacylated sucrose derivatives from the bark of Prunus padus. Chem. Pharm. Bull. 1990, 38, 415–417. [Google Scholar] [CrossRef]
- Bashir, A.; Hamburger, M.; Msonthi, J.D.; Hostettmann, K. Sinapoic acid esters from Polygala virgata. Phytochemistry 1993, 32, 741–745. [Google Scholar] [CrossRef]
- Qian-Cutrone, J.; Huang, S.; Trimble, J.; Li, H.; Lin, P.F.; Alam, M.; Klohr, S.E.; Kadow, K.F. Niruriside, a new HIV REV/RRE binding inhibitor from Phyllanthus niruri. J. Nat. Prod. 1996, 59, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Gao, W.; Zhang, Y.; Wang, Y. A new phenylpropanoid glycosides from Paris polyphylla var. yunnanensis. Fitoterapia 2008, 79, 306–307. [Google Scholar] [CrossRef] [PubMed]
- Takashi, H.; Yoshiyasu, F.; Toshihide, Y.; Kazuyuki, N. Structures of magnolosides B and C, novel phenylpropanoid glycosides with allopyranose as core the sugar unit. Chem. Pharm. Bull. 1988, 36, 1245–1248. [Google Scholar]
- Miyase, T.; Mimatsu, A. Acylated Iridoid and Phenylethanoid Glycosides from the Aerial Parts of Scrophularia nodosa. J. Nat. Prod. 1999, 62, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Karasawa, H.; Miyase, T.; Fukushima, S. Studies on the contituents of Cistanchis Herba. IV. Isolation and structures of two new phenylpropanoid glycosides, cistanosides C and D. Chem. Pharm. Bull. 1984, 32, 3880–3885. [Google Scholar] [CrossRef]
- Benkrief, R.; Ranarivelo, Y.; Skaltsounis, A.L.; Tillequin, F.; Koch, M.; Pusset, J.; Sévenet, T. Monoterpene alkaloids, iridoids and phenylpropanoid glycosides from Osmanthusaustrocaledonica. Phytochemistry 1998, 47, 825–832. [Google Scholar] [CrossRef]
- Su, B.N.; Ma, L.P.; Jia, Z.J. Iridoid and Phenylpropanoid Glycosides from Pedicularis artselaeri. J. Planta Med. 1998, 64, 720–723. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.M.; Jia, Z.G. Phenylpropanoid and iridoid glycosides from Pedicularis striata. Phytochemistry 1991, 30, 1341–1344. [Google Scholar] [PubMed]
- Kanchanapoom, T.; Kasai, R.; Yamasaki, K. Phenolic glycosides from Markhamia stipulata. Phytochemistry 2002, 59, 557–563. [Google Scholar] [CrossRef]
- Kanchanapoom, T.; Kasai, R.; Yamasaki, K. Lignan and phenylpropanoid glycosides from Fernandoa adenophylla. Phytochemistry 2001, 57, 1245–1248. [Google Scholar] [CrossRef]
- De Santos Galindez, J.; Diaz-Lanza, A.M.; Fernández Matellano, L.; Rumbero Sánchez, A. A new phenylpropanoid glycoside isolated from Scrophularia scorodonia L. Magn. Reson. Chem. 2000, 38, 688–691. [Google Scholar] [CrossRef]
- Skrzypek, Z.; Wysokinska, H.; Swia̧tek, L.; Wróblewski, A.E. Phenylpropanoid Glycosides from Penstemon serrulatus. J. Nat. Prod. 1999, 62, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.C.; Chen, C.M.; Li, Z.Q.; Row, L.C. Phenylpropanoid glycosides from the parasitic plant, Aeginetia indica. J. Chin. Chem. Soc. 2004, 51, 1073–1076. [Google Scholar] [CrossRef]
- Jia, Z.J.; Liu, Z.M.; Wang, C.Z. Phenylpropanoid and iridoid glycosides from Pedicularis lasiophrys. Phytochemistry 1992, 31, 263–266. [Google Scholar] [PubMed]
- Nonaka, G.; Nishioka, I. Bitter phenylpropanoid glycosides from Conandron ramoidioides. Phytochemistry 1977, 16, 1265–1267. [Google Scholar] [CrossRef]
- Çaliş, İ.; Taşdemir, D.; Wright, A.D.; Sticher, O. Lagotoside: A new phenylpropanoid glycoside from Lagotis stolonifera. Helv. Chim. Acta 1991, 74, 1273–1276. [Google Scholar] [CrossRef]
- Kang, K.H.; Jang, S.K.; Kim, B.K.; Park, M.K. Antibacterial phenylpropanoid glycosides from Paulownia tomentosa Steud. Arch. Pharm. Res. 1994, 17, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.J.; Gao, J.J. Phenylpropanoid glycosides from Pedicularis striata pallssp. Arachnoidea. Phytochemistry 1993, 34, 1188–1190. [Google Scholar]
- Jia, Z.J.; Liu, Z.M.; Wang, C.Z. Phenylpropanoid and iridoid glycosides from Pedicularis spicata. Phytochemistry 1991, 30, 3745–3747. [Google Scholar] [PubMed]
- Jia, Z.J.; Liu, Z.M. Phenylpropanoid and iridoid glycosides from Pedicularis longiflora. Phytochemistry 1992, 31, 3125–3127. [Google Scholar]
- Ersoz, T.; SaracogluA, İ.; Harput, Ü.Ş.; Çalis, İ.; Donmez, A.A. Iridoid and phenylpropanoid glycosides from Phlomis grandiflora var. fimbrilligera and Phlomis fruticosa. Turk. J. Chem. 2002, 26, 171–178. [Google Scholar]
- Kobayashi, H.; Karasawa, H.; Miyase, T.; Fukushima, S. Studies on the constituents of Cistanchis herba. III. Isolation and structures of new phenylpropanoid glycosides, cistanosides A and B. Chem. Pharm. Bull. 1984, 32, 3009–3014. [Google Scholar] [CrossRef]
- He, Z.D.; Yang, C.R. Brandioside, a phenylpropanoid glycoside from Brandisia hancei. Phytochemistry 1991, 30, 701–702. [Google Scholar] [PubMed]
- Çaliş, İ. Two phenylpropanoid glycosides from Leonurus glaucescens. Phytochemistry 1992, 31, 357–359. [Google Scholar] [CrossRef]
- Sticher, O.; Rüedi, P. Phlinosides A, B and C, three phenylpropanoid glycosides from Phlomis linearis. Phytochemistry 1990, 29, 1253–1257. [Google Scholar]
- Kobayashi, H.; Karasawa, H.; Miyase, T.; Fukushima, S. Studies on the constituents of Cistanchis Herba. V. Isolation and structures of two new phenylpropanoid glycosides, cistanosides E and F. Chem. Pharm. Bull. 1985, 33, 1452–1457. [Google Scholar] [CrossRef]
- Yi, J.H.; Zhang, G.L.; Li, B.G.; Chen, Y.Z. Phenylpropanoid glycosides from Lamiophlomis rotata. Phytochemistry 1999, 51, 825–828. [Google Scholar] [CrossRef]
- Calis, I.; Lahloub, M.F.; Rogenmoser, E.; Sticher, O. Isomartynoside, a phenylpropanoid glycoside from Galeopsis pubescens. Phytochemistry 1984, 23, 2313–2315. [Google Scholar] [CrossRef]
- Jimenez, C.; Villaverde, M.C.; Riguera, R.; Castedo, L.; Stermitz, F.R. Five phenylpropanoid glycosides from Mussatia. Phytochemistry 1988, 27, 2947–2951. [Google Scholar] [CrossRef]
- Hosoya, T.; Yun, Y.S.; Kunugi, A. Antioxidant phenylpropanoid glycosides from the leaves of Wasabia japonica. Phytochemistry 2008, 69, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Nahrstedt, A.; Rockenbach, J.; Wray, V. Phenylpropanoid glycosides, a furanone glucoside and geniposidic acid from members of the rubiaceae. Phytochemistry 1995, 39, 375–378. [Google Scholar] [CrossRef]
- Afifi, M.S.; Lahloub, M.F.; El-Khayaat, S.A.; Anklin, C.G.; Rüegger, H.; Sticher, O. Crenatoside: A Novel Phenylpropanoid Glycoside from Orobanche crenata. Planta Med. 1993, 59, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Miyase, T.; Kuroyanagi, M.; Umehara, K.; Noguchi, H. Oligosaccharide polyesters from roots of Polygala fallax. Phytochemistry 1997, 45, 733–741. [Google Scholar] [CrossRef]
- Kobayashi, S.; Miyase, T.; Noguchi, H. Polyphenolic Glycosides and Oligosaccharide Multiesters from the Roots of Polygala dalmaisiana. J. Nat. Prod. 2002, 65, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Seligmann, O.; Wagner, H.; Bauer, R. Paucifloside, A New Phenylpropanoid Glycoside from Lysionotus pauciflorus. Nat. Prod. Lett. 1995, 7, 23–28. [Google Scholar] [CrossRef]
- Saracoglu, I.; Harput, U.S.; Inoue, M.; Ogihara, Y. New phenylethanoid glycosides from Veronica pectinata var. glandulosa and their free radical scavenging activities. Chem. Pharm. Bull. 2002, 50, 665–668. [Google Scholar] [PubMed]
- Rønsted, N.; Bello, M.A.; Jensen, S.R. Aragoside and iridoid glucosides from Aragoa cundinamarcensis. Phytochemistry 2003, 64, 529–533. [Google Scholar] [CrossRef]
- Thuan, N.D.; Thuong, P.T.; Na, M.K.; Bae, K.; Lee, J.P.; Lee, J.H.; Seo, H.W.; Min, B.S.; Kim, J.C.; Bae, K.H. A phenylpropanoid glycoside with antioxidant activity from Picria tel-ferae. Arch. Pharm. Res. 2007, 30, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Shyr, M.H.; Tsai, T.H.; Lin, L.C. Rossicasins A, B and rosicaside F, three new phenylpropanoid glycosides from Boschniakia rossica. Chem. Pharm. Bull. 2006, 54, 252–254. [Google Scholar] [CrossRef] [PubMed]
- Boros, C.A.; Marshall, D.R.; Caterino, C.R.; Stermitz, F.R. Iridoid and phenylpropanoid glycosides from Orthocarpus spp. Alkaloid content as a consequence of parasitism on Lupinus. J. Nat. Prod. 1991, 54, 506–513. [Google Scholar] [CrossRef]
- Budzianowski, J.; Skrzypczak, L. Phenylpropanoid esters from Lamium album flowers. Phytochemistry 1995, 38, 997–1001. [Google Scholar] [CrossRef]
- Gross, G.A.; Lahloub, M.F.; Anklin, C.; Schulten, H.R.; Sticher, O. Teucrioside, a phenylpropanoid glycoside from Teucrium chamaedrys. Phytochemistry 1988, 27, 1459–1463. [Google Scholar] [CrossRef]
- Çalis, İ.; Başaran, A.A.; Saracog̈lu, İ.; Sticher, O. Phlinosides D and E, phenylpropanoid glycosides, and iridoids from Phlomis linearis. Phytochemistry 1991, 30, 3073–3075. [Google Scholar] [CrossRef]
- Yang, H.; Hou, A.J.; Mei, S.X.; Peng, L.Y.; Sun, H.D. A new phenylpropanoid glycoside: Serratumoside A from Clerodendrum serratum. Chin. Chem. Lett. 2000, 11, 323–326. [Google Scholar]
- Jiménez, C.; Villaverde, M.C.; Riguera, R.; Castedo, L.; Stermitz, F. Phenylpropanoid glycosides from Mussatia hyacinthina. J. Nat. Prod. 1989, 52, 408–410. [Google Scholar] [CrossRef]
- Saitoh, H.; Miyase, T.; Ueno, A. Senegoses F-I, Oligosaccharide Multi-Esters from the Roots of Polygala senega var latifolia Torr. Et Gray. Chem. Pharm. Bull. 1993, 41, 2125–2128. [Google Scholar] [CrossRef] [PubMed]
- Çalış, I.; Kırmızıbekmez, H. Glycosides from Phlomis lunariifolia. Phytochemistry 2004, 65, 2619–2625. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, H.; Miyase, T.; Ueno, A.; Atarashi, K.; Saiki, Y. Senegoses J-O, oligosaccharide multi-esters from the roots of Polygala senega L. Chem. Pharm. Bull. 1994, 42, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, H.; Miyase, T.; Ueno, A. Senegoses A-E, Oligosaccharide Multi-Esters from Polygala senega var. latifolia Torr.et Gray. Chem. Pharm. Bull. 1993, 41, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Murakami, T.; Ueno, T.; Kadoya, M.; Matsuda, H.; Yamahara, J.; Murakami, N. Bioactive Saponins and Glycosides. I. Senegae Radix. (1): E-Senegasaponins a and b and Z-Senegasaponins a and b. Their Inhibitory Effect on Alcohol Absorption and Hypoglycemic Activity. Chem. Pharm. Bull. 1995, 43, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zuo, L.; Yang, J.; Chen, R.; Zhang, D. Oligosaccharide polyester and triterpenoid saponins from the roots of Polygala japonica. Phytochemistry 2008, 69, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Miyase, T.; Kuroyanagi, M.; Umehara, K.; Noguchi, H. Polygalasaponins XLII–XLVI from roots of Polygala glomerata. Phytochemistry 1998, 47, 459–466. [Google Scholar] [CrossRef]
- Ikeya, Y.; Takeda, S.; Tunakawa, M.; Karakida, H.; Toda, K.; Yamaguchi, T.; Aburada, M. Cognitive Improving and Cerebral Protective Effects of Acylated Oligosaccharides in Polygala tenuifolia. Biol. Pharm. Bull. 2004, 27, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, M.; Liu, P.; Gao, D.H.; Wei, R.B.; Rahman, K. Possible mechanism of the antidepressant effect of 3,6′-disinapoyl sucrose from Polygala tenuifolia Willd. J. Pharm. Parmacol. 2011, 63, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, M.Y.; Liu, P.; Dong, X.Z.; Boran, A.D.W. Neuroprotective Effects of 3,6′-Disinapoyl Sucrose Through Increased BDNF Levels and CREB Phosphorylation via the CaMKII and ERK1/2 Pathway. J. Mol. Neurosci. 2014, 53, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Fabre, N.; Urizzi, P.; Souchard, J.P.; Fréchard, A.; Claparols, C.; Fourasté, I.; Moulis, C. An antioxidant sinapic acid ester isolated from Iberis amara. Fitoterapia 2000, 71, 425–428. [Google Scholar] [CrossRef]
- Wang, M.; Shao, Y.; Li, J.; Zhu, N.; Rangarajan, M.; LaVoie, E.J.; Ho, C.T. Antioxidative phenolic glycosides from sage (Salvia officinalis). J. Nat. Prod. 1999, 62, 454–456. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.C.; Wang, Y.W.; Hou, Y.C.; Chang, S.; Liou, K.T.; Chou, Y.C.; Wang, W.Y.; Shen, Y.C. The inhibitory effect of phenylpropanoid glycosides and iridoid glucosides on free radical production and β2 integrin expression in human leucocytes. J. Pharm. Parmacol. 2006, 58, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, P.; Abad, M.J.; Díaz, A.M.; Fernández, L.; De Santos, J.; Sanchez, S.; Villaescusa, L.; Carrasco, L.; Irurzun, A. Antiviral Activity of Seven Iridoids, Three Saikosaponins and One Phenylpropanoid Glycoside Extracted from Bupleurumrigidum and Scrophularia scorodonia. Planta Med. 2002, 68, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Sahpaz, S.; Garbacki, N.; Tits, M.; Bailleul, F. Isolation and pharmacological activity of phenylpropanoid esters from Marrubium vulgare. J. Ethnopharmacol. 2002, 79, 389–392. [Google Scholar] [CrossRef]
- Kawai, Y.; Kumagai, H.; Kurihara, H.; Yamazaki, K.; Sawano, R.; Inoue, N. β-Glucosidase inhibitory activities of phenylpropanoid glycosides, vanicoside A and B from Polygonum sachalinense rhizome. Fitoterapia 2006, 77, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Ismailoglu, U.B.; Saracoglu, I.; Harput, U.S.; Sahin-Erdemli, I. Effects of phenylpropanoid and iridoid glycosides on free radical-induced impairment of endothelium-dependent relaxation in rat aortic rings. J. Ethnopharmacol. 2002, 79, 193–197. [Google Scholar] [CrossRef]
- Kako, M.; Miura, T.; Nishiyama, Y.; Ichimaru, M.; Moriyasu, M.; Kato, A. Hypoglycemic activity of some triterpenoid glycosides. J. Nat. Prod. 1997, 60, 604–605. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds not avalible are available from the authors.
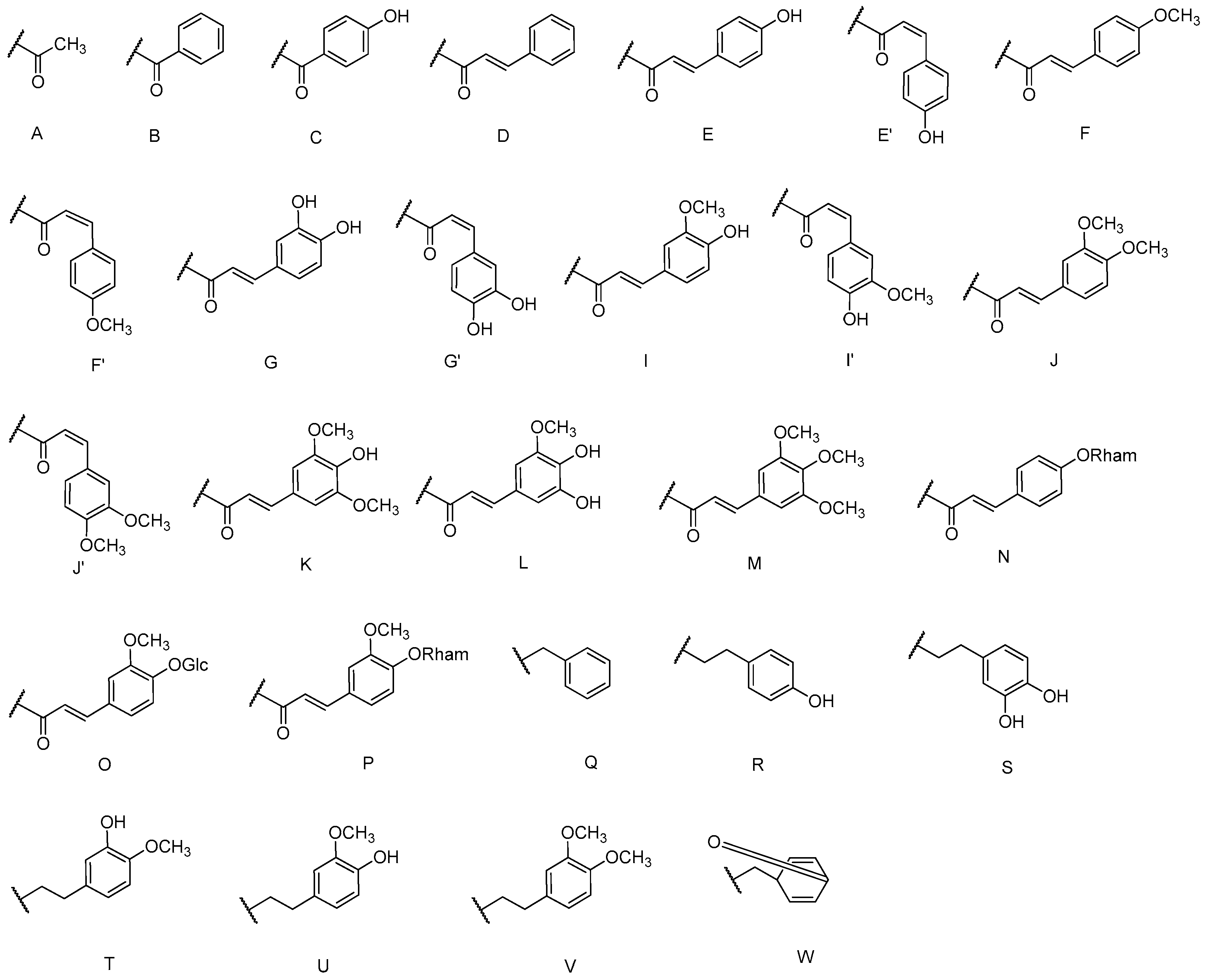
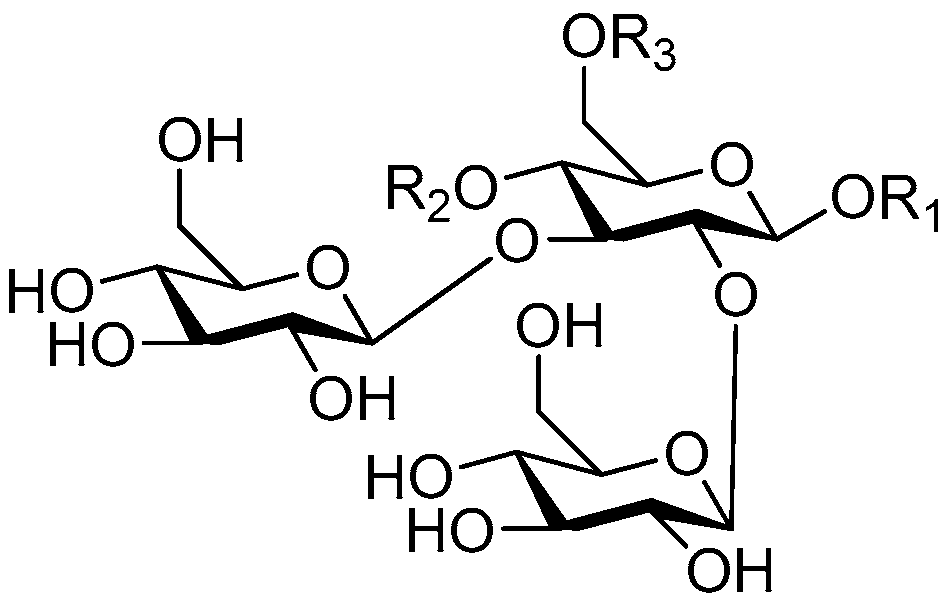
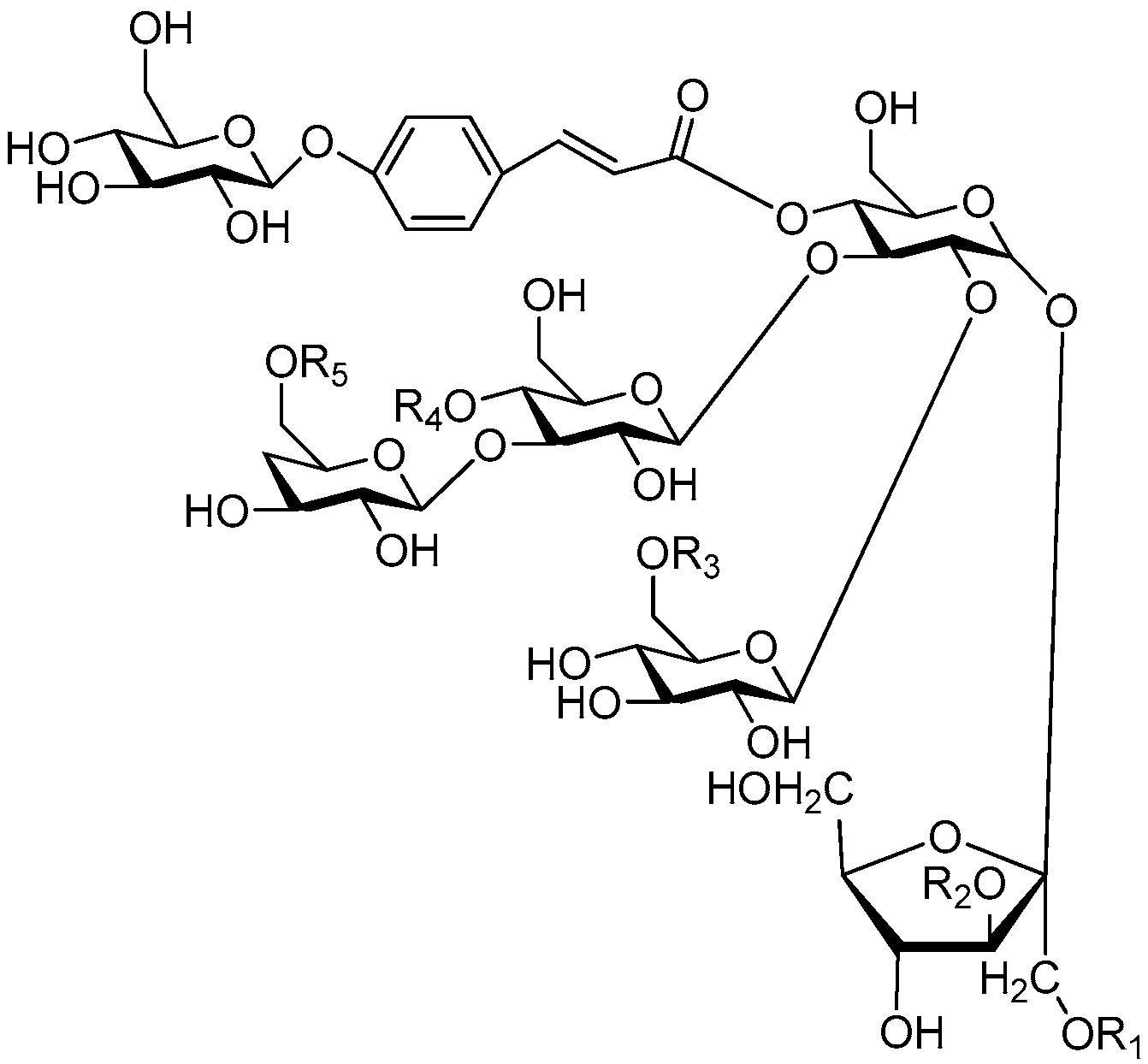
| Cpd. | R1 | R2 | R3 | R4 | R5 | Cpd. | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | E | H | H | H | G | 6 | R | H | H | E | H |
| 2 | G | H | H | H | G | 7 | S | H | H | H | G |
| 3 | S | H | H | H | I | 8 | S | H | H | G | H |
| 4 | I | H | H | H | I | 9 | T | H | H | H | G |
| 5 | R | H | H | H | E | 10 | V | H | H | H | G |

| Cpd. | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 11 | H | H | H | F |
| 12 | H | A | F | H |
| 13 | H | A | F | F |
| 14 | H | F | A | H |
| 15 | H | A | F′ | H |

| Cpd. | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|
| 16 | H | H | H | H | E |
| 17 | H | H | H | H | G |
| 18 | H | H | H | H | K |
| 19 | H | H | H | E | H |
| 20 | H | H | G′ | H | G |
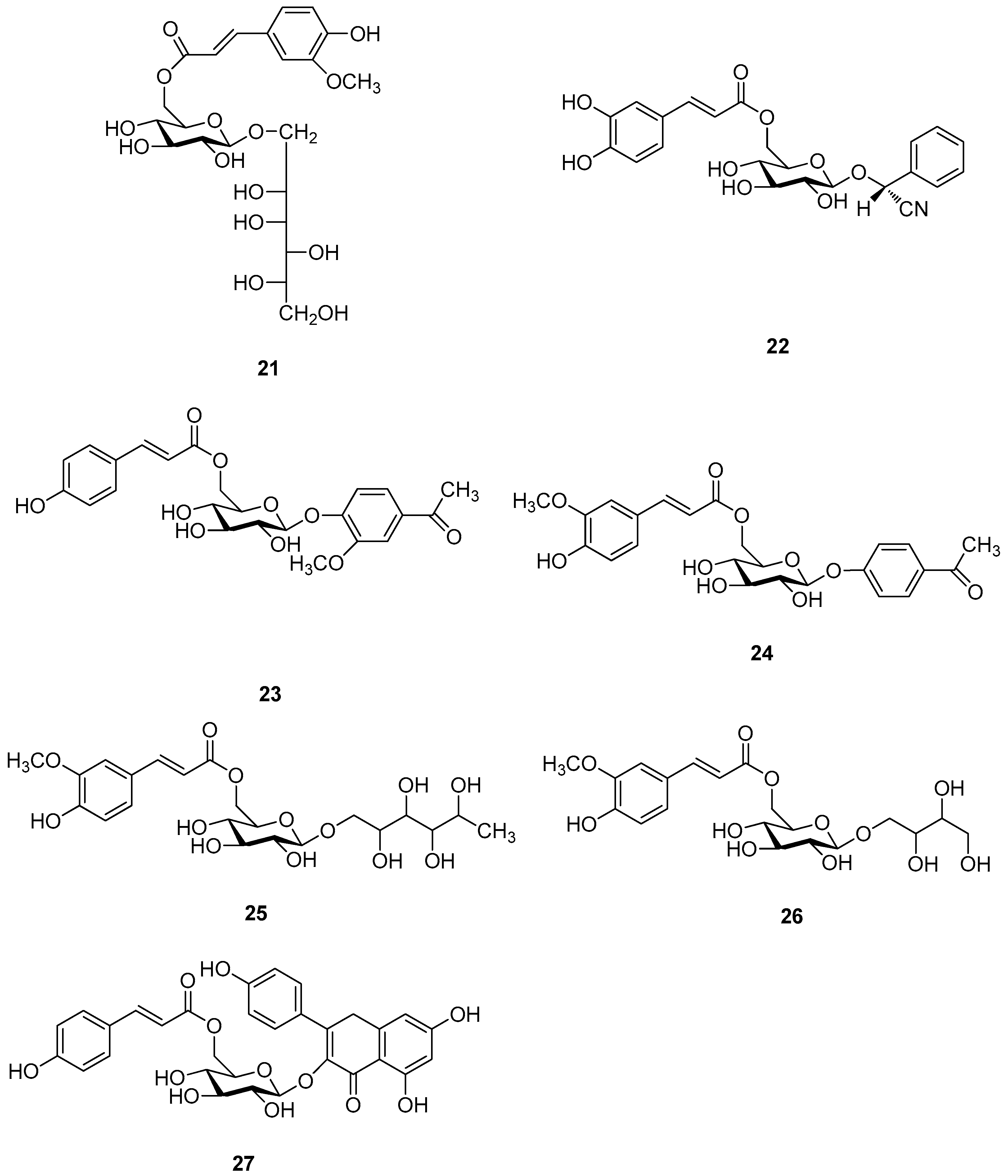
| Cpd. | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | Cpd. | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 28 | H | H | H | H | H | H | H | I | 75 | H | H | H | K | H | H | H | H |
| 29 | H | H | H | H | H | H | H | K | 76 | H | H | H | K | H | H | H | I |
| 30. | H | H | H | H | H | H | H | M | 77 | H | H | H | M | H | H | H | M |
| 31 | H | H | H | H | H | E | H | E | 78 | H | H | H | M | H | H | H | H |
| 32 | H | H | H | H | H | I | H | I | 79 | H | H | A | A | H | I | H | I |
| 33 | H | H | H | H | H | I | H | E | 80 | H | H | A | B | H | H | H | M |
| 34 | H | H | H | H | K | H | H | I | 81 | H | H | A | I | H | H | H | I |
| 35 | H | H | H | H | K | H | H | K | 82 | H | H | A | K | H | H | H | K |
| 36 | H | H | H | H | A | H | H | I | 83 | H | H | B | H | H | H | H | I |
| 37 | H | H | H | H | E | E | H | E | 84 | H | H | B | H | H | H | H | M |
| 38 | H | H | H | H | E | I | H | E | 85 | H | H | E | A | H | I | H | I |
| 39 | H | H | H | H | E | I | H | I | 86 | H | H | I | H | H | I | H | I |
| 40 | H | H | H | H | E | I | A | I | 87 | H | H | I | A | H | I | H | I |
| 41 | H | H | H | H | I | I | H | E | 88 | H | H | K | H | H | H | H | K |
| 42 | H | H | H | H | I | I | H | I | 89 | H | A | H | A | A | H | H | E |
| 43 | H | H | H | A | H | H | H | M | 90 | H | A | H | A | H | I | H | I |
| 44 | H | H | H | A | H | H | H | I | 91 | H | A | H | I | E | E | H | E |
| 45 | H | H | H | A | H | I | H | I | 92 | H | A | H | I | H | A | H | I |
| 46 | H | H | H | A | E | I | H | E | 93 | H | A | H | I | H | A | A | I |
| 47 | H | H | H | A | E | I | H | I | 94 | H | A | H | K | H | H | H | K |
| 48 | H | H | H | B | H | H | H | I | 95 | H | I | H | H | H | I | H | H |
| 49 | H | H | H | B | H | H | H | M | 96 | H | A | A | A | A | H | H | E |
| 50 | H | H | H | B | H | H | H | K | 97 | A | H | H | A | A | I | H | I |
| 51 | H | H | H | C | H | H | H | M | 98 | A | H | H | A | H | I | H | I |
| 52 | H | H | H | C | H | H | H | K | 99 | A | H | H | A | I | I | H | E |
| 53 | H | H | H | D | H | H | H | H | 100 | A | H | H | A | I | I | H | I |
| 54 | H | H | H | E | H | H | H | K | 101 | A | H | H | A | E | I | H | I |
| 55 | H | H | H | E | H | H | H | M | 102 | A | H | H | I | E | E | H | E |
| 56 | H | H | H | E | H | H | I | I | 103 | A | H | H | I | I | E | H | E′ |
| 57 | H | H | H | E | E | E | H | E | 104 | A | H | H | H | E | E | H | E |
| 58 | H | H | H | E | E | I | H | E | 105 | A | H | H | H | E | I | H | I |
| 59 | H | H | H | G | H | H | H | H | 106 | A | H | H | H | E | I | H | E |
| 60 | H | H | H | G | H | H | I | I | 107 | A | H | H | H | H | I | H | I |
| 61 | H | H | H | I | H | H | H | M | 108 | A | H | A | A | H | I | H | I |
| 62 | H | H | H | I | H | H | H | H | 109 | A | H | A | A | A | D | H | D |
| 63 | H | H | H | I | I | E | H | E | 110 | A | H | A | A | A | I | H | I |
| 64 | H | H | H | I | I | I | H | E | 111 | A | H | A | A | I | I | H | I |
| 65 | H | H | H | I | H | E | H | E | 112 | A | H | A | A | E | I | H | I |
| 66 | H | H | H | I | E | E | H | H | 113 | A | H | A | I | E | E | H | E |
| 67 | H | H | H | I | E | E | H | E | 114 | A | H | E | H | H | I | H | I |
| 68 | H | H | H | I | H | H | A | I | 115 | A | H | I | H | H | I | H | I |
| 69 | H | H | H | I | H | A | H | I | 116 | A | A | A | A | H | H | H | E |
| 70 | H | H | H | I | H | A | A | I | 117 | A | A | H | A | A | H | H | E |
| 71 | H | H | H | I | H | H | H | I | 118 | E | H | A | H | A | E | H | E |
| 72 | H | H | H | K | H | H | H | M | 119 | I | H | H | H | H | I | H | I |
| 73 | H | H | H | K | H | H | H | K | 120 | A | A | A | A | H | H | H | G |
| 74 | H | H | H | K | H | H | K | K |

| Cpd. | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 121 | S | G | H | H |
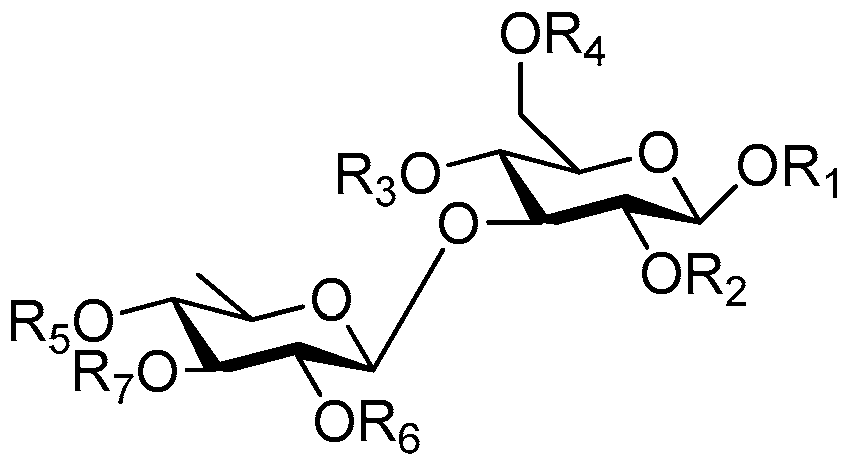
| Cpd. | R1 | R2 | R3 | R4 | R5 | R6 | R7 | Cpd. | R1 | R2 | R3 | R4 | R5 | R6 | R7 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 122 | R | H | H | E | H | H | H | 132 | S | H | G′ | H | H | H | H |
| 123 | R | H | E | H | H | H | H | 133 | S | H | I | H | H | H | H |
| 124 | G | H | T | H | H | H | H | 134 | S | H | I′ | H | H | H | H |
| 125 | Q | H | G | H | H | H | H | 135 | S | A | G | H | H | H | A |
| 126 | R | H | E | H | H | H | H | 136 | S | A | G | H | H | H | H |
| 127 | S | H | H | E | H | H | H | 137 | T | H | A | I | H | A | H |
| 128 | S | H | H | G | H | H | H | 138 | T | H | I | H | H | H | H |
| 129 | S | H | E′ | H | H | H | H | 139 | T | H | I′ | H | H | H | H |
| 130 | S | H | G | D | H | H | H | 140 | U | H | I | H | H | H | H |
| 131 | S | H | G | H | H | H | H |

| Cpd. | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 141 | S | H | G | H |
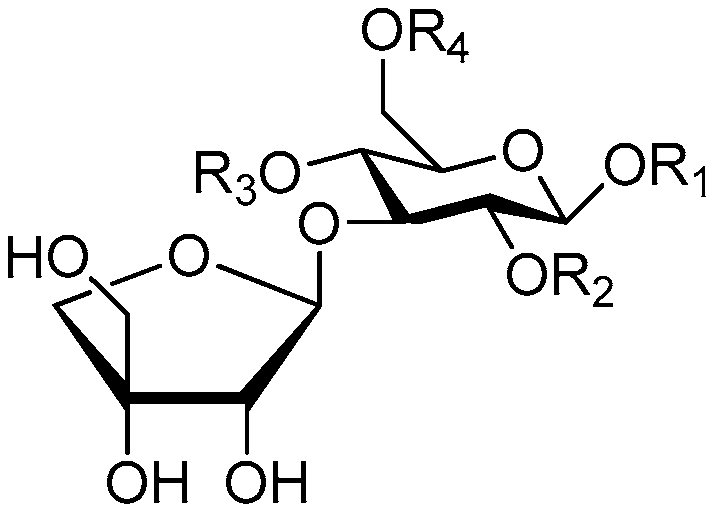
| Cpd. | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 142 | S | H | H | G |
| 143 | S | H | G | H |
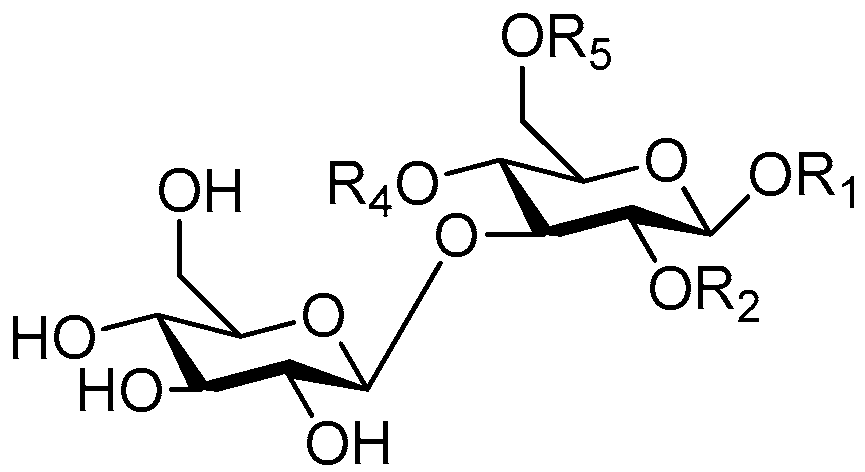
| Cpd. | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 144 | S | H | G | H |

| Cpd. | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 145 | H | H | H | E |
| 146 | H | H | G | H |
| 147 | T | H | H | I |
| 148 | S | H | H | G |
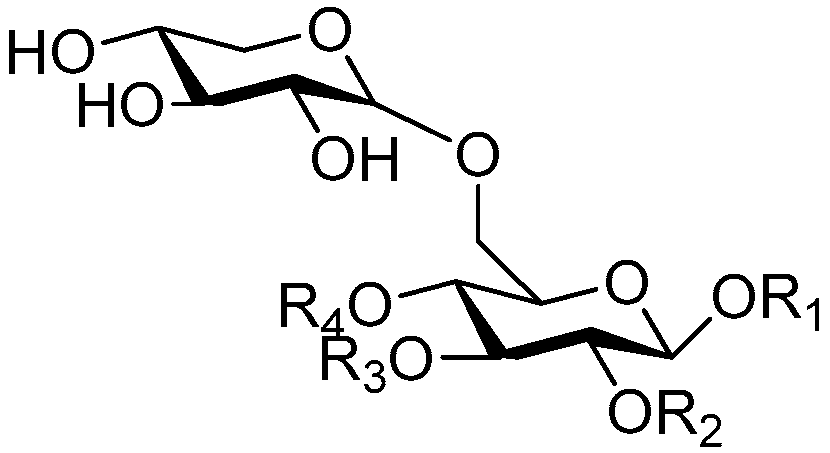
| Cpd. | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 148 | S | H | H | G |
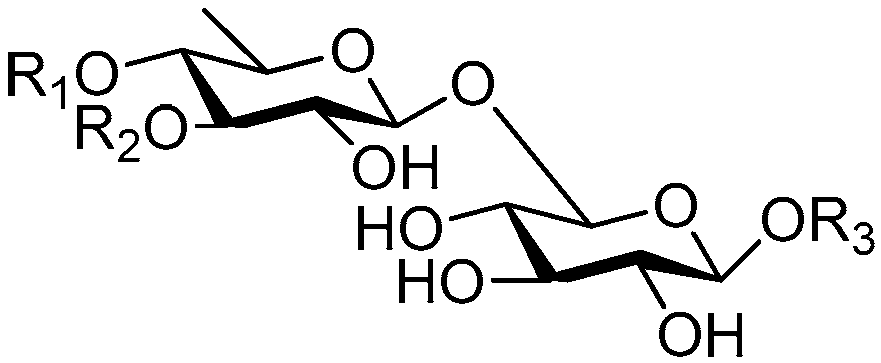
| Cpd. | R1 | R2 | R3 |
|---|---|---|---|
| 149 | D | H | R |
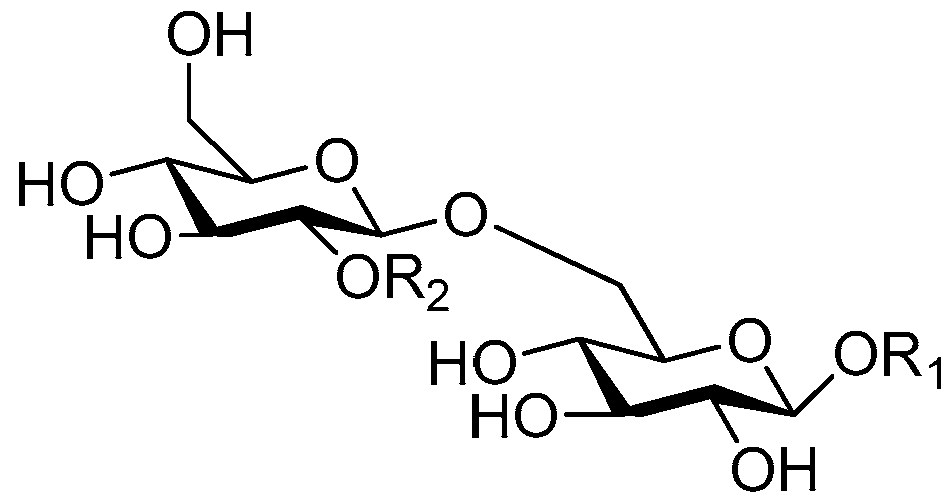
| Cpd. | R1 | R2 |
|---|---|---|
| 150 | G | K |
| 151 | I | K |
| 152 | K | K |
| 153 | L | I |
| 154 | L | K |
| 155 | L | L |
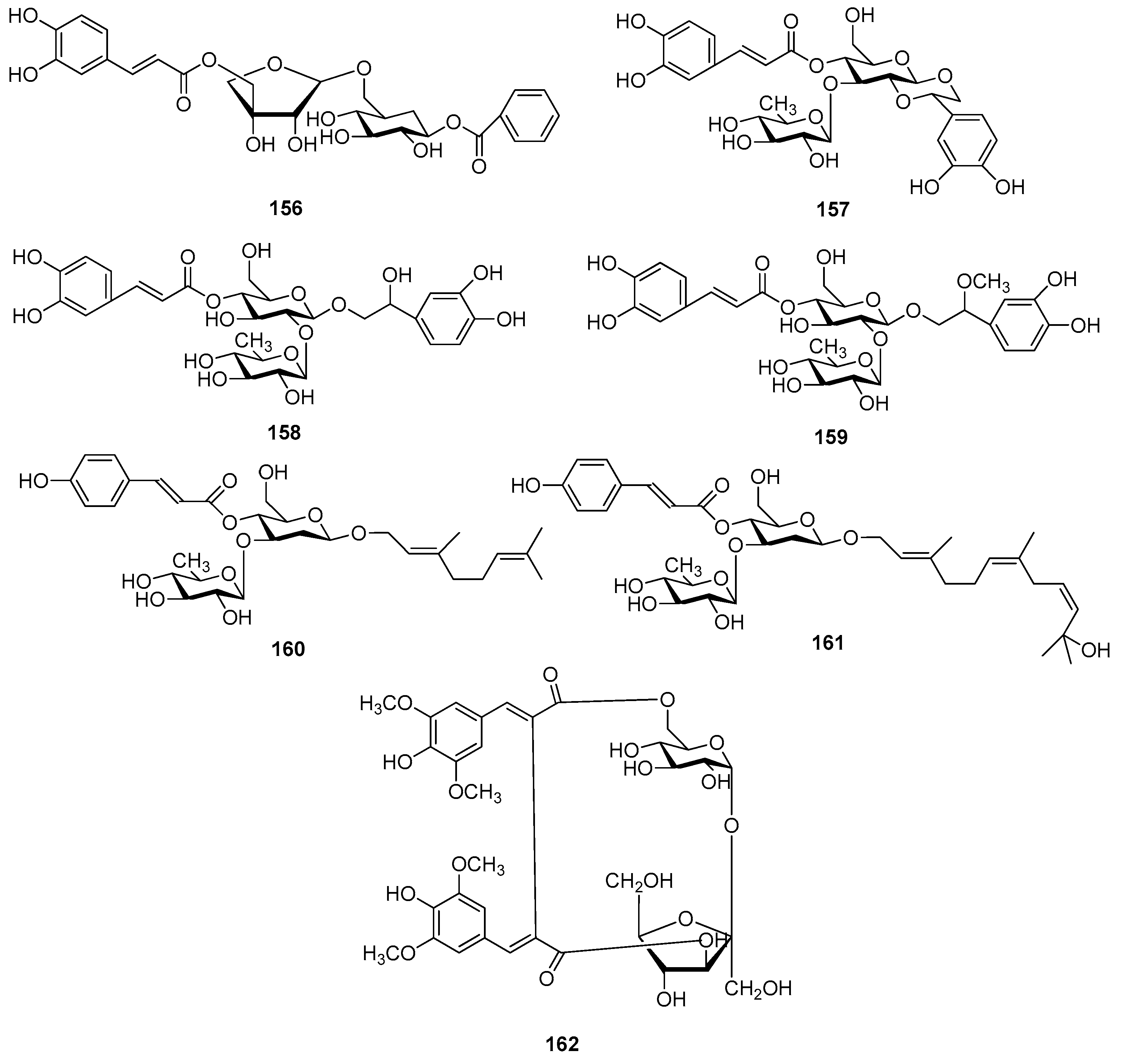
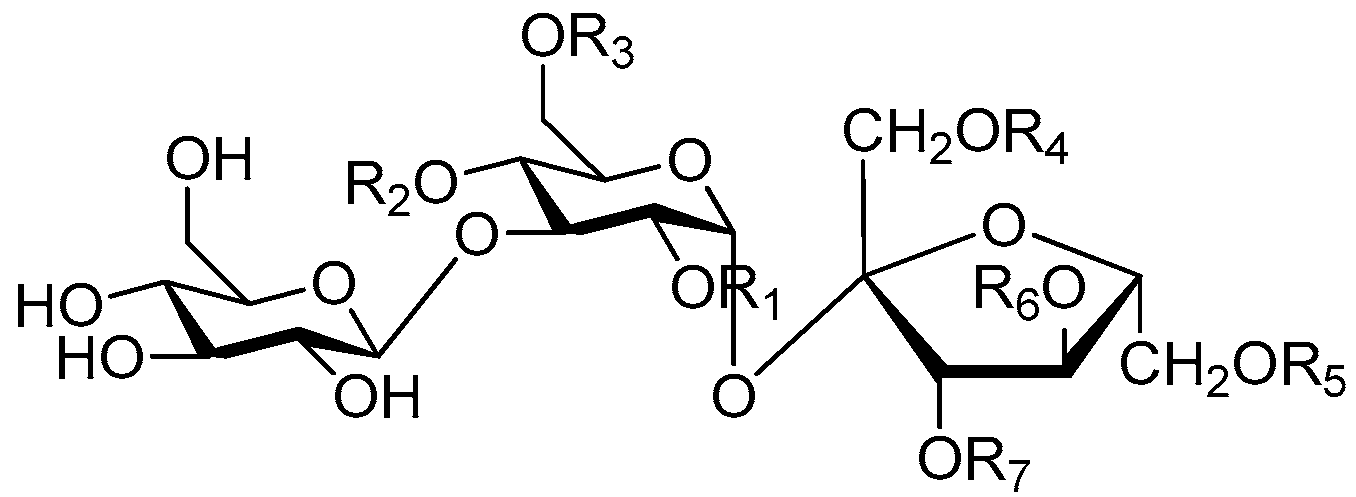
| Cpd. | R1 | R2 | R3 | Cpd. | R1 | R2 | R3 |
|---|---|---|---|---|---|---|---|
| 163 | K | H | K | 165 | M | I | K |
| 164 | M | H | K | 166 | M | K | K |

| Cpd. | R1 | R2 | R3 | R4 | R5 | R6 | R7 |
|---|---|---|---|---|---|---|---|
| 167 | H | H | I | H | H | H | K |
| 168 | H | H | K | H | H | H | K |
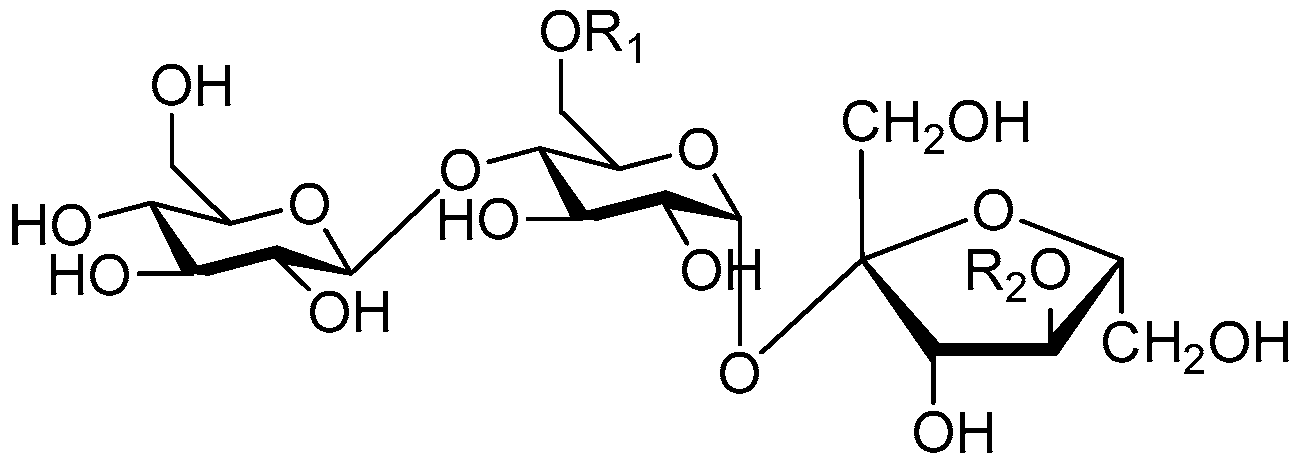
| Cpd. | R1 | R2 | Cpd. | R1 | R2 |
|---|---|---|---|---|---|
| 169 | I | H | 171 | H | I |
| 170 | K | H | 172 | H | K |
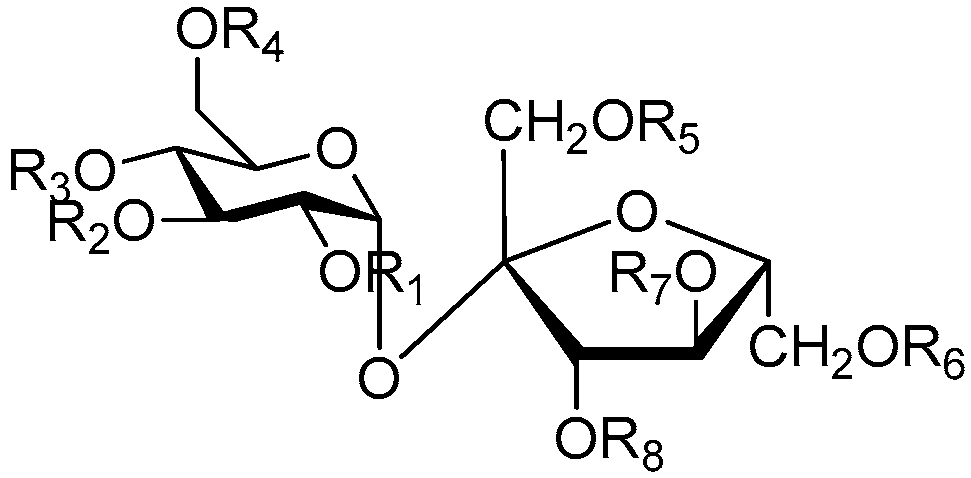
| Cpd. | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 |
|---|---|---|---|---|---|---|---|---|
| 173 | H | H | H | O | H | H | A | I |
| 174 | H | H | P | B | H | H | H | B |

| Cpd. | R1 | R2 | R3 |
|---|---|---|---|
| 175 | E | A | D |
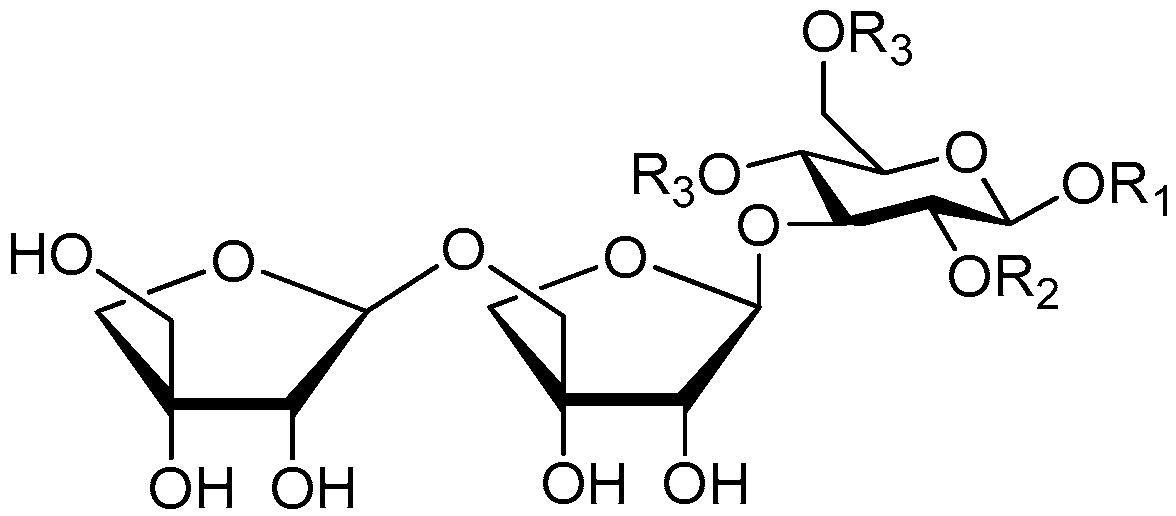
| Cpd. | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 176 | S | H | H | G |
| 177 | S | H | G | H |
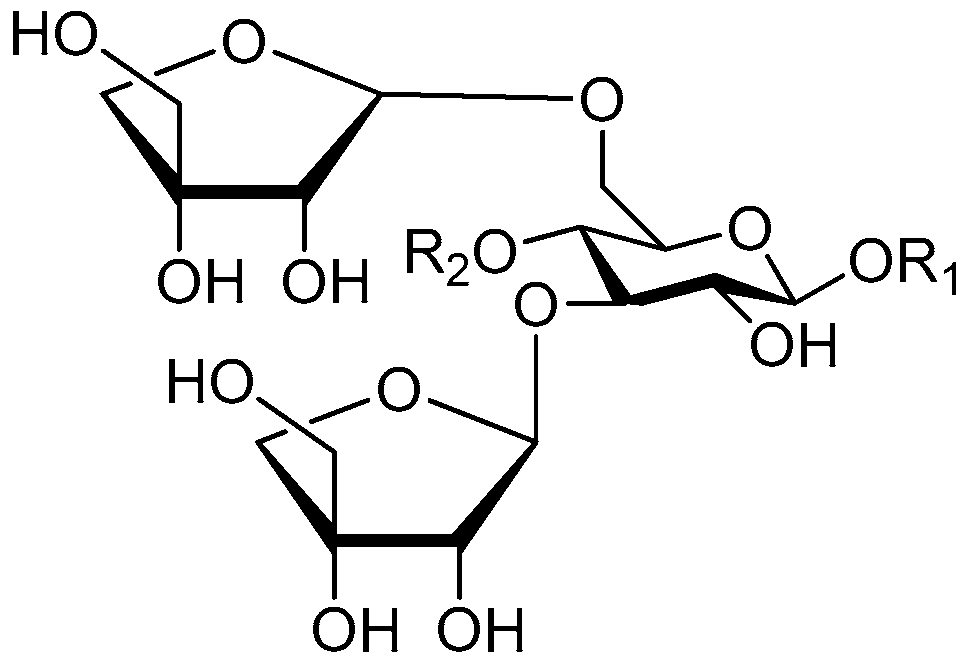
| Cpd. | R1 | R2 |
|---|---|---|
| 178 | S | G |

| Cpd. | R1 | R2 |
|---|---|---|
| 179 | S | G |
| 180 | S | I |
| 181 | T | I |
| 182 | U | I |
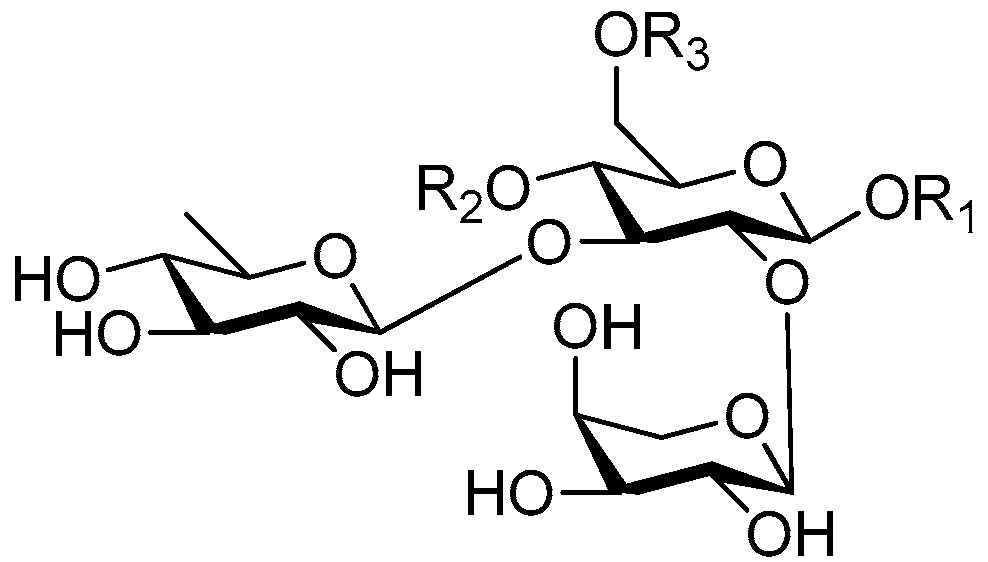
| Cpd. | R1 | R2 | R3 |
|---|---|---|---|
| 183 | S | H | G |
| 184 | S | G | H |
| 185 | S | G | A |
| 186 | S | I | H |
| 187 | T | I | H |
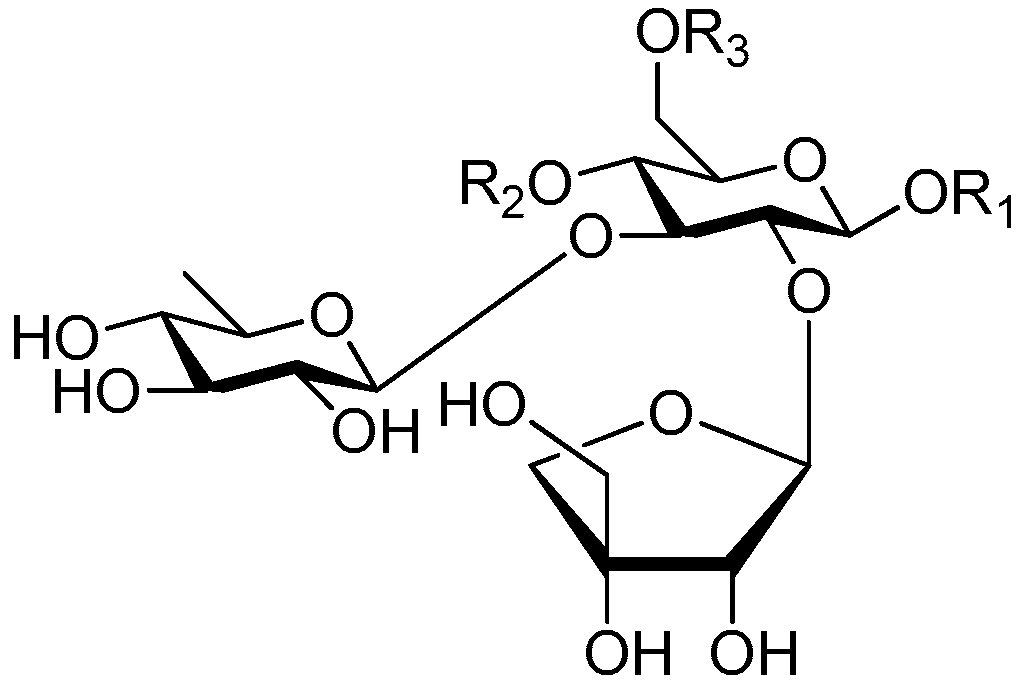
| Cpd. | R1 | R2 | R3 |
|---|---|---|---|
| 188 | S | G | H |
| 189 | S | G | A |
| 190 | S | H | G |
| 191 | S | H | I |
| 192 | T | H | I |
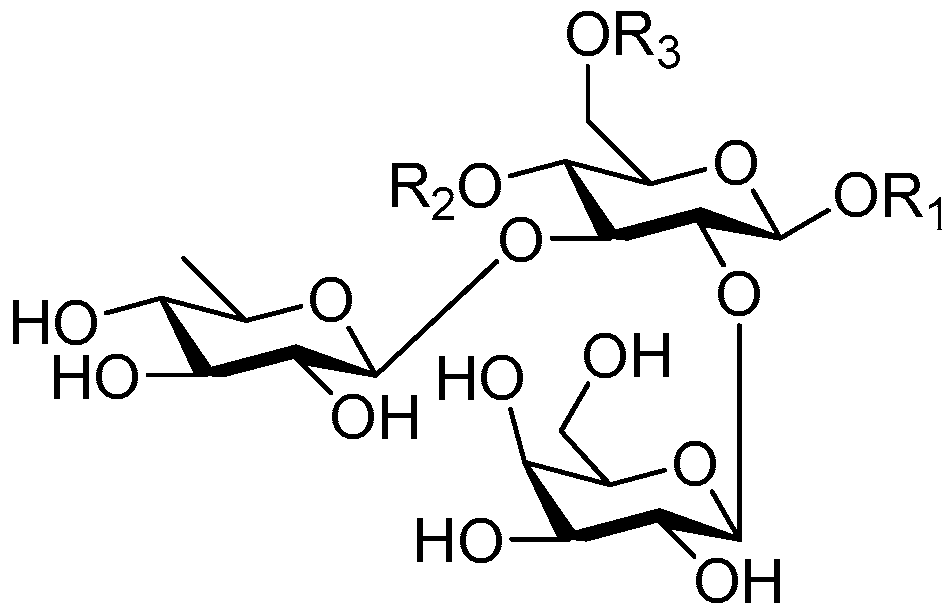
| Cpd. | R1 | R2 | R3 |
|---|---|---|---|
| 193 | S | G | A |
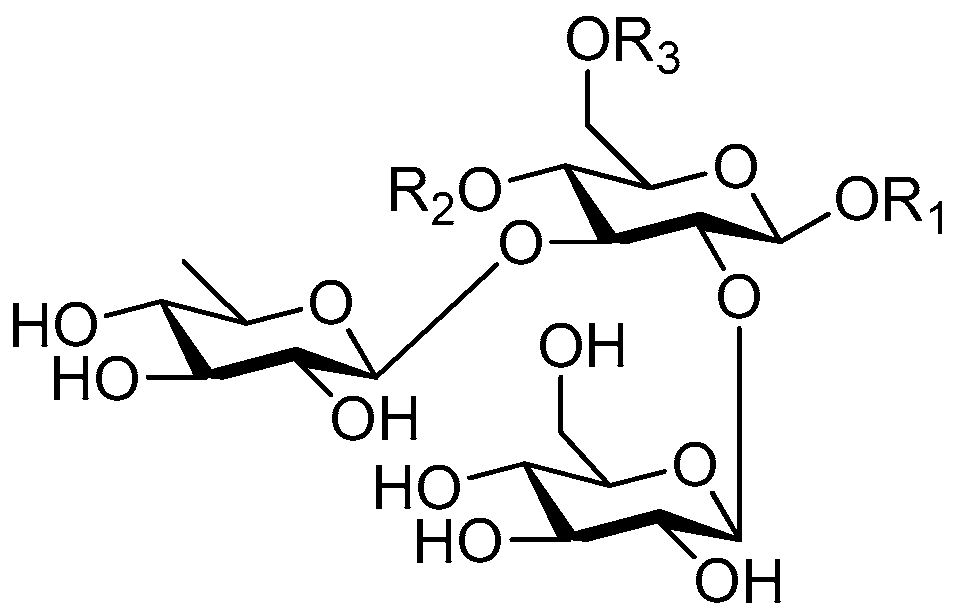
| Cpd. | R1 | R2 | R3 |
|---|---|---|---|
| 194 | S | G | H |
| 195 | S | I | H |
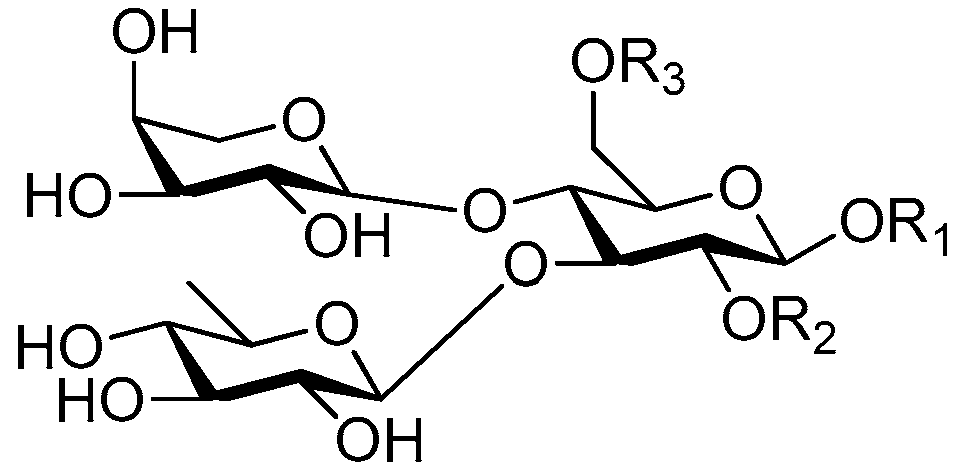
| Cpd. | R1 | R2 | R3 |
|---|---|---|---|
| 196 | U | H | I |
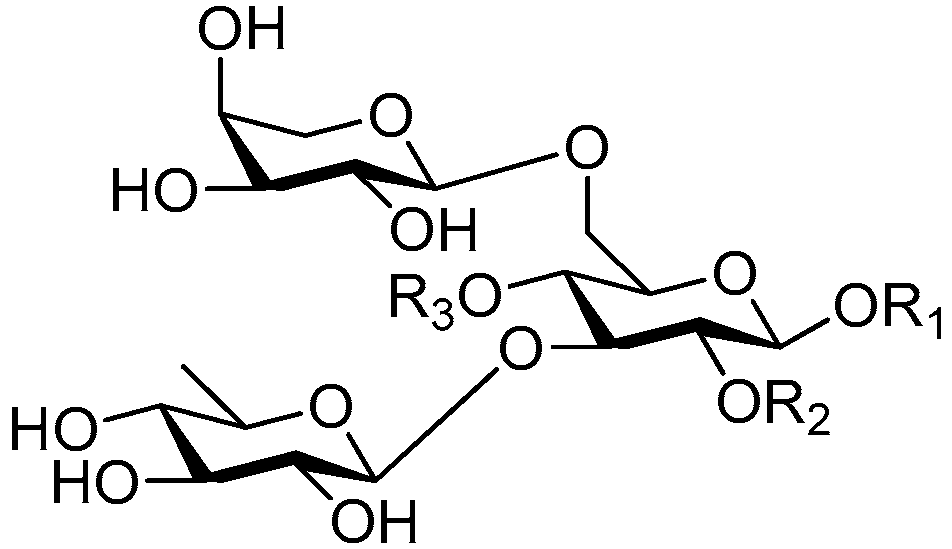
| Cpd. | R1 | R2 | R3 |
|---|---|---|---|
| 197 | S | H | G |
| 198 | S | H | I |
| 199 | S | H | I′ |
| 200 | S | H | I |
| 201 | T | H | I |

| Cpd. | R1 | R2 | R3 |
|---|---|---|---|
| 202 | S | H | G |
| 203 | U | H | G |
| 204 | U | H | I |
| 205 | U | H | I′ |
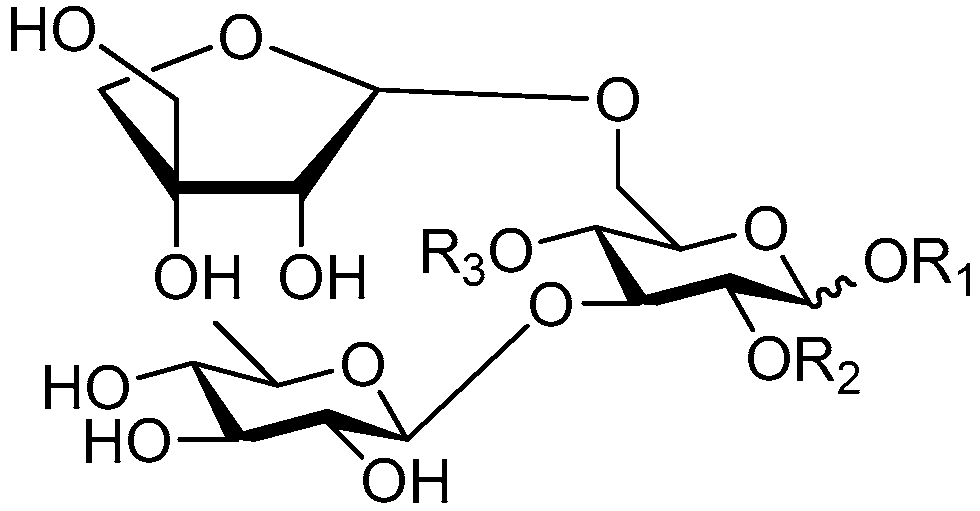
| Cpd. | R1 | R2 | R3 |
|---|---|---|---|
| 206 | S | H | G |
| 207 | S | H | I |
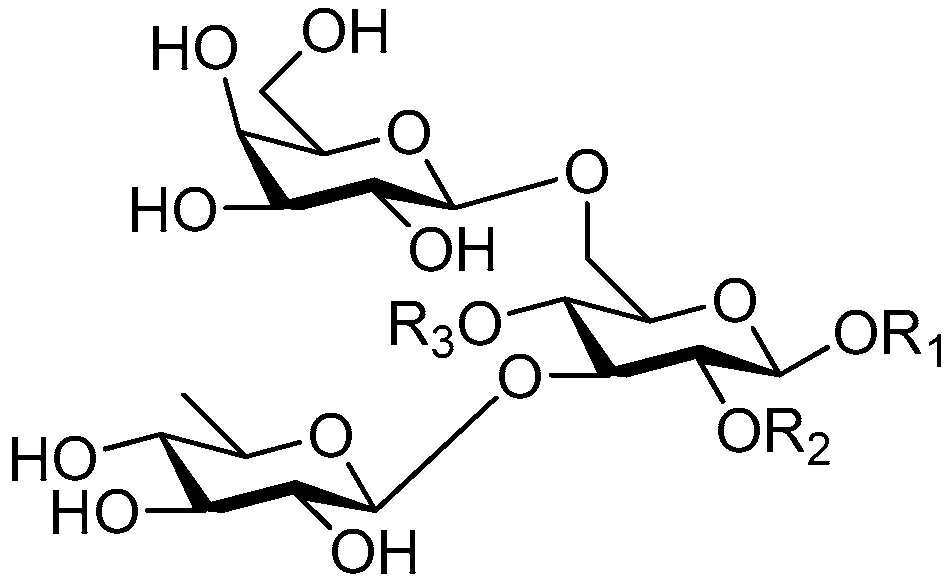
| Cpd. | R1 | R2 | R3 |
|---|---|---|---|
| 208 | S | H | E |
| 209 | S | H | G |
| 210 | S | H | I |
| 211 | S | H | I′ |
| 212 | U | H | I |
| 213 | U | H | I′ |
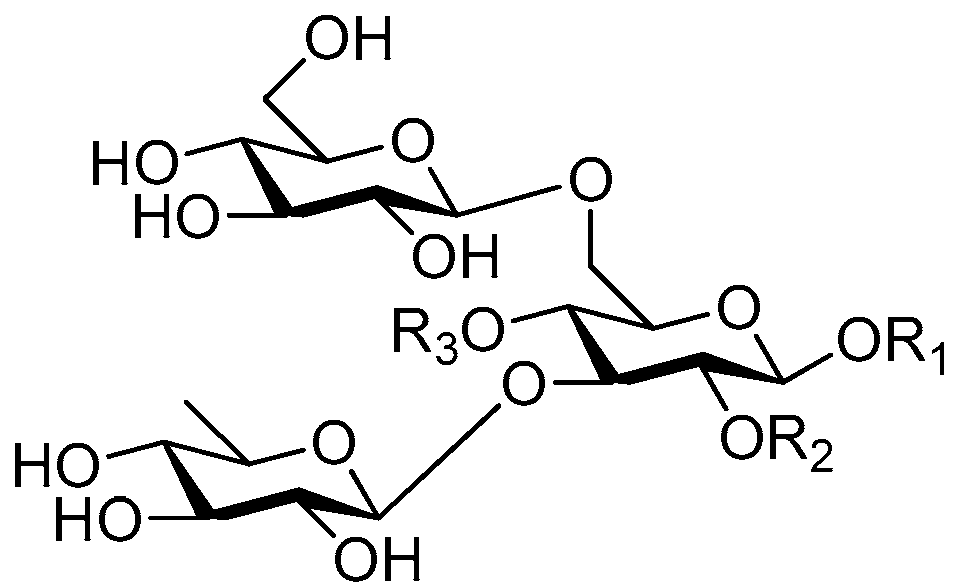
| Cpd. | R1 | R2 | R3 |
|---|---|---|---|
| 214 | S | H | G |
| 215 | U | H | G |
| 216 | U | H | I |
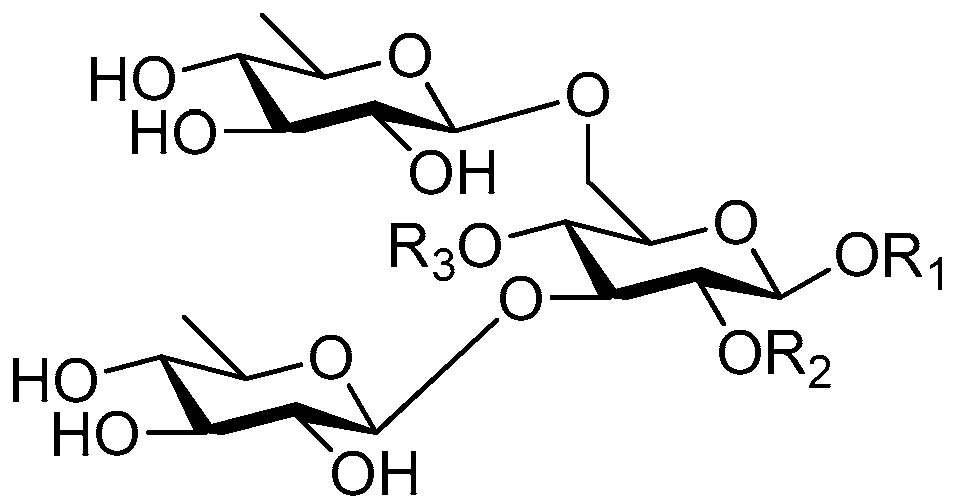
| Cpd. | R1 | R2 | R3 |
|---|---|---|---|
| 217 | S | H | G |
| 218 | S | A | G |
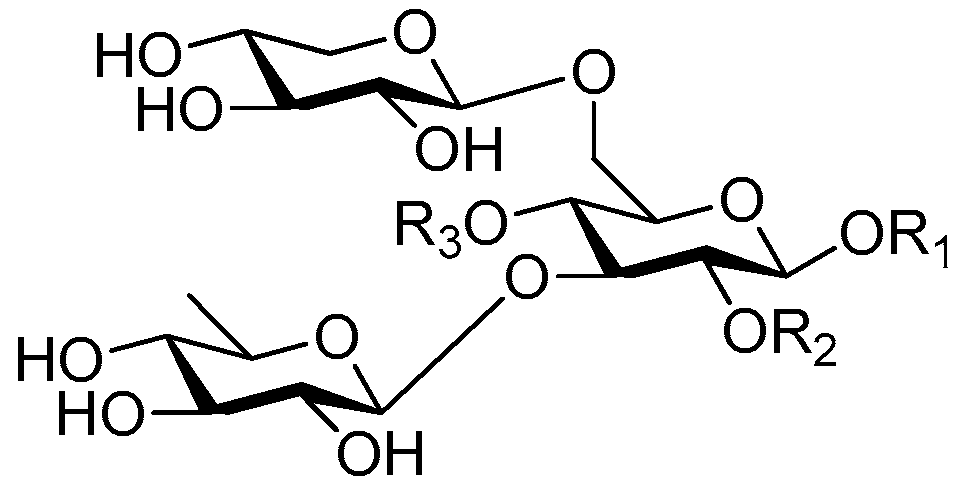
| Cpd. | R1 | R2 | R3 |
|---|---|---|---|
| 219 | S | H | G |

| Cpd. | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 220 | S | H | G | H |

| Cpd. | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 221 | E | H | I | H |
| 222 | S | H | G | H |
| 223 | S | A | G | H |
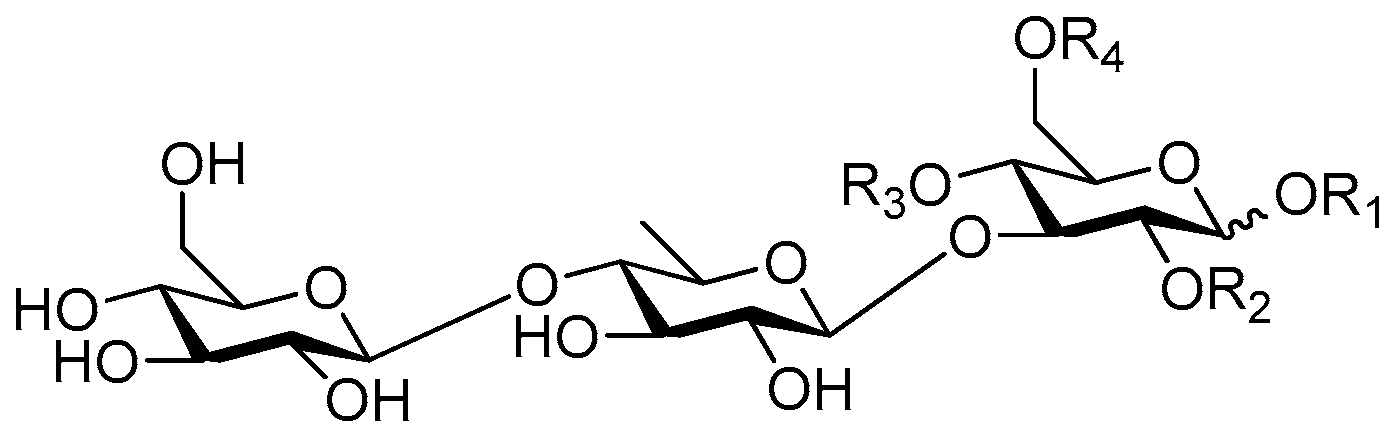
| Cpd. | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 224 | H | H | I | H |

| Cpd. | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 225 | S | H | G | H |
| 226 | S | H | I | H |
| 227 | T | H | I | H |

| Cpd. | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 228 | S | H | G | H |
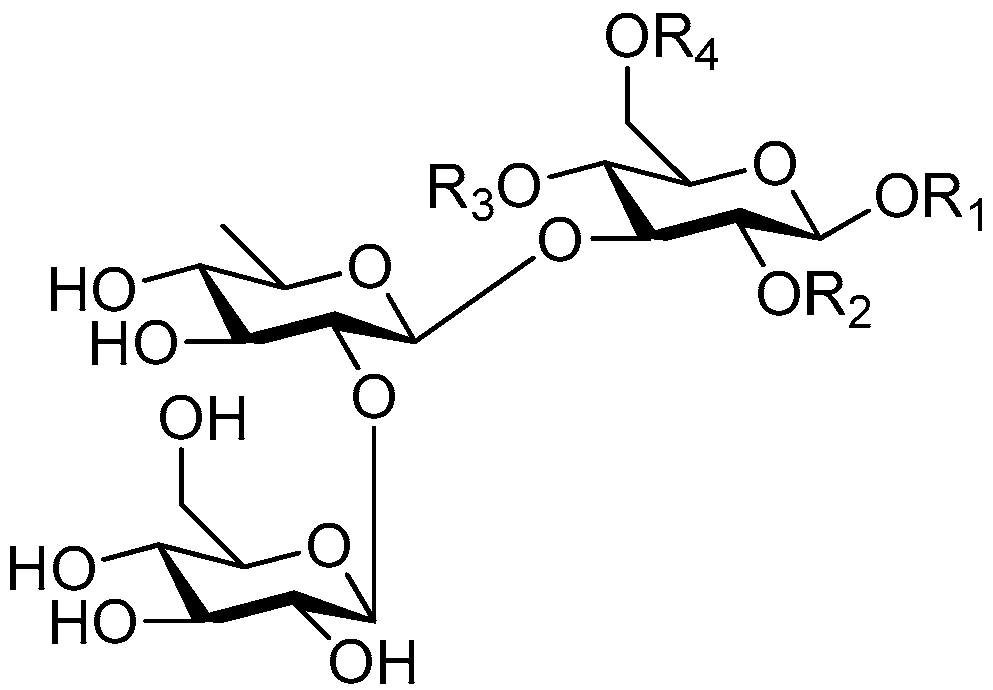
| Cpd. | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 229 | S | H | G | H |
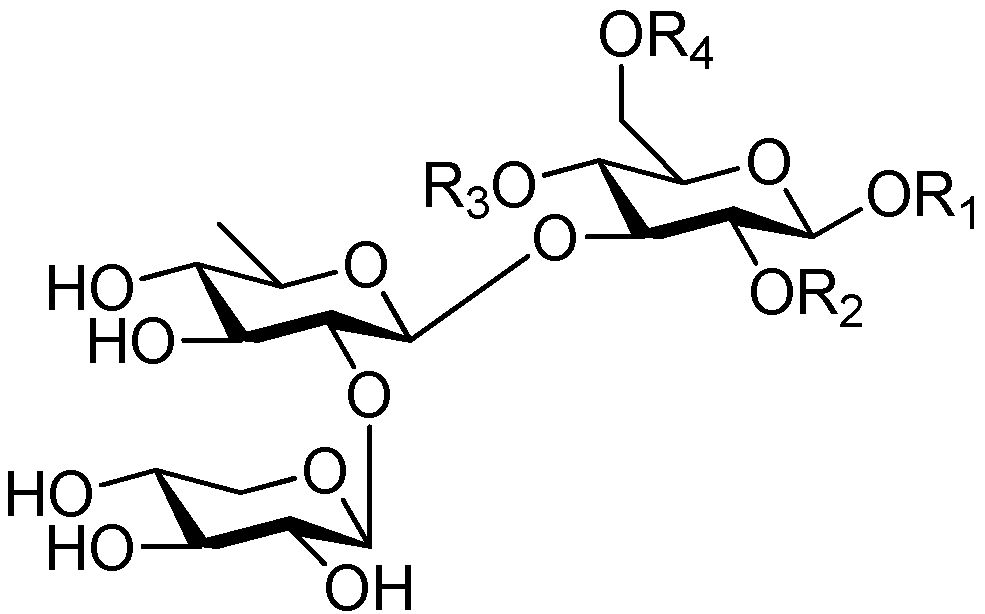
| Cpd. | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 230 | S | H | G | H |
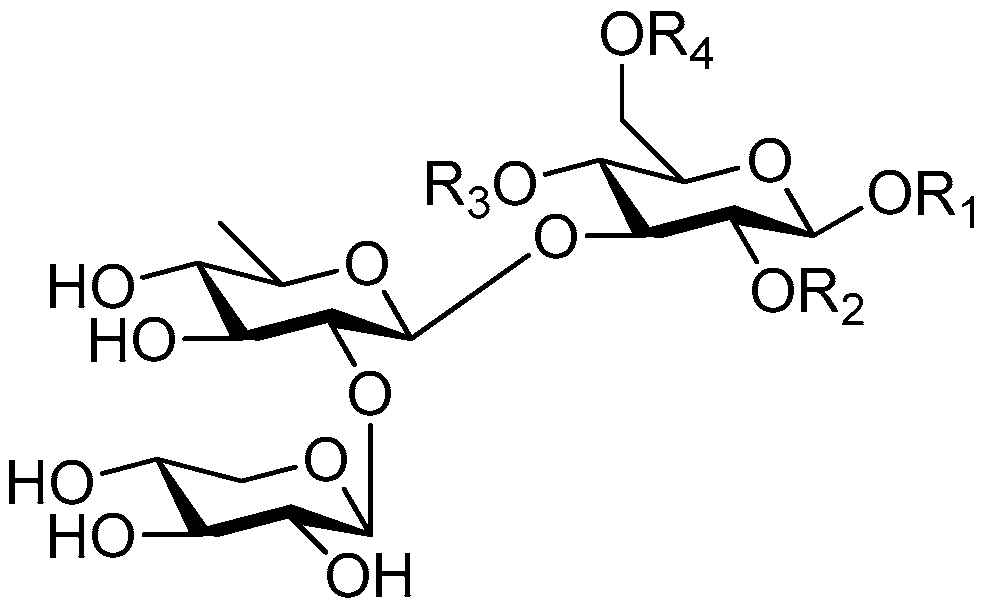
| Cpd. | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 231 | S | H | G | H |
| 232 | S | H | I | H |
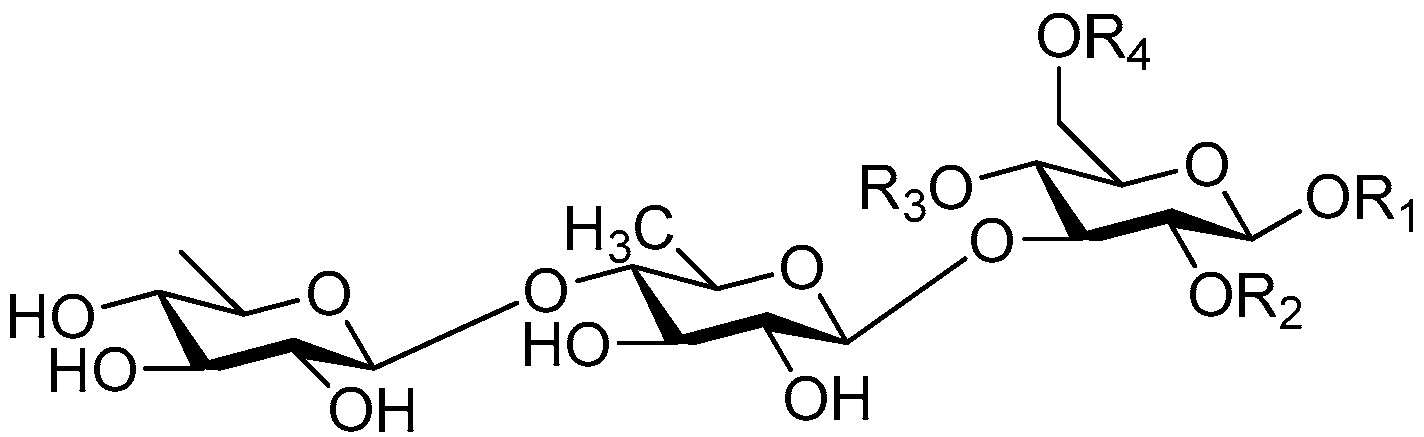
| Cpd. | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 233 | R | H | H | E |
| 234 | R | H | H | G |
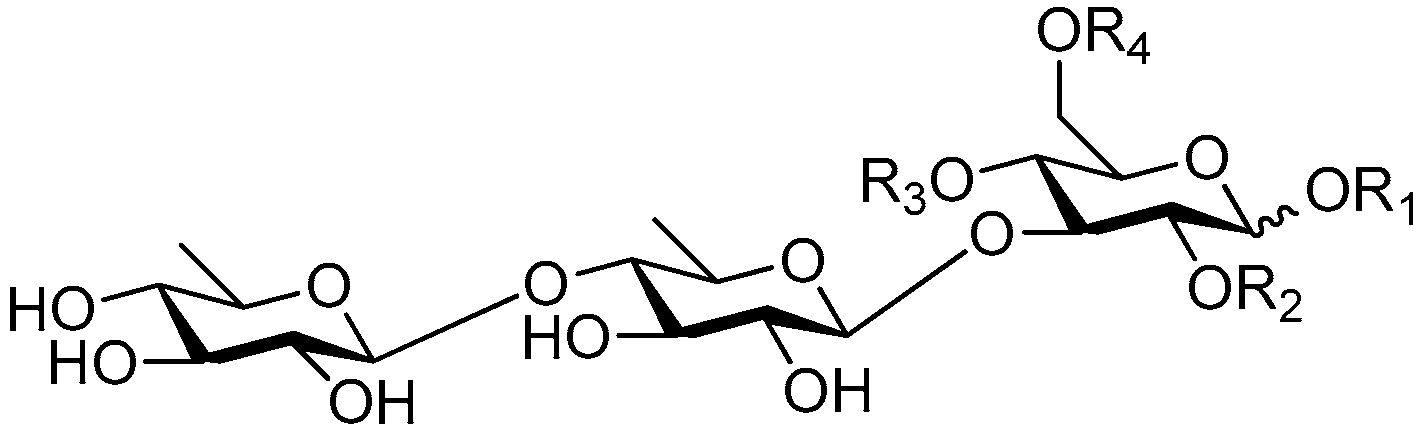
| Cpd. | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 235 | H | H | G | H |
| 236 | R | H | E | H |
| 237 | R | H | G | H |
| 238 | S | H | G | H |
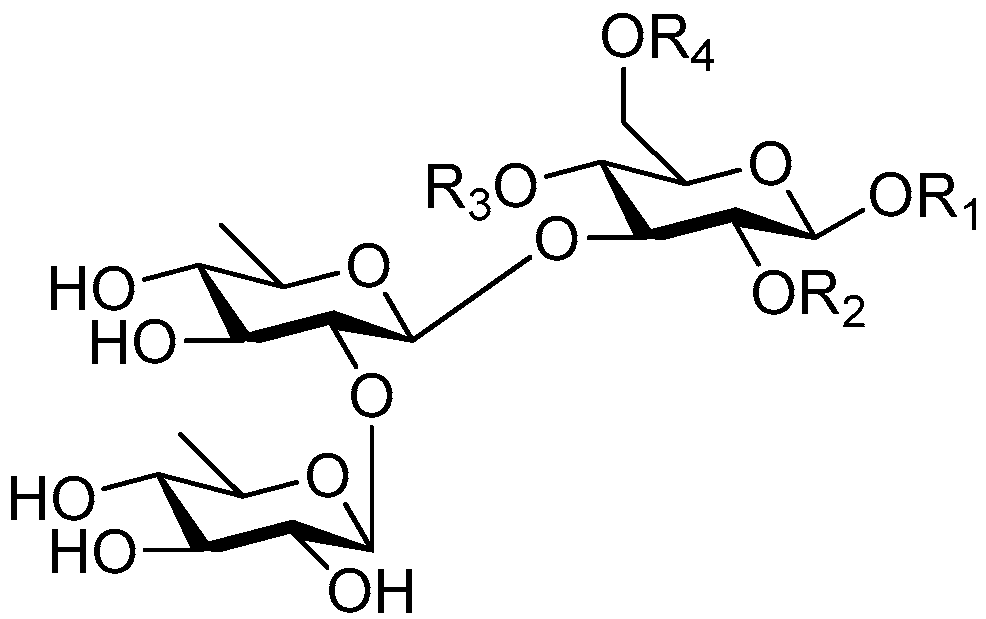
| Cpd. | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 239 | S | H | G | H |
| 240 | S | H | I | H |
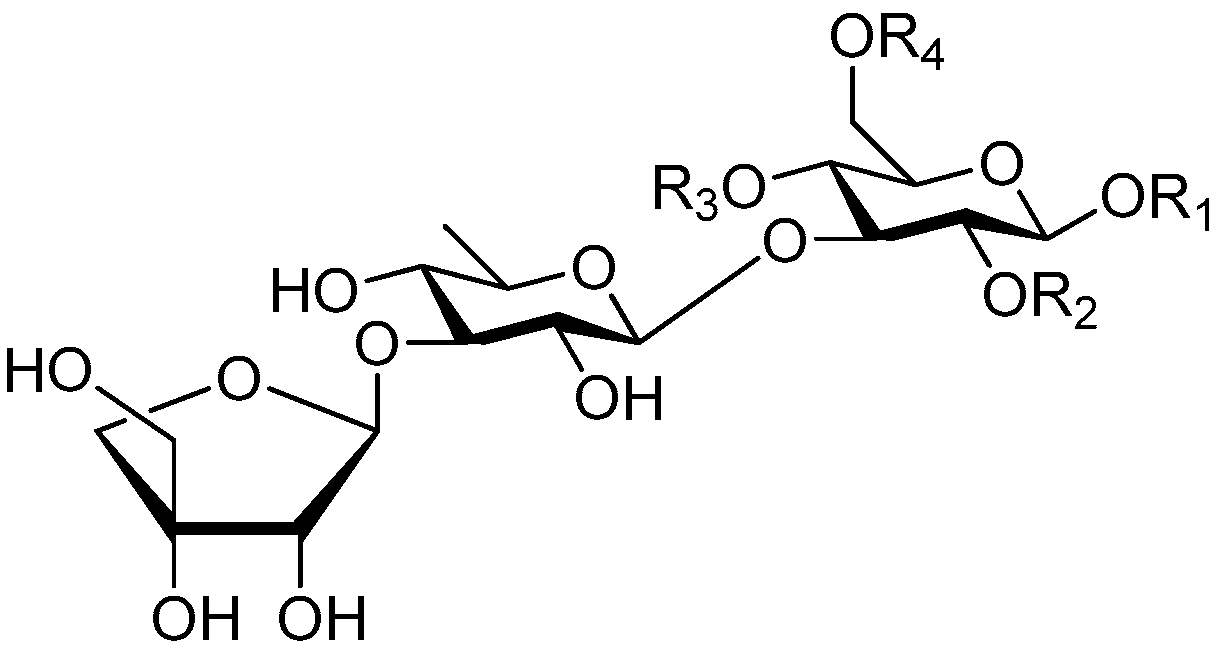
| Cpd. | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 241 | S | H | G | H |
| 242 | T | H | I | H |
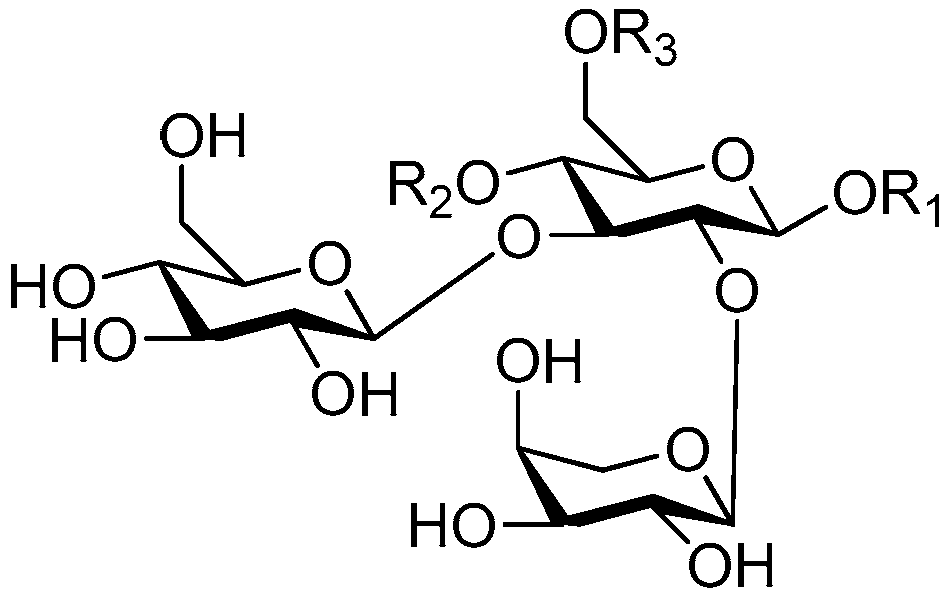
| Cpd. | R1 | R2 | R3 |
|---|---|---|---|
| 243 | S | G | H |
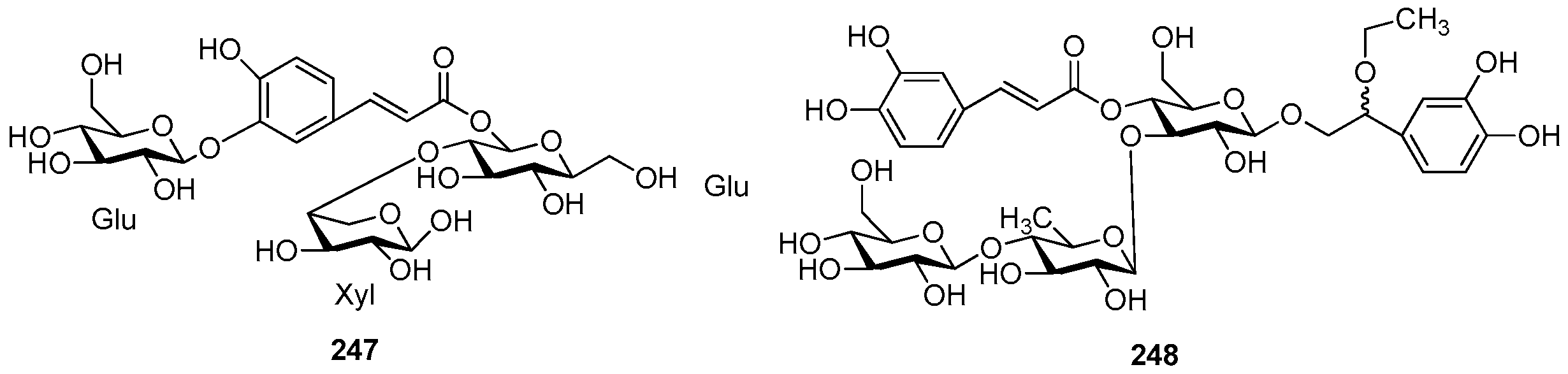
| Cpd. | R1 | R2 | R3 |
|---|---|---|---|
| 244 | S | G | H |

| Cpd. | R1 | R2 | R3 |
|---|---|---|---|
| 245 | T | H | I |

| Cpd. | R1 | R2 | R3 |
|---|---|---|---|
| 246 | S | G | H |

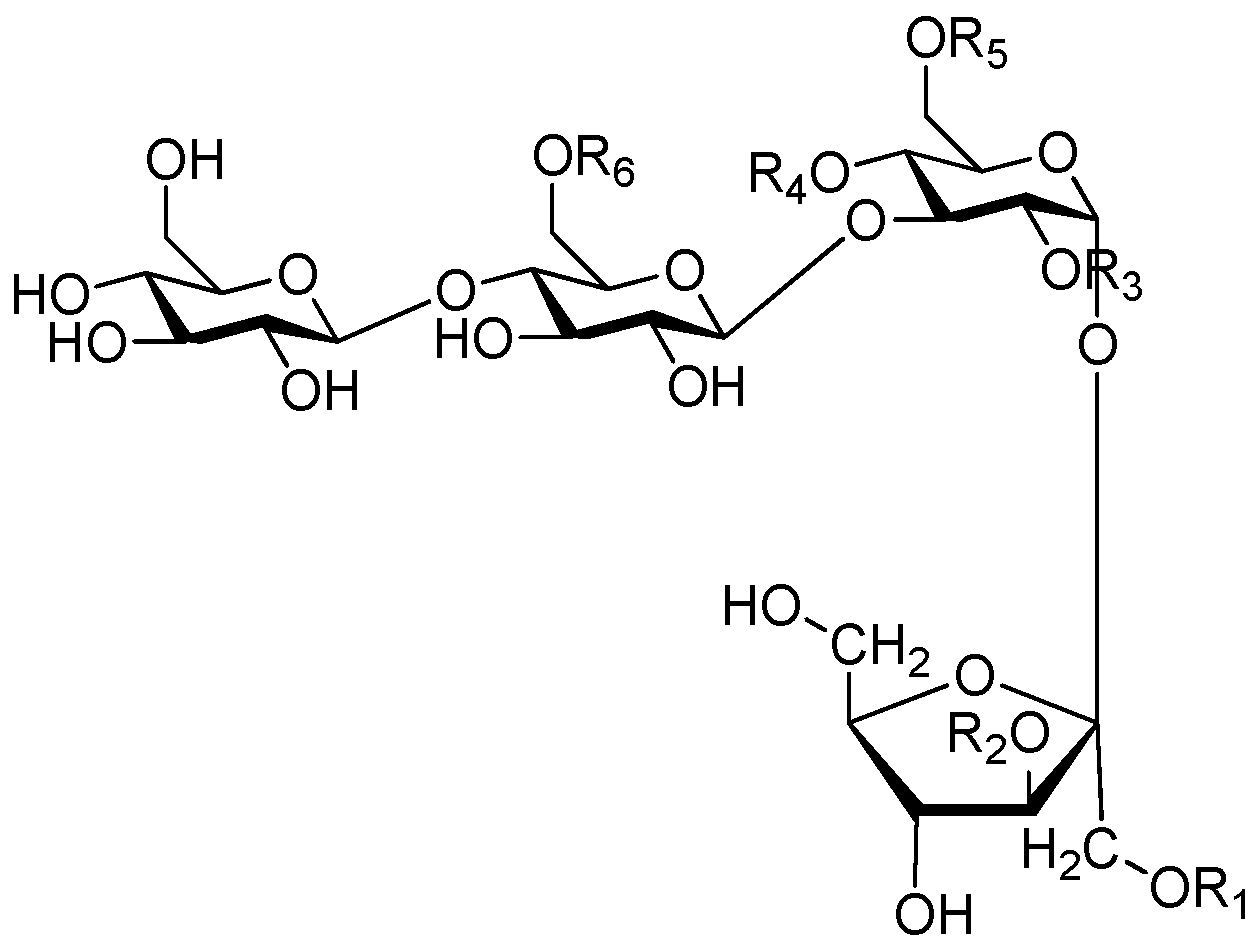
| Cpd. | R1 | R2 | Cpd. | R1 | R2 |
|---|---|---|---|---|---|
| 249 | D | R | 252 | F | R |
| 250 | E | R | 253 | I | R |
| 251 | E′ | R | 254 | J | R |

| Cpd. | R1 | R2 | R3 | R4 | Cpd. | R1 | R2 | R3 | R3 |
|---|---|---|---|---|---|---|---|---|---|
| 255 | K | H | H | K | 258 | K | K | H | K |
| 256 | K | E | H | K | 259 | M | I | H | K |
| 257 | K | I | H | K | 260 | M | K | H | K |
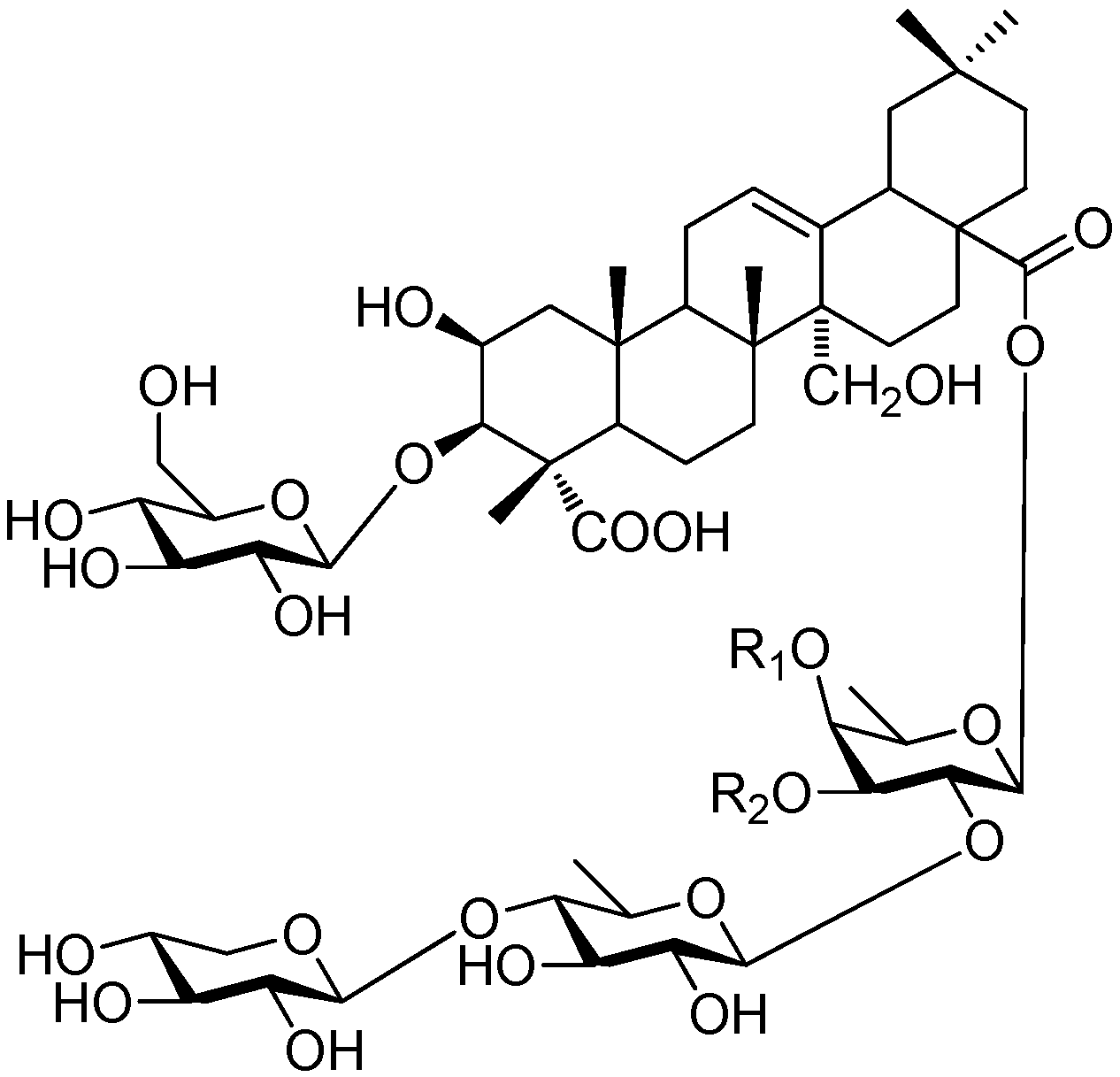
| Cpd. | R1 | R2 | R3 | R4 | R5 | R6 |
|---|---|---|---|---|---|---|
| 261 | I | B | H | I | A | A |

| Cpd. | R1 | R2 | R3 | R4 | R5 | Cpd. | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 262 | E | B | I | A | A | 269 | E | B | I | H | I |
| 263 | E | B | H | E | A | 270 | E | B | I | A | I |
| 264 | E | B | E | H | A | 271 | I | B | E | A | A |
| 265 | E | B | E | A | A | 272 | I | B | I | H | A |
| 266 | E | B | E | A | E | 273 | I | B | I | A | H |
| 267 | E | B | I | H | A | 274 | I | B | I | A | A |
| 268 | E | B | I | H | E |
| Cpd. | R1 | R2 | R3 |
|---|---|---|---|
| 275 | J | H | H |
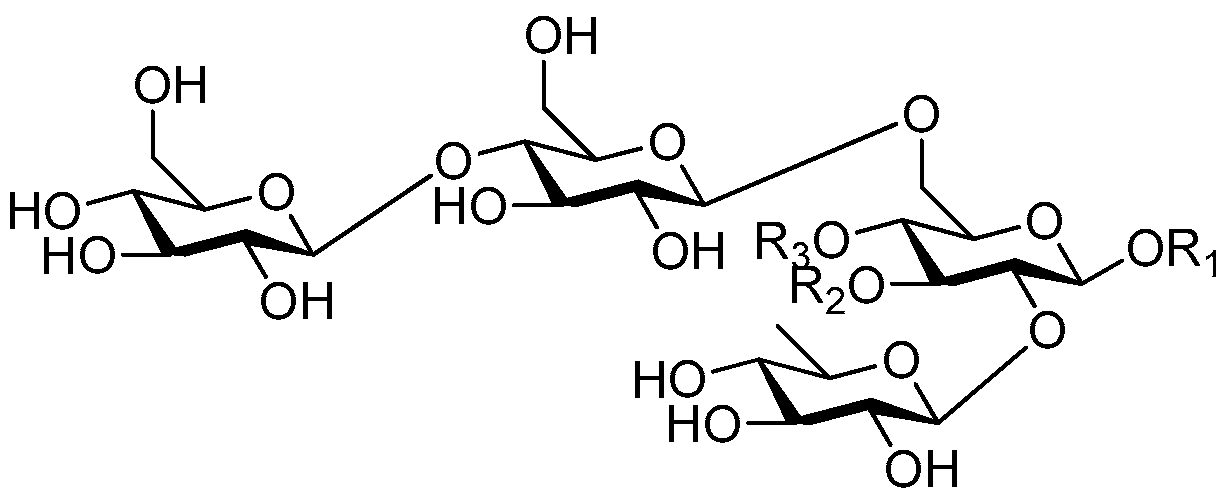
| Cpd. | R1 | R2 | R3 |
|---|---|---|---|
| 276 | S | G | H |


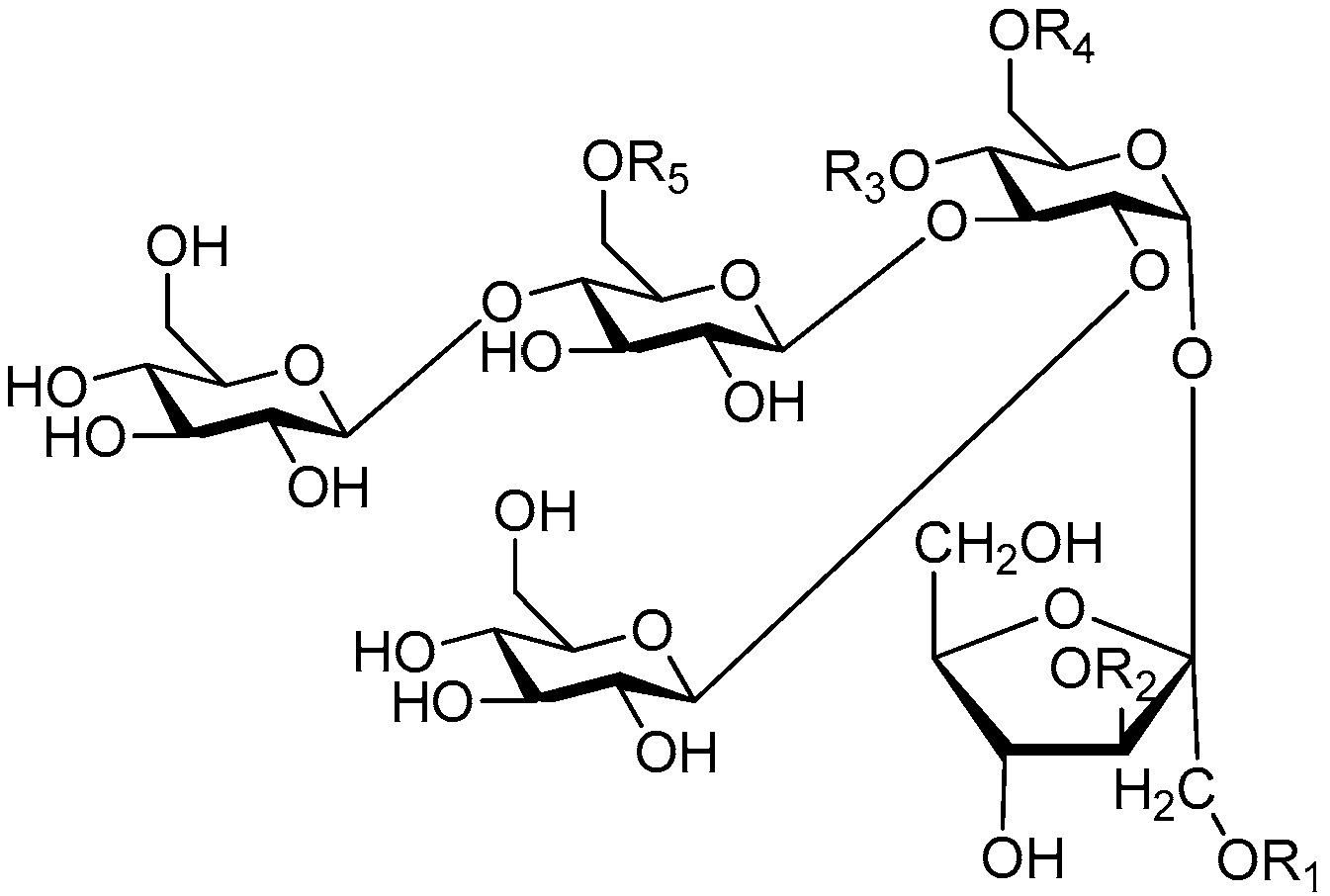
| Cpd. | R1 | R2 | R3 | R4 | R5 | R6 | Cpd. | R1 | R2 | R3 | R4 | R5 | R6 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 280 | E | B | E | H | H | A | 288 | E | B | I | A | A | A |
| 281 | E | B | E | H | A | A | 289 | I | B | I | H | H | A |
| 282 | E | B | E | A | H | A | 290 | I | B | I | H | A | A |
| 283 | E | B | E | A | A | A | 291 | I | B | I | A | H | A |
| 284 | E | B | I | H | H | A | 292 | I | B | I | A | A | A |
| 285 | E | B | I | H | A | A | 293 | I | B | E′ | A | H | A |
| 286 | E | B | I | A | H | A | 294 | I | B | H | I | H | A |
| 287 | E | B | I | A | H | H |
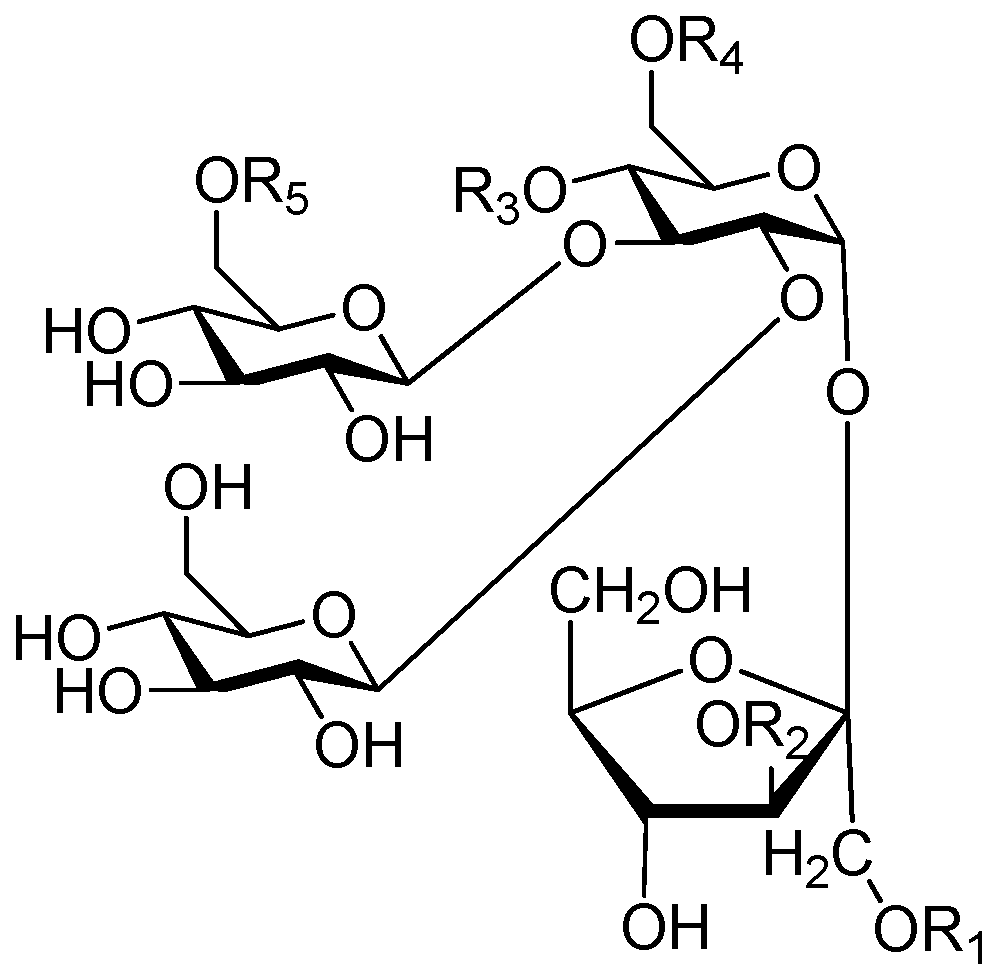
| Cpd. | R1 | R2 | R3 | R4 | R5 | Cpd. | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 295 | E | B | I | A | H | 301 | I | B | I | H | H |
| 296 | E | B | I | A | A | 302 | I | B | I | H | A |
| 297 | E | B | I′ | A | A | 303 | I | B | I | A | H |
| 298 | E′ | B | I | A | A | 304 | I | B | I | A | A |
| 299 | I | B | E | A | H | 305 | I | B | I′ | A | A |
| 300 | I | B | E | A | A |
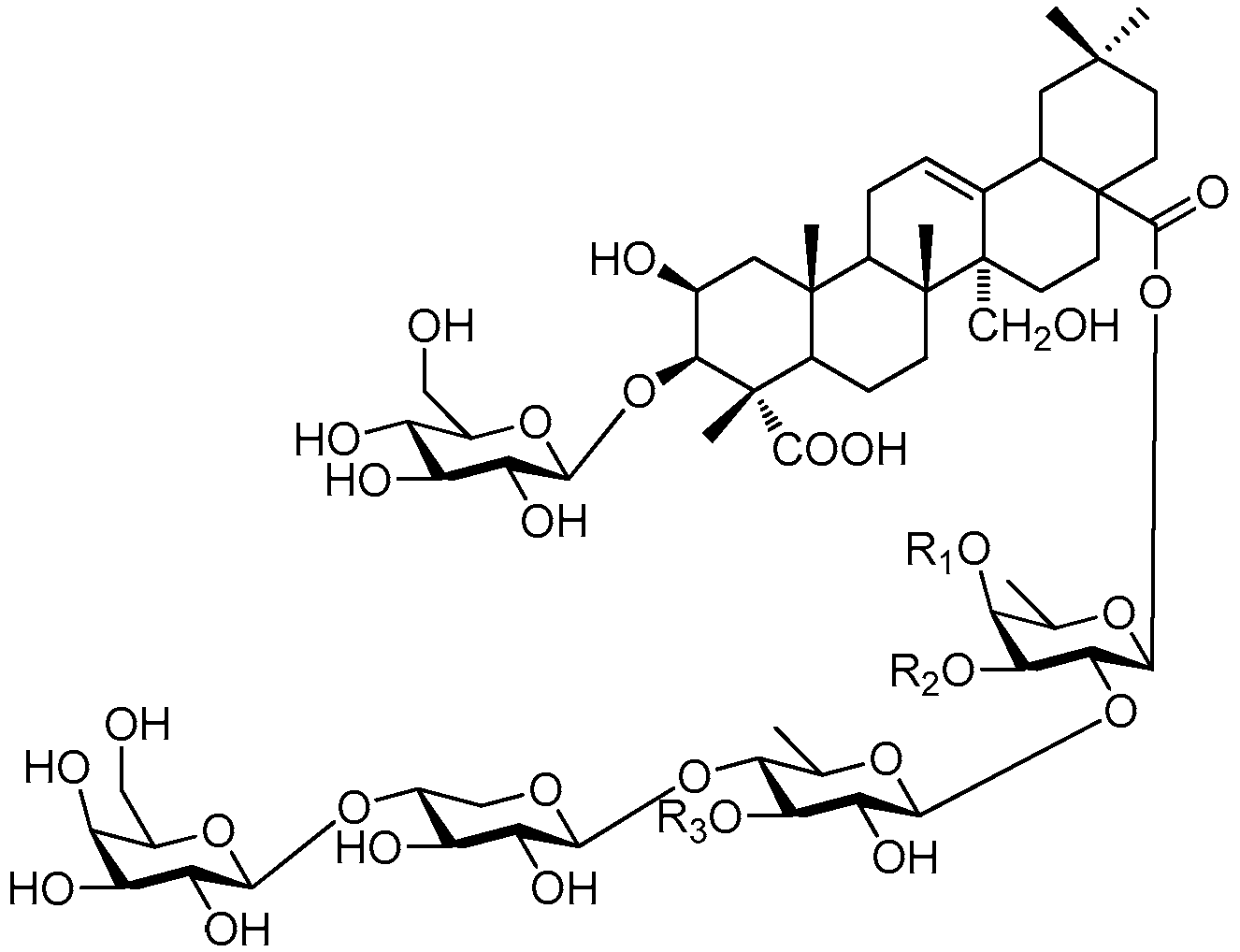
| Cpd. | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|
| 306 | E | B | N | A | H |
| 307 | E | B | N | A | A |
| 308 | E | B | P | A | H |
| 309 | E | B | P | A | E |
| 310 | E | B | P | A | I |
| 311 | I | B | N | A | H |
| 312 | I | B | P | H | I |
| 313 | I | B | P | A | H |
| 314 | I | B | P | A | A |
| 315 | I | B | P | A | E |
| 316 | I | B | P | A | I |
| Cpd. | R1 | R2 | R3 |
|---|---|---|---|
| 317 | F | H | H |
| 318 | F′ | H | H |
| 319 | J | H | H |
| 320 | J′ | H | H |

| Cpd. | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|
| 321 | E | B | A | H | A |
| 322 | E | B | A | A | A |

| Cpd. | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|
| 323 | E | B | A | H | A |
| 324 | E | B | A | A | A |
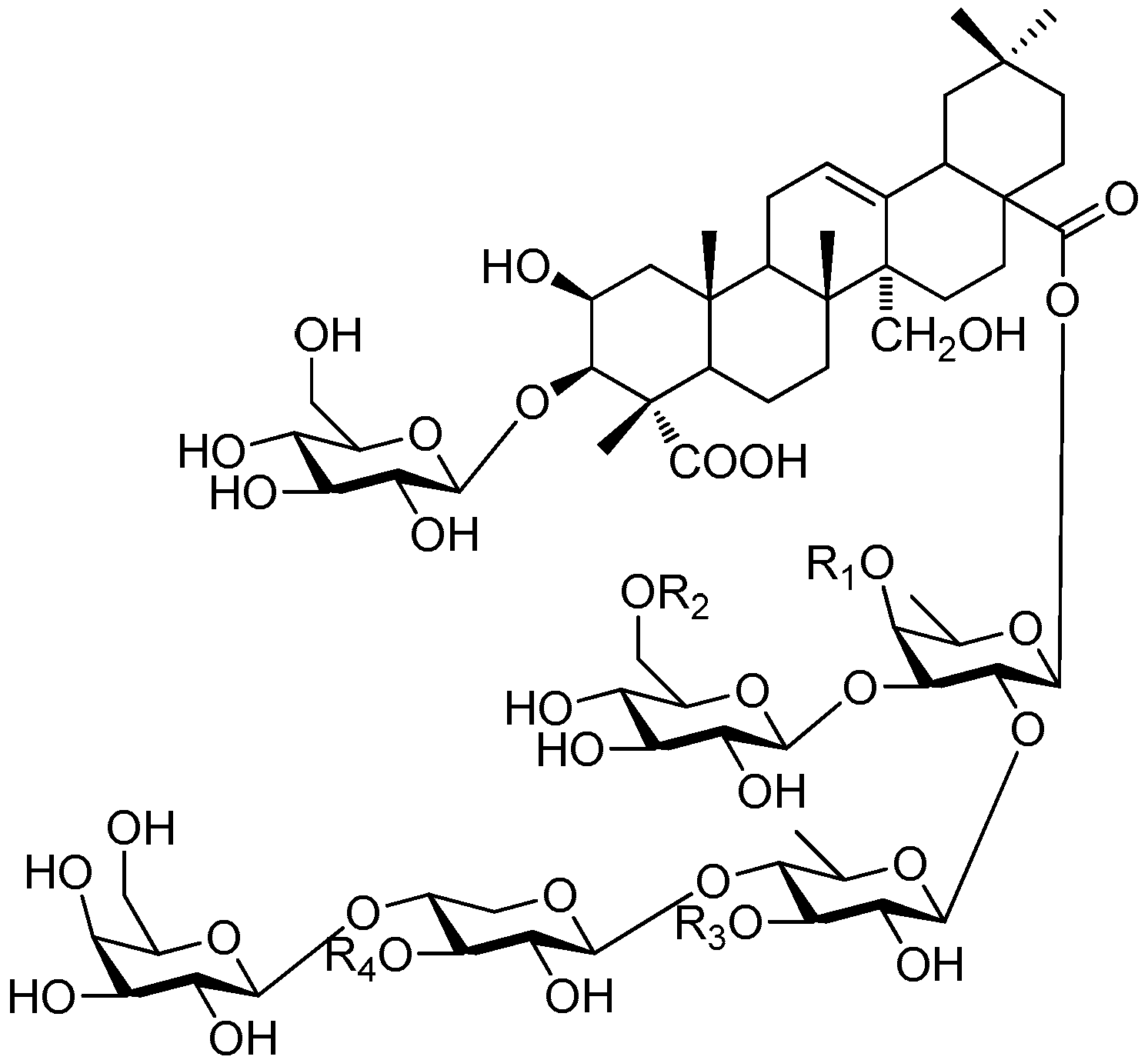
| Cpd. | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 325 | J | H | H | H |
| 326 | J | A | H | H |
| 327 | J′ | A | H | H |
| 328 | F | H | H | H |
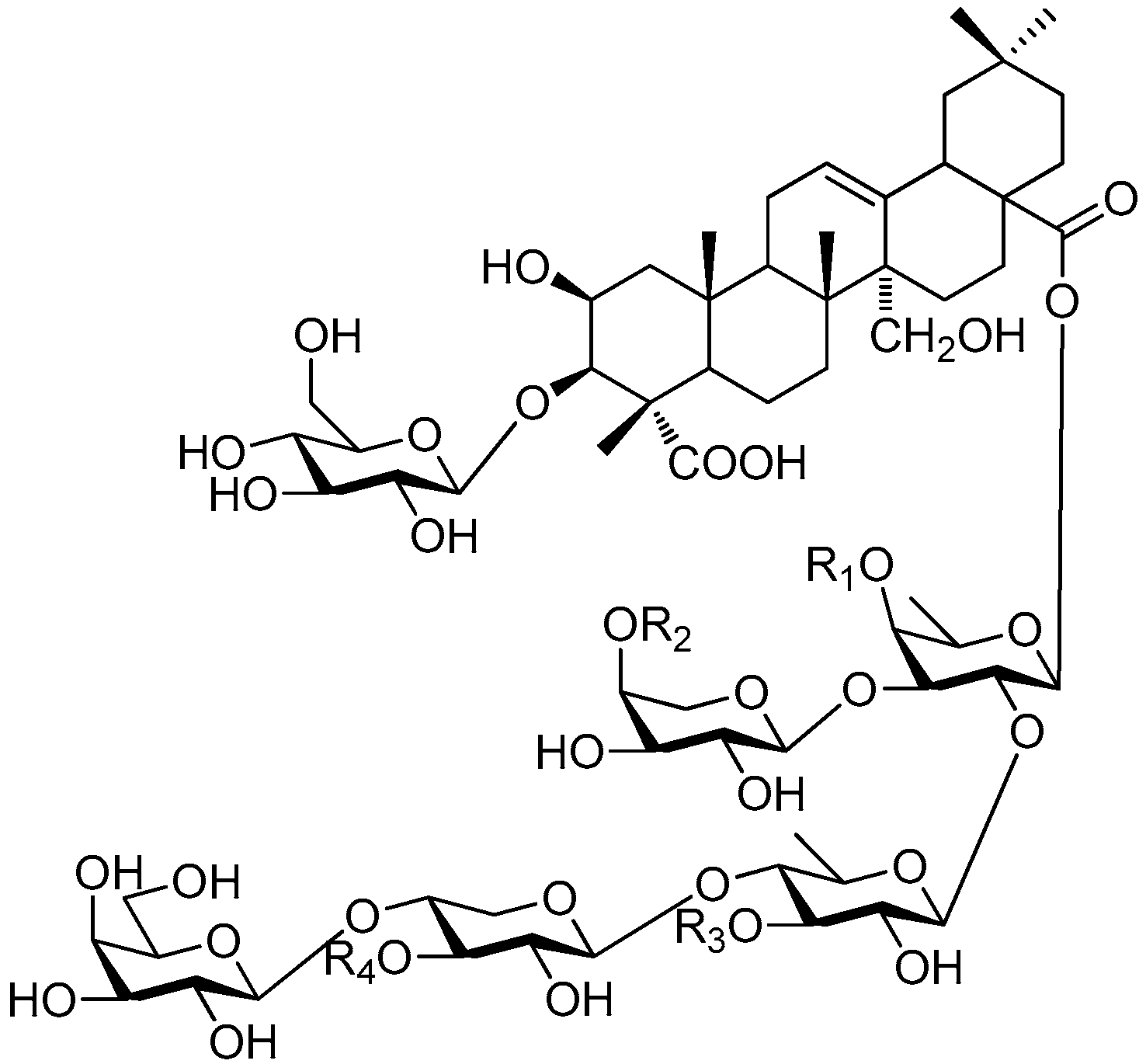
| Cpd. | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| 329 | F | H | H | H |
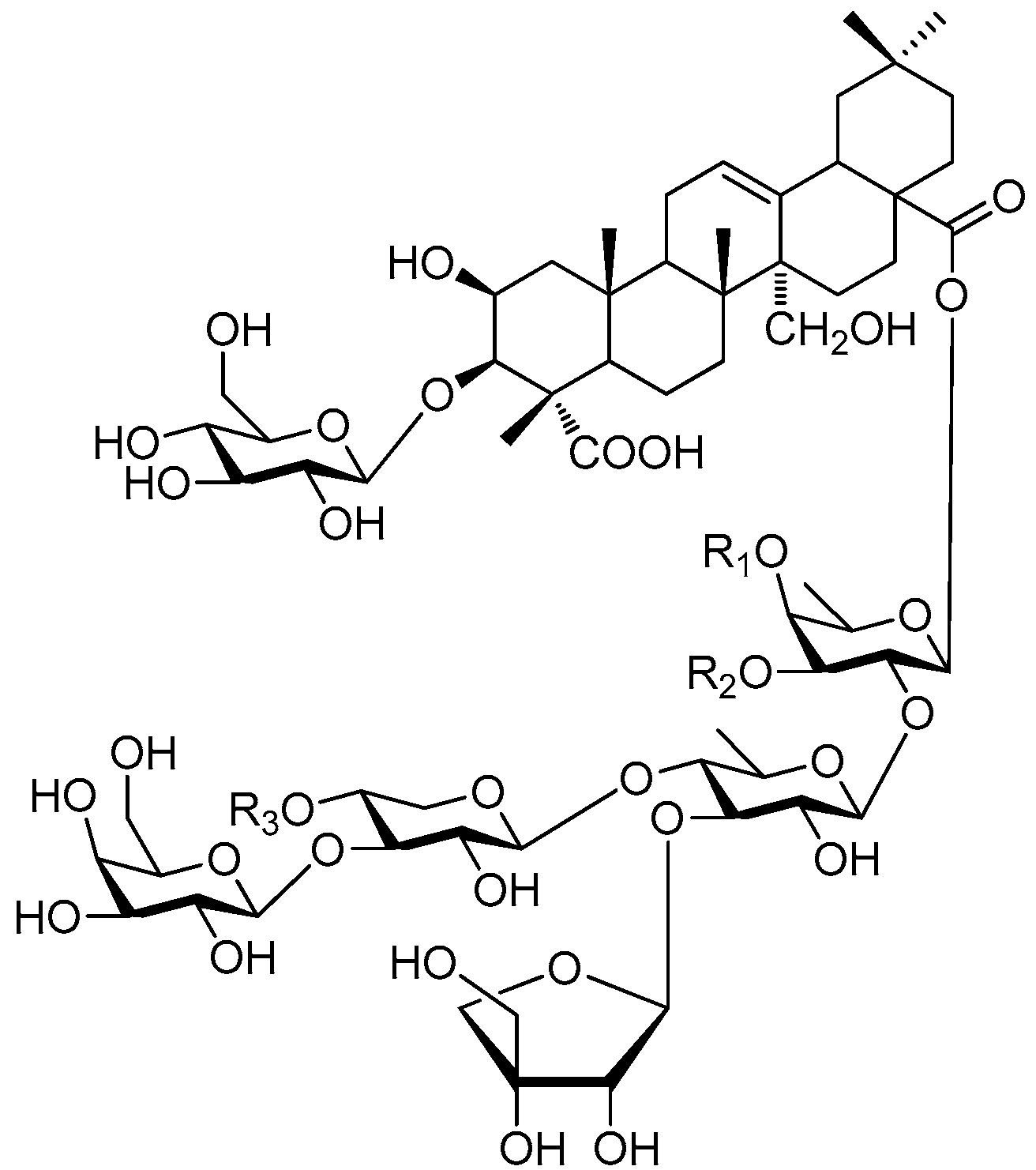
| Cpd. | R1 | R2 | R3 |
|---|---|---|---|
| 330 | M | H | H |
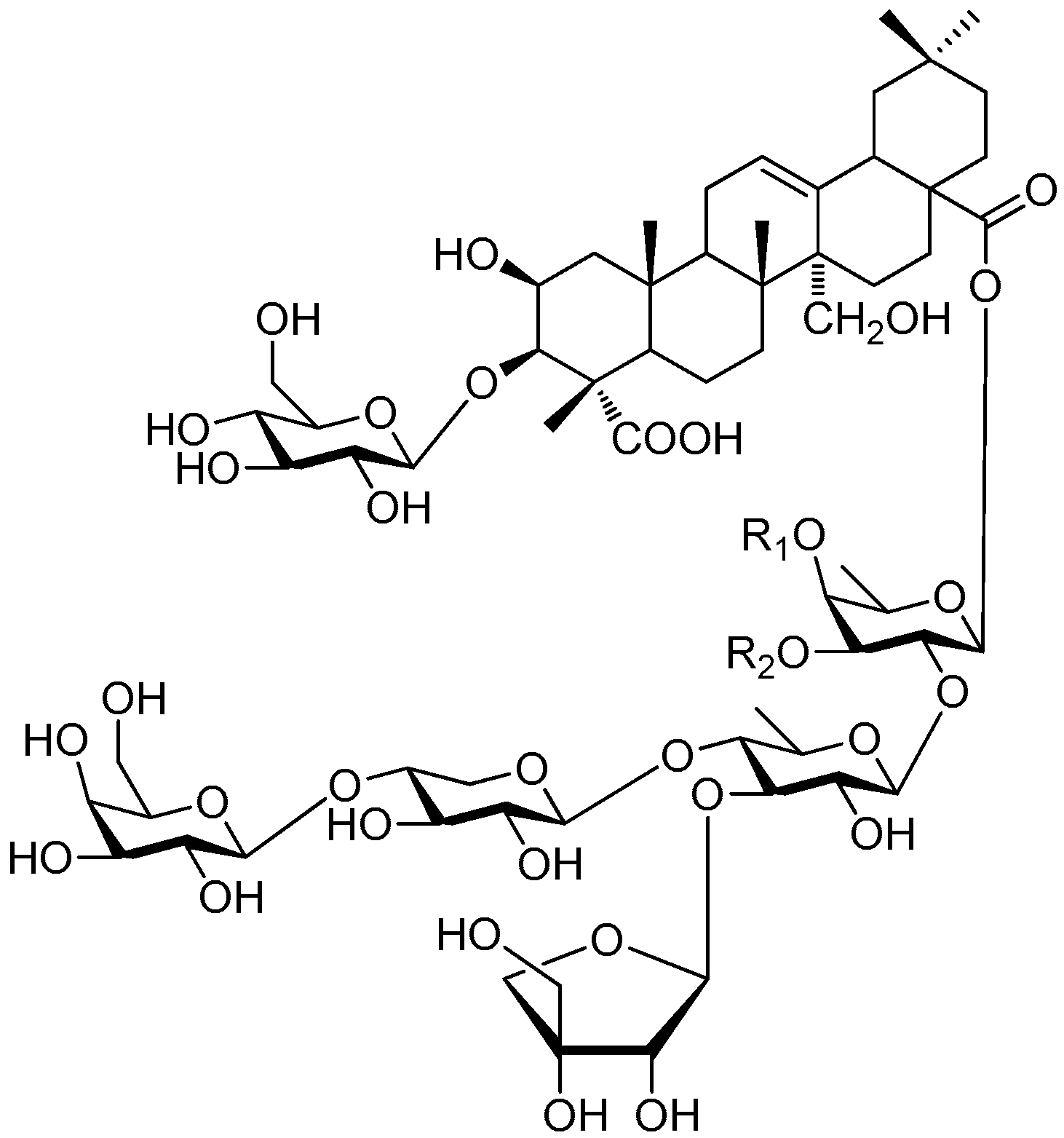
| Cpd. | R1 | R2 |
|---|---|---|
| 331 | F | H |
| 332 | F′ | H |
| Cpd. | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|
| 333 | E | B | A | A | A |
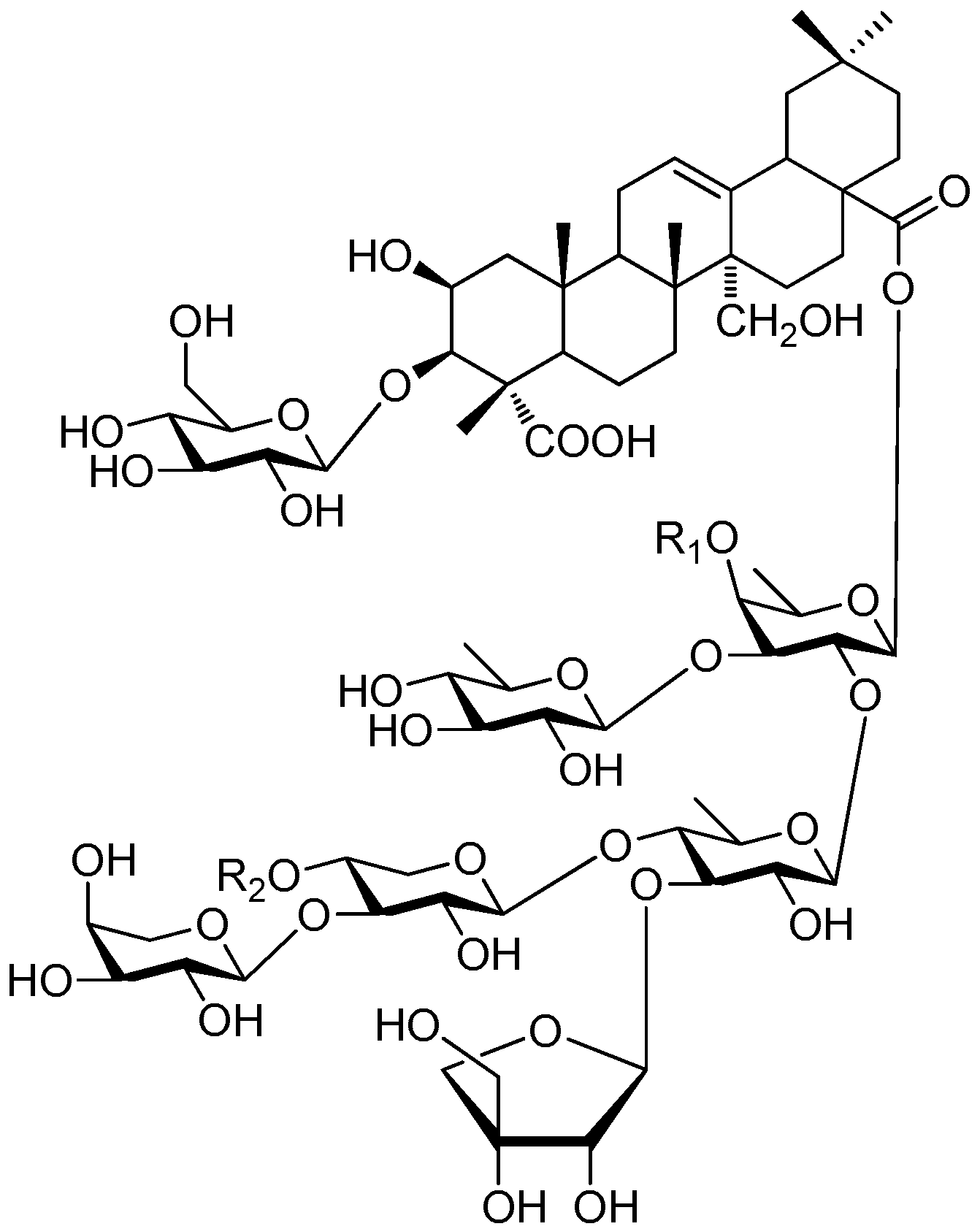
| Cpd. | R1 | R2 |
|---|---|---|
| 334 | F | H |
| Family | Number | Family | Number |
|---|---|---|---|
| Asclepiadaceae | 1 | Gesneriaceae | 8 |
| Hymenophyllaceae | 1 | Labiatae | 11 |
| Sterculiaceae | 1 | Lamiaceae | 11 |
| Amaranthaceae | 2 | Rosaceae | 13 |
| Smilacaeae | 3 | Orobanchaceae | 16 |
| Magnoliaceae | 3 | Polygonaceae | 19 |
| Rubiaceae | 5 | Oleaceae | 20 |
| Plantaginaceae | 6 | Bignoniaceae | 22 |
| Cruciferae | 6 | Liliaceae | 34 |
| Verbenaceae | 7 | Scrophulariaccae | 58 |
| Polygalaceae | 126 |
| Name in TCM | Sources | Traditional Effect | Medicinal Parts | Compounds | Activity | Refs. |
|---|---|---|---|---|---|---|
| Polygalae Radix | Polygala tenuifolia Willd. | Common wisdom calms the nerves, restoring normal coordination between heart and kidney, Expectoration, subsidence of a swelling | Root | 51, 52, 72, 73, 280–290, 292, 321–324 | Anti-depression activity, neuroprotective activity | [10,11,12] |
| Polygala sibirica L. | 28–30, 50, 51, 73, 75, 78, 88 | Anti-depression activity, neuroprotective activity, antioxidant activity | [13] | |||
| Smilacis China Rhizoma | Smilaz china L. | Syphilis, gout, and rheumatism | Root | 39, 40, 45, 47, 79, 98, 99, 101, 107 | Anticancer activity | [14] |
| Smilax bracteata C. Presl | 38, 41, 42, 45–47, 105, 106 | Antioxidant activity | [15] | |||
| Scrophula-riae Radix | Scrophularia ningpoensis Hemsl. | Clearing heat and cooling blood, nourishing yin to reduce pathogenic fire, detoxicating and resolving a mass | Root | 14, 53, 59, 132 | Antioxidative activity | [16,17] |
| Scrophula-riae Radix | Scrophularia buergeriana Miq. | Clearing heat and cooling blood, nourishing yin to reduce pathogenic fire, detoxicating and resolving a mass | Root | 11, 12, 13, 15 | Neuroprotective | [18] |
| Rehmann-ia Radix | Rehmannia glutinosa var. Purpurea | Clearing heat and cooling blood, promoting the secretion of saliva or body fluid | Root | 124, 125, 131, 133, 136, 138, 207–212 | PKC inhibitory activity, antiinflammatory effects, antiviral activity, antibacterial activity | [19] |
| No. | Name | Source | Refs. |
|---|---|---|---|
| 1 | 6-O-Caffeoyl-1-O-p-coumaroyl-β-d-glucopyranose | Prunus buergeriana | [20] |
| 2 | 1,6-Di-O-caffeoyl-β-d-glucopyranose | Prunus buergeriana; Coussarea hydrangeifolia | [20,21] |
| 3 | Osmanthuside E | Osmanthus asiaticus | [22] |
| 4 | 1,6-Diferuloyl glucose | Sterculia foetida | [23] |
| 5 | Eutigoside A | Ligustrum purpurascens | [24] |
| 6 | Osmanthuside A | Ligustrum purpurascens | [24] |
| 7 | 2-(3,4-Dihydroxyphenyl)-ethyl-(6-O-caffeoyl)-β-d-glucopyranoside or calceolarioside B | Calceolaria hypericina; Prunus ssiori; Paraboea glutinosa | [25,26] |
| 8 | 3,4-Dihydroxyphenethyl alcohol 4-O-Caffeoyl-β-d-allopyranoside or calceolarioside A or derhamnosylverbascoside | Trichomanes reniforme Forst.f; Calceolaria hypericina; Lantana camaro L. | [25,27,28] |
| 9 | 1′-O-β-d-(1-Hydroxy-4-oxo-2,5-cyclohexadien)-ethyl-6′-O-caffeoylglucopyranoside or calceolarioside D | Calceolaria hypericina | [25] |
| 10 | 2-(3,4-Methylenedioxyphenyl)-ethyl-(6-O-caffeoyl)-β-d-glucopyranoside | Prunus ssiori | [29] |
| 11 | 4-O-(E)-p-Methoxycinnamoyl-α-l-rhamno-pyranoside or buergeriside C3 | Scrophularia buergeriana | [18] |
| 12 | 2-O-Acetyl-3-O-(E)-p-methoxycinnamoyl-α-l-rhamnopyranoside or buergeriside B1 | Scrophularia buergeriana | [18] |
| 13 | 2-O-Acetyl-3,4-di-O-(E)-p-methoxycinnamoyl-α-l-rhamnopyranoside or buergeriside A1 | Scrophularia buergeriana | [18] |
| 14 | 3-O-Acetyl-2-O-p-methoxycinnamoyl-α(β)-l-rhamnopyranose or ningposide D | Scrophularia ningpoensis | [16] |
| 15 | 2-O-Acetyl-3-O-(Z)-p-methoxycinnamoyl-α-l-rhamnopyranoside or buergeriside B2 | Scrophularia buergeriana | [18] |
| 16 | 6-O-p-Coumaroyl-d-glucopyranose | Prunus buergeriana | [20] |
| 17 | 6-O-Caffeoyl-d-glucopyranose or 6-O-Caffeoyl-d-glucopyranoside | Prunus buergeriana; Prunus ssiori | [20,29] |
| 18 | 6-O-[E]-Sinapoyl-(α- and β-)-d-glucopyranoside | Cynanchum hancockianum | [30] |
| 19 | O-Acylglycoses | Ligustrum purpurascens | [24] |
| 20 | 3,6-di-O-Caffeoyl-(α/β)-glucose | Rubus sanctus | [31] |
| 21 | 6-O-Feruloyl-β-d-glucopyranosyl-(1→6)-glucitol or globularitol | Globularia orientalis | [32] |
| 22 | (2R)-[(6-O-Caffeoyl)-β-d-glucopyranosyloxy]-benzeneacetonitrile or grayanin | Prunus buergeriana | [20] |
| 23 | Scrophyloside A | Neopicrorhiza scrophulariiflora | [33] |
| 24 | Scrophyloside B | Neopicrorhiza scrophulariiflora | [33] |
| 25 | Hexane-1,2,3,4,5-pentanol 1-O-β-(6-O-(E)-feruloyl) glucopyranoside or paederol A | Paederia scandens | [34] |
| 26 | Butane-1,2,3,4-tetraol 1-O-β-(6-O-(E)-feruloyl) glucopyranoside or paederol B | Paederia scandens | [34] |
| 27 | Kaempferol 3-O-β-d-(6-O-p-E-Coumaroyl)-glucopyranoside | Froelichia floridana | [35] |
| 28 | 3-O-Feruloylsucrose or sibiricose A5 | Trillium kamtschaticum; Polygala sibirica | [13,36] |
| 29 | 3′-Sinapoyl sucrose or sibiricose A6 | Polygala sibirica; Polygala tricornis | [13,37] |
| 30 | 3-O-[(E)-3,4,5-Trimethoxycinnamoyl]-β-d-fructo-furanosyl-(2→1)-α-d-glucopyranoside or glomeratose A | Polygala sibirica; Polygala tricornis; Polygala glomerata | [13,37,38] |
| 31 | 3,6-Di-p-coumaroyl sucrose or lapathosides D | Polygonum lapathifolium | [39] |
| 32 | Heronioside A | Trillium kamtschaticum; Smilax glabra | [36,40] |
| 33 | Parispolyside F | Paris polyphylla var. yunnanensis | [41] |
| 34 | β-d-(1-Sinapoyl-3-feruloyl)-α-d-glucopyranoside | Polygala chamaebuxus | [42] |
| 35 | β-d-(l-Acetyl-3-feruloyl)-fructofuranosyl-α-d-gluco-pyranoside | Polygala chamaebuxus | [42] |
| 36 | β-d-(1,3-Disinapoyl)-fructofuranosyl-d-gluco-pyranoside | Polygala chamaebuxus | [42] |
| 37 | β-d-(1,3,6-Tri-p-coumaryl)-fructofuranosyl-α-d-glucopyranoside or hydropiperoside | Polygonum hydropiperitum; Polygonurn hydropiper | [39,43] |
| 38 | (1,3-O-di-p-Coumaroyl-6-O-feruloyl)-β-d-fructo-furanosyl-(2→1)-α-d-glucopyranoside or smilaside G | Smilax bracteata | [15] |
| 39 | 1-p-Coumaroyl-3,6-diferuloyl sucrose or smilaside C | Smilax china | [14] |
| 40 | 1-p-Coumaroyl-3,6-diferuloyl-4-acetyl sucrose or smilaside D | Smilax china | [14] |
| 41 | (3-O-p-Coumaroyl-1,6-O-diferuloyl)-β-d-fructo-furanosyl-(2→1)-α-d-glucopyranoside or smilaside J | Smilax bracteata | [15] |
| 42 | 1,3,6-O-Triferuloyl-β-d-fructofuranosyl-(2→1)-α-d-glucopyranoside or smilaside L | Smilax bracteata | [15] |
| 43 | 3-O-[(E)-3,4,5-Trimethoxycinnamoyl]-β-d-fructo-furanosyl-(2→1)-(6-O-acetyl)-α-d-glucopyranoside or tricornose A | Polygala tricornis | [37] |
| 44 | Regaloside A | Trillium kamtschaticum | [36] |
| 45 | 6′-Acetyl-3,6-diferuloylsucrose or helonioside B | Smilax china; Smilax bracteata; Polygonum perfoliatum; Heterosmilax erythrantha | [14,15,44,45] |
| 46 | (1,3-O-di-p-Coumaroyl-6-O-feruloyl)-β-d-fructo-furanosyl-(2→1)-(6-O-acetyl)-α-d-glucopyranoside or smilaside I | Smilax bracteata | [15] |
| 47 | 1-p-Coumaroyl-3,6-diferuloyl-6′-acetyl sucrose or smilaside E | Smilax china; Smilax bracteata | [14,15] |
| 48 | Reiniose C | Polygala reinii Fr.et Sav | [46] |
| 49 | 6-O-Benzoyl-3′-O-3,4,5-trimethoxycinnamoyl-sucrose or 3-O-[(E)-3,4,5-trimethoxy-cinnamoyl]-β-d-fructofuranosyl-(2→1)-(6-O-benzoyl)-α-d-glucopyranoside or [3-O-(3,4,5-trimethoxycinnamoyl]-β-d-fructo-furanosyl-(6-O-benzoyl)-α-d-glucopyranoside | Polygala tricornis; Polygala glomerata; Polygala reinii Fr.et Sav | [37,38,46] |
| 50 | 3′-Sinapoyl-6-benzoyl sucrose or 6-O-benzoyl-3′-O-sinapoylsucrose 6-O-benzoyl-3′-O-sinapoylsucrose or (3-O-[(2E)-3-(4-hydroxy-3,5-dimethoxyphenyl)-1-oxoprop-2-enyl]-β-d-fructofuranosyl 6-O-benzoyl-α-d-glucopyranoside) | Polygala sibirica; Polygala tricornis; Polygala telephioidesWilld. | [13,37,47] |
| 51 | β-d-[3-O-(3,4,5-Trimethoxycinnamoyl)]-fructo-furanosyl-α-D-[6-O-(p-hydroxybenzoyl)]-gluco-pyranoside or tenuifoliside A | Polygala tenuifolia; Polygala sibirica | [10,11,12,13] |
| 52 | β-d-(3-O-Sinapoyl)-fructofuranosyl-α-d-(6-O-(p-hydroxybenzoyl)]-glucopyranoside or tenuifoliside B | Polygala tenuifolia | [10] |
| 53 | Sibirioside A | Scrophularia ningpoensis Hemsl | [17] |
| 54 | 3-O-(E)-Sinapoyl-β-d-fructofuranosyl-(2→1)-[6-O-(E)-p-coumaroyl]-α-d-glucopyranoside or glomeratose B | Polygala glomerata | [38] |
| 55 | 3-O-[(E)-3,4,5-Trimethoxycinnamoyl]-β-d-fructo-furanosyl-(2→1)-[6-O-(E)-p- coumaroyl] -α-d-glucopyranoside or glomeratose C | Polygala glomerata | [38] |
| 56 | 3,4-O-β-d-Di-feruloyl-fructofuranosyl-6-O-α-d-(p-coumaroyl)-glucopyranoside | Monnina obtusifolia H.B.K. | [48] |
| 57 | 6′-O-p-Coumarylhydropiperoside or vanicoside D | Polygonum pensylvanicum | [49] |
| 58 | 1,3,6′-Tri-p-coumaroyl-6-feruloyl sucrose or diboside A | Fagopyrum dibotrys (D. Don.) Hara. | [50] |
| 59 | 6-O-Caffeoyl-β-d-fructofuranosyl-(2→1)-α-d-gluco-pyranoside | Scrophularia ningpoensis Hemsl; Globularia orientalis | [17,32] |
| 60 | 3,4-O-β-d-Di-feruloyl-fructofuranosyl-6-O-α-d-(caffeoyl)-glucopyranoside | Monnina obtusifolia H.B.K. | [48] |
| 61 | Reiniose A | Polygala reinii Fr.et Sav | [46] |
| 62 | 6-O-Feruloyl-β-d-fructofuranosyl-(2→1)-α-d-glucopyranoside or β-d-fructofuranosyl-6-O-feruloyl-α-d-glucopyranoside or arillatose B | Globularia orientalis; Polygala arillata | [32,51] |
| 63 | 1,6′-Diferuloyl-3,6-di-p-coumaroylsucrose or lapathoside A | Polygonum lapathifolium | [39] |
| 64 | 1,6,6′-Triferuloyl-3-p-coumaroyl sucrose or lapathoside B | Polygonum lapathifolium | [39] |
| 65 | 6′-Feruloyl-3,6-di-p-coumaroyl sucrose or lapathoside C | Polygonum lapathifolium | [39] |
| 66 | 6′-Feruloyl-1,6-di-p-coumaroyl sucrose or hydropiperoside A | Polygonum hydropiper L. | [52] |
| 67 | Vanicoside B | Polygonum perfoliatum; Polygonum pensylvanirum | [44,53] |
| 68 | 4-Acetyl-3,6′-diferuloylsucrose | Lilium speciosum var. rubrum; Lilium longiflorum | [54,55] |
| 69 | 6-Acetyl-3,6′-diferuloylsucrose | Lilium speciosum var. rubrum | [54] |
| 70 | 4,6-Diacetyl-3,6′-diferuloylsucrose | Lilium speciosum var. rubrum | [54] |
| 71 | 3,6′-Diferuloylsucrose | Lilium speciosum var. rubrum;Lilium longiflorum | [54,55] |
| 72 | β-d-[3-O-(3,4,5-Trimethoxycinnamoyl)]-fructo-furanosyl-α-d-(6-O-sinapoyl)-glucopyranoside or tenuifoliside C | Polygala tenuifolia; Polygala tricornis; Polygala glomerata; Polygala reinii Fr.et Sav; Polygala japonica Houtt. | [10,37,38,46,56] |
| 73 | 3′,6-Disinapoyl sucrose or 3-O-(E)-sinapoyl-β-d-fructofuranosyl-(2→1)-[6-O-(E)-sinapoyl]-α-d-glucopyranoside | Polygala tenuifolia; Polygala sibirica; Polygala tricornis; Polygala glomerata; Polygala reinii Fr.et Sav; Securidaca longipedunculata; Polygala virgata | [10,13,37,38,46,57,58] |
| 74 | β-D-(3,4-Disinapoyl)fructofuranosyl-α-d-(6-sinapoyl)glucopyranoside | Securidaca longipedunculata | [57] |
| 75 | 6-O-Sinapoylsucrose or sibiricose A1 | Polygala sibirica | [13] |
| 76 | 3-O-Feruloyl-β-d-fructofuranosyl-(6-O-sinapoyl)-α-d-glucopyranoside | Polygala reinii Fr.et Sav | [46] |
| 77 | 3-O-[(E)-3,4,5-Trimethoxycinnamoyl]-β-d-fructo-furanosyl-(2→1)-[6-O-(E)-p-coumaroyl]-α-d-glucopyranoside or glomeratose D | Polygala glomerata | [38] |
| 78 | 6-O-3,4,5-Trimethoxycinnamoyl sucrose or sibiricose A2 | Polygala sibirica | [13] |
| 79 | 3,6-Diferuloyl-4′,6′-diacetylsucrose or smilaside A | Smilax china | [14] |
| 80 | 3-O-[(E)-3,4,5-Trimethoxycinnamoyl]-β-d-fructo-furanosyl-(2→1)-(4-O-acetyl)-(6-O-benzoyl)-α-d-glucopyranoside or tricornoses B | Polygala tricornis | [37] |
| 81 | 4′-Acetyl-3,6′-diferuloylsucrose | Lilium speciosum var. rubrum | [54] |
| 82 | β-d-(3-O-Sinapoyl)fructofuranosyl-α-d-(4-O-acetyl-6-O-sinapoyl)glucopyranoside | Polygala virgata | [58] |
| 83 | Reiniose B | Polygala reinii Fr.et Sav | [46] |
| 84 | 4-O-Benzoyl-3′-3,4,5-trimethoxycinnamoylsucrose or [3-O-(3,4,5-trimethoxycinnamoyl)]-β-d-fructofuranosyl-(4-O-benzoyl)-α-d-gluco-pyranoside | Polygala tricornis; Polygala reinii Fr.et Sav | [37,46] |
| 85 | (3,6-O-Diferuloyl)-β-d-fructofuranosyl-(2→1)-(4-O-p-coumaroyl-6-O-acetyl)-α-d-glucopyranoside or quiquesetinerviuside D | Calamus quiquesetinervius Burret | [4] |
| 86 | (3,6-O-Diferuloyl)-β-d-fructofuranosyl-(2→1)-(4-O-feruloyl)-α-d-glucopyranoside or quiquesetinerviuside A | Calamus quiquesetinervius Burret | [4] |
| 87 | (3,6-O-Diferuloyl)-β-d-fructofuranosyl-(2→1)-(4-O-feruloyl-6-O-acetyl)-α-d-glucopyranoside or quiquesetinerviuside B | Calamus quiquesetinervius Burret | [4] |
| 88 | 3′,4-O-Disinapoylsucrose or sibiricose A4 | Polygala sibirica | [13] |
| 89 | 1-O-Acetyl-3-O-p-coumaroyl-β-d-fructofuranosyl-3,6-di-O-acetyl-α-d-glucopyranoside | Prunus padus | [58] |
| 90 | (3,6-Di-O-feruloyl)-β-d-fructofuranosyl-(3,6-di-O-acetyl)-α-d-glucopyranoside | Smilax glabra | [40] |
| 91 | 3′-O-Acetylvanicoside B or vanicoside F | Polygonum pensylvanicum | [49] |
| 92 | 6,3′-Diacetyl-3,6′-diferuloylsucrose | Lilium speciosum var. rubrum | [54] |
| 93 | 4,6,3′-Triacetyl-3,6′-diferuloylsucrose | Lilium speciosum var. rubrum | [54] |
| 94 | β-d-(3-O-Sinapoyl)fructofuranosyl-α-d-(3-O-acetyl-6-O-sinapoyl)glucopyranoside | Polygala virgata | [59] |
| 95 | Heterosmilaside | Heterosmilax erythrantha | [45] |
| 96 | 1-O-Acetyl-3-O-p-coumaroyl-β-d-fructofuranosyl-3,4,6-tri-O-acetyl-α-d-glucopyranoside | Prunus padus | [58] |
| 97 | 1,2′,6′-Triacetyl-3,6-diferuloylsucrose | Polygonum perfoliatum | [44] |
| 98 | 2′,6′-Diacetyl-3,6-diferuloylsucrose | Polygonum perfoliatum; Smilax china; Heterosmilax erythrantha | [14,44,45] |
| 99 | 1,3-Di-p-coumaroyl-6-feruloyl-2′,6′-diacetylsucrose or smilaside F | Smilax china | [14] |
| 100 | Smiglaside B | Smilax glabra | [40] |
| 101 | Smiglaside E | Smilax china; Smilax glabra | [14,40] |
| 102 | Vanicoside A | Polygonum perfoliatum; Polygonum pensylvanirum | [44,53] |
| 103 | 2′-Acetyl-1,6′-diferuloyl-3,6-di-p-coumaroyl sucrose or hydropiperoside B | Polygonum hydropiper L. | [52] |
| 104 | 2′-O-Acetylhydropiperoside or vanicoside C | Polygonum pensylvanirum | [49] |
| 105 | 1-O-p-Coumaroyl-3,6-O-diferuloyl-β-d-fructo-furanosyl-(2→1)-(2-O-acetyl)-α-d-glucopyranoside or smilaside K | Smilax bracteata | [15] |
| 106 | (1,3-O-Di-p-coumaroyl-6-O-feruloyl)-β-d-fructo-furanosyl-(2→1)-(2-O-acetyl)-α-d-glucopyranoside or smilaside H | Smilax bracteata | [15] |
| 107 | 3,6-Diferuloyl-2′-acetyl sucrose or smilaside B | Smilax china | [14] |
| 108 | 2′,4′,6′-Triacetyl-3,6-diferuloylsucrose or smiglaside C | Smilax glabra; Polygonum perfoliatum | [40,44] |
| 109 | β-d-(1-O-Acetyl-3,6-O-trans-dicinnamoyl)fructo-furanosyl-α-d-(2,4,6-O-triacetyl)glucopyranoside or niruriside | Phyllanthus niruri L. | [60] |
| 110 | 1,2′,4′,6′-Tetraacetyl-3,6-diferuloylsucrose | Polygonum perfoliatum | [44] |
| 111 | Smiglaside A | Smilax glabra | [40] |
| 112 | Smiglaside D | Smilax glabra | [40] |
| 113 | 4′-O-Acetylvanicoside A or vanicoside E | Polygonum pensylvanicum | [49] |
| 114 | (3,6-O-Diferuloyl)-β-d-fructofuranosyl-(2→1)-(4-O-p-coumaroyl-2-O-acetyl)-α-d-glucopyranoside or quiquesetinerviuside E | Calamus quiquesetinervius Burret | [4] |
| 115 | (3,6-O-Diferuloyl)-β-d-fructofuranosyl-(2→1)-(4-O-feruloyl-2-O-acetyl)-α-d-glucopyranoside or quiquesetinerviuside C | Calamus quiquesetinervius Burret | [4] |
| 116 | 3-O-p-Coumaroyl-β-d-fructofuranosyl2,3,4,6-tetra-O-acetyl-α-d-glucopyranoside | Prunus padus | [58] |
| 117 | 1-O-Acetyl-3-O-p-coumaroyl-β-d-fructofuranosyl 2,3,6-tri-O-acetyl-α-d-glucopyranoside | Prunus padus | [58] |
| 118 | β-d-(1-O-Acetyl-3,6-O-p-E-dicoumaroyl)-fructo-furanosyl-α-d-(4′-O-acetyl-2′-O-p-E-coumaroyl)-glucopyranoside | Froelichia floridana | [35] |
| 119 | 2-Feruloyl-O-α-d-glucopyranoyl-(1′→2)-3,6-O-feruloyl-β-d-fructofuranoside | Paris polyphylla var. yunnanensis | [61] |
| 120 | 3-O-Caffeoyl-β-d-fructofuranosyl 2,3,4,6-tetra-O-acetyl-α-d-glucopyranoside | Prunus ssiori | [24] |
| 121 | Magnoloside A | Magnolia obovata Thunb | [62] |
| 122 | β-(p-Hydroxyphenyl)ethyl O-α-l-rhamno-pyranosyl-(1→3)-6-O-trans-p-coumaroyl-β-d-gluco-pyranoside or osmanthuside B6 | Osmanthus asiaticus; Ligustrum purpurascens | [22,24] |
| 123 | β-(p-Hydroxyphenyl)ethyl O-α-l-rhamno-pyranosyl-(1→3)-4-O-cis-p-coumaroyl-β-d-gluco-pyranoside or osmanthuside D | Osmanthus asiaticus | [22] |
| 124 | Jionoside D | Rehmannia glutinosa var. Purpurea; Scrophularia nodosa L. | [19,63] |
| 125 | 2-Phenylethyl O-α-l-rhamnopyranosyl-(1→3)-4-O-caffeoyl-β-d-glucopyranoside or jionoside C | Rehmannia glutinosa var. Purpurea | [19] |
| 126 | Osmanthuside B | Ligustrum purpurascens; cistanche salsa | [24,64] |
| 127 | Lipedoside A-II | Ligustrum purpurascens | [24] |
| 128 | Isoverbascoside | Lantana camaro L.; Pedicularis artselaeri; Pedicularis striata; Markhamia stipulate; Fernandoa adenophylla; Markhamia lutea; Scrophularia scorodonia | [15,29,65,66,67,68,69] |
| 129 | Scrophularia nodosa L. | [63] | |
| 130 | 6′-O-(E)-Cinnamoyl verbascoside | Osmanthus austrocaledonica | [65] |
| 131 | Acteoside or verbascoside | Rehmannia glutinosa var. Purpurea; Ligustrum purpurascens; Calceolaria hypericina; Lantana camaro L.; Scrophularia nodosa L.; Pedicularis artselaeri; Pedicularis striata; Markhamia stipulate; Fernandoa adenophylla; Markhamia lutea; Scrophularia scorodonia; Penstemon serrulatus Menz; Aeginetia indica Linn; Pedicularis lasiophrys; Lagotis stolonifera; Conandron ramoidioides; Paulownia tomentosa stem; Phlomis grandiflora; Pedicularis spicata; Pedicularis bngijora; cistanche salsa; Brandisia hancei; Phlomis linearis | [15,19,24,25,29,63,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84] |
| 132 | cis-Acteoside or cisacteoside | Scrophularia ningpoensis Hemsl; Scrophularia nodosa L.; Penstemon serrulatus Menz | [17,63,71] |
| 133 | Cistanoside C or leucosceptoside A or trans-leucosceptoside A | Rehmannia glutinosa var. Purpurea; Fernandoa adenophylla; Penstemon serrulatus Menz; Pedicularis bngijora; cistanche salsa; Lamiophlomis rotata | [19,69,71,79,85,86] |
| 134 | cis-Leucosceptoside A | Penstemon serrulatus Menz | [71] |
| 135 | 2′′,3′′′-Diacetyl acteoside | Aeginetia indica Linn | [72] |
| 136 | 2′-Acetyl acteoside | Rehmannia glutinosa var. Purpurea; cistanche salsa; Aeginetia indica Linn; Brandisia hancei | [19,64,72,82] |
| 137 | l′-O-β-d-(3-Methoxy-4-hydroxy-β-phenyl)-ethyl-6′-O-feruloyl-α-l-(2-acetyl)-rhamnosyl-(1→3′)-4′-acetylglucopyranoside or pedicularioside E | Pedicularis lasiophrys | [73] |
| 138 | Martynoside or trans-martynoside | Rehmannia glutinosa var. Purpurea; Pedicularis artselaeri; Fernandoa adenophylla; Penstemon serrulatus Menz; Paulownia tomentosa stem; Galeopws pubescens | [19,66,69,71,76,87] |
| 139 | cis-Martynoside | Penstemon serrulatus Menz | [71] |
| 140 | 2-(4-Hydroxy-3-methoxyphenyl)ethyl O-α-l-rhamnopyranosyl-(1→3)-O-(4-O-feruloyl)-β-d-glucopyranoside or cistanoside D | cistanche salsa; Pedicularis artselaeri; Pedicularis lasiophrys; Pedicularis bngijora | [64,66,73,79] |
| 141 | 2-(3′,4′-Dihydroxyphenyl)-ethanol 1-O-β-d-xylosyl-(1→3)-β-d-(4-caffeyl)-glucoside or conandroside | Conandron ramoidioides | [74] |
| 142 | Isonuomioside A | Paraboea glutinosa; Lantana camaro L. | [27,29] |
| 143 | Calceolarioside E | Paraboea glutinosa; Lantana camaro L. | [27,29] |
| 144 | Plantamajoside | Lagotis stolonifera | [75] |
| 145 | Isocistanoside F | Ligustrum purpurascens | [24] |
| 146 | α-l-Rhamnopyranosyl(1→3)-O-(4-O-caffeoyl)-d-glucopyranoseor cistanoside F | cistanche salsa | [85] |
| 147 | 3-Hydroxy-4-methoxy-β-phenylethoxy-O-α-l-rhamnopyranosyl-(1→3)-6-O-feruloyl-β-d-gluco-pyranoside or isomartynoside | Galeopws pubescens | [87] |
| 148 | 1′-O-β-d-(3,4-Dihydroxy-β-phenyl)-ethyl-4′-O-caffeoyl-β-d-xylopyranosyl-(1′′′→6′)-glucopyran oside or calceolarioside C | Calceolaria hypericina | [25] |
| 149 | 4-Cinnamoyl desxylosyl mussatioside | Mussatia | [88] |
| 150 | 1-O-trans-Caffeoyl-2′-O-trans-sinapoylgentiobiose. | Wasabia japonica Matsumura | [89] |
| 151 | 1-O-trans-Feruloyl-2′-O-trans-sinapoylgentiobiose | Wasabia japonica Matsumura | [89] |
| 152 | 1,2′-di-O-trans-sinapoylgentiobiose | Wasabia japonica Matsumura | [89] |
| 153 | 1-(3′′,4′′-Dihydroxy-5′′-methoxy)-O-trans-cinnamoyl-2′-O-trans-feruloyl gentiobiose | Wasabia japonica Matsumura | [89] |
| 154 | 1-(3′′,4′′-Dihydroxy-5′′-methoxy)-O-trans-cinnamoyl-2′-O-trans-sinapoylgentiobiose | Wasabia japonica Matsumura | [89] |
| 155 | 1,2′-Di-(3′′,4′′-dihydroxy-5′′-methoxy)-O-trans-cinnamoyl gentiobiose | Wasabia japonica Matsumura | [89] |
| 156 | (5-O-E-Caffeoyl)-β-d-apio-d-furanosyl-(1→6)-β-d-glucopyranosyl benzoic acid ester or psydroside | Psydrax livida | [90] |
| 157 | Crenatoside | Orobanche crenata | [91] |
| 158 | Campneoside II or orobanchoside | Paulownia tomentosa stem; Orobanche crenata | [76,91] |
| 159 | Campneoside I | Paulownia tomentosa stem | [76] |
| 160 | Ligurobustoside C | Ligustrum purpurascens | [24] |
| 161 | Ligurobustoside I | Ligustrum purpurascens | [24] |
| 162 | 1-O-{6-O-[3-O-(E,E)-(β,β′-bis-Sinapoyl)-β-d-fructo-furanosyl]}-α-d-glucopyranoside intramolecular ester or glomeratose E | Polygala glomerata | [38] |
| 163 | 3-O-[(E)-Sinapoyl]-β-d-fructofuranosyl-(2→1)-[β-d-glucopyranosyl-(1→2)]-[6-O-(E)-sinapoyl]-α-d-glucopyranosideor tricornose D | Polygala tricornis | [37] |
| 164 | 3-O-[(E)-3,4,5-Trimethoxycinnamoyl]-β-d-fructo-furanosyl-(2→1)-[β-d-glucopyranosyl-(1→2)]-[6-O-(E)-sinapoyl]-α-d-glucopyranoside or tricornose C | Polygala tricornis | [37] |
| 165 | 3-O-(E)-3,4,5-Trimethoxycinnamoyl-[4-O-(E)-feruloyl]-β-d-fructofuranosyl-(2→1)-[β-d-gluco-pyranosyl-(1→2)]-[6-O-(E)-sinapoyl]-α-d- gluco-pyranoside or tricornose F | Polygala tricornis | [37] |
| 166 | 3-O-(E)-3,4,5-Trimethoxycinnamoyl-[4-O-(E)-sinapoyl]-β-d-fructofuranosyl-(2→1)-[β-d-glucopyranosyl-(1→2)]-[6-O-(E)-sinapoyl]-α-d-glucopyranoside or tricornose E | Polygala tricornis | [37] |
| 167 | Reiniose E | Polygala reinii Fr.et Sav | [46] |
| 168 | Reiniose F | Polygala reinii Fr.et Sav | [46] |
| 169 | O-β-d-Glucopyranosyl-(1→3)-6-O-feruloyl -α-d-glucopyranosyl β-d-fructofuranoside or arillatose C | Polygala arillata | [51] |
| 170 | O-β-d-Glucopyranosyl-(1→3)-6-O-sinapoyl-α-d-glucopyranosyl β-d-fructofuranoside or arillatose D | Polygala arillata | [51] |
| 171 | O-β-d-Glucopyranosyl-(1→3)-α-d-gluco-pyranosyl-3′-O-feruloyl-β-d-fructofuranoside or arillatose E | Polygala arillata | [51] |
| 172 | O-β-d-Glucopyranosyl-(1→3)-α-d-gluco-pyr anosyl-3′-O-sinapoyl-β-d-fructofuranoside or arillatose F | Polygala arillata | [51] |
| 173 | 3-Feruloyl-4-acetyl-6′-(13′-O-β-d-gluco-pyranosyl)feruloylsucrose | Lilium longiflorum | [55] |
| 174 | Reiniose D | Polygala reinii Fr.et Sav; Polyyala fallax | [46,92] |
| 175 | Dalmaisiose A | Polygala dalmaisiana | [93] |
| 176 | 3,4-Dihydroxyphenylethanol-6-O-trans-caffeoyl-β-d-apiofuranosyl(1→5)-β-d-apiofuranosyl(1→3)-β-d-glucopyranoside or paraboside B | Paraboea glutinosa | [27] |
| 177 | 3,4-Dihydroxyphenylethanol-4-O-trans-caffeoyl-β-d-apiofuranosyl(1→5)-β-d-apiofuranosyl(1→3)-β-d-glucopyranosideor paraboside A | Paraboea glutinosa | [27] |
| 178 | 2-(3,4-Dihydroxyphenyl)ethyl 3,6-O-bis(β-d-apiofranosyl)-4-O-caffeoyl-β-d-glucopyranoside or paucifloside | Lysionotus pauciflorus | [94] |
| 179 | l′-O-β-d-(3,4-Dihydroxy-β-phenyl)-ethyl-4′-O-caffeoyl-β-d-apiosyl-(l→3′)-α-l-rhamnosyl-(l→6′)-glucopyranoside or pedicularioside A | Pedicularis striata; Markhamia lutea; Pedicularis striata pall ssp. arachnoidea; Pedicularis spicata | [5,67,77,78] |
| 180 | l′-O-β-d-(3,4-Dihydroxy-β-phenyl)-ethyl-4′-O-feruloyl-β-d-apiosyl(1→3′)-α-l-rhamnosyl-(1→6′)-glucopyranoside or pedicularioside M | Pedicularis striata pall ssp. arachnoidea | [77] |
| 181 | l′-O-β-d-(3-hydroxy-4-methoxy-β-phenyl)-ethyl-4′-feruloyl-β-d-apiosyl(l→3′)-α-l-rhamnosyl-(l→6′)-glucopyranoside or pedicularioside N | Pedicularis artselaeri; Pedicularis striata pall ssp. arachnoidea | [66,77] |
| 182 | l′-O-β-d-(3-Methoxy-4-hydroxy-β-phenyl)-ethyl-4′-O-feruloyl-β-d-apiosyl-(1→3′)-α-l-rhamnos yl-(1→6′)-glucopyranoside or pedicularioside H | Pedicularis spicata | [78] |
| 183 | 3,4-Dihydroxy-β-phenylethoxy-O-[α-arabino-pyranosyl-(1′′′′→2′′)-α-rhamnopyranosyl-(1′′′→3′′)-6′′-O-caffeoyl-β-glucopyranoside] or markhamioside C | Markhamia stipulata | [68] |
| 184 | Ehrenoside | Veronica pectinata var. glandulosa; Aragoa cundinamarcensis | [75,95,96] |
| 185 | 3,4-Dihydroxy-β-phenylethoxy-O-[α-arabino-pyranosyl-(1′′′′→2′′)-α-rhamnopyranosyl-(1′′′→3′′)-4-O-caffeoyl-6-O-acetyl-β-glucopyranoside or markhamioside D | Markhamia stipulata | [68] |
| 186 | 2-(3,4-Dihydroxyphenyl)ethyl-O-α-l-arabino-pyranosyl-(1→2)-[α-l-rhamnopyranosyl-(1→3)]-(4-O-trans-feruloyl)-β-d-glucopyranoside or verpectoside A | Veronica pectinata var. glandulosa | [95] |
| 187 | Lagotoside | Lagotis stolonifera | [75] |
| 188 | 3,4-Dihydroxy-β-phenylethoxy-O-β-apiofuranosyl-(1→2)-α-rhamnopyranosyl-(1→3)-4-O-caffeoyl-β-glucopyranosideor 2′′-O-β-apiosylverbascoside | Markhamia stipulata ; Fernandoa adenophylla | [68,69] |
| 189 | 1-O-(3,4-Dihydroxyphenyl)ethyl β-d-apiofuranosyl(1→2)-α-l-rhamnopyranosyl (1→3)-4-O-caffeoyl-6-acetyl-β-d-glucopyrano sideor luteoside A | Markhamia stipulate; Markhamia lutea | [5,68] |
| 190 | 1-O-(3,4-Dihydroxyphenyl)ethyl β-d-apio-furanosyl(1→2)-α-l-rhamnopyranosyl(1→3)-6-O-caffeoyl-β-d-glucopyranosideor luteoside B | Markhamia stipulate; Markhamia lutea | [5,68] |
| 191 | 1-O-(3,4-Dihydroxyphenyl)ethyl β-d-apio-furan osyl(1→2)-α-l-rhamnopyranosyl(1→3)-6-O-feruloyl-β-d-glucopyranoside or luteoside C | Markhamia lutea | [5] |
| 192 | 3-Hydroxy-4-methoxy-β-phenylethoxy-O-[β-apio-furanosyl-(1′′′′→2′′)-α-rhamnopyranosyl-(1′′′→3′′)-6′′-O-feruloyl-β-glucopyranoside] or markhamioside B | Markhamia stipulate | [68] |
| 193 | 3,4-Dihydroxy-β-phenylethoxy-O-[β-galacto-pyranosyl-(1′′′′→2′′)-α-rhamnopyranosyl-(1′′′→3′′)-4-O-caffeoyl-6-O-acetyl-β-glucopyranoside] or markhamioside E | Markhamia stipulate | [68] |
| 194 | 2-(3,4-Dihydroxyphenyl)ethyl-O-β-d-gluco-pyranosyl-(1→2)-[α-l-rhamnopyranosyl-(1→3)]-(4-O-trans-caffeoyl)-β-d-glucopyranoside or verpectoside B | Veronica pectinata var. glandulosa | [95] |
| 195 | 2-(3,4-Dihydroxyphenyl)ethyl-O-β-d-gluco-pyranosyl-(1→2)-[α-l-rhamnopyranosyl-(1→3)]-(4-O-trans-feruloyl)-β-d-glucopyranoside or verpectoside C | Veronica pectinata var. glandulosa | [95] |
| 196 | 1′-O-β-d-(3-Methoxy-4-hydroxy-phenyl)-ethyl-α-l-rhamnosyl-(1→3′)-α-l-arabinosyl-(1→4′)-6′-O-feruloyl-glucopyranoside or pedicularioside I | Pedicularis bngijora | [79] |
| 197 | Angoroside A | Scrophularia nodosa L.; Scrophularia scorodonia | [63,70] |
| 198 | Scrophuloside B1 | Scrophularia nodosa L. | [63] |
| 199 | Scrophuloside B2 | Scrophularia nodosa L. | [63] |
| 200 | 3,4-Dihydroxy-β-phenylethoxy-O-α-l-arabino-pyranosyl-(1→6)-α-l-rhamnopyranosyl-(1→3)-4-O-feruloyl-β-d-glucopyranoside or angoroside D | Scrophularia scorodonia | [70] |
| 201 | Angoroside C | Scrophularia nodosa L. | [63] |
| 202 | Forthysioside B | Markhamia lutea | [5] |
| 203 | 6′-β-d-Apiofuranosyl cistanoside C | Lamiophlomis rotata | [86] |
| 204 | Lamiophlomiside A | Lamiophlomis rotata | [86] |
| 205 | cis-Lamiophlomiside A | Lamiophlomis rotata | [86] |
| 206 | Forsythoside B | Phlomis grandiflora; Phlomis fruticosa | [80] |
| 207 | Alyssonoside | Phlomis grandiflora; Phlomis fruticosa | [80] |
| 208 | 2-(3,4-Dihydroxyphenyl)ethyl O-α-rhamno-pyranosyl-(1→3)-[β-d-galactopyranosyl-(l→6)]-(4-O-p-coumaroyl)-β-d-glucopyranoside or jionoside E | Rehmannia glutinosa var. Purpurea | [19] |
| 209 | Purpureaside C | Rehmannia glutinosa var. Purpurea; Scrophularia nodosa L. | [19,63] |
| 210 | Jionoside A1 | Rehmannia glutinosa var. Purpurea | [19] |
| 211 | Jionoside A2 | Rehmannia glutinosa var. Purpurea | [19] |
| 212 | Jionoside B1 | Rehmannia glutinosa var. Purpurea | [19] |
| 213 | Jionoside B2 | Rehmannia glutinosa var. Purpurea | [19] |
| 214 | Echinacoside | Ligustrum purpurascens; cistanche salsa | [24,81] |
| 215 | 2-(4-Hydroxy-3-methoxyphenyl)ethyl O-α-l-rhamnopyranosyl-(1→3)-O-[β-d-glucopyrano syl(1→6)]-(4-O-caffeoyl)-β-d-glucopyranosideor cistanoside A | Ligustrum purpurascens | [81] |
| 216 | 2-(4-Hydroxy-3-methoxyphenyl)ethyl O-α-l-rhamnopyranosyl-(1→3)-O-[β-d-glucopyran osyl(1→6)]-(4-O-feruloyl)-β-d-glucopyranoside or cistanoside B | Ligustrum purpurascens | [81] |
| 217 | Poliumoside | Brandisia hancei | [82] |
| 218 | [β-(3′,4′-Dihydroxylphenyl)-ethyl]-(2-O-acetyl)-(3,6-O-di-α-l-rhamnopyranosyl-(4-O-caffeoyl)β-d-glucopyranoside or brandioside | Brandisia hancei | [82] |
| 219 | Arenarioside | Scrophularia nodosa L. | [63] |
| 220 | 1-O-3,4-(Sihydroxyphenyl)-ethyl-β-d-apiofuranosyl-(1→4)-α-l-rharmnopyranosyl-(1→3)-4-O-caffeoyl-β-d-glucopyranoside or myricoside | Markhamia lutea; Picria tel-ferae Lour. | [5,97] |
| 221 | Rossicaside B | Boschniakia rossica | [98] |
| 222 | Rossicaside A | Boschniakia rossica | [98] |
| 223 | 2-O-Acetylrossicaside A | Ortbocarpus densiflourus var. gracilis | [99] |
| 224 | β-d-glucopyranosyl(1→4)-α-l-rhamnopyranosyl-(1→3)-(4-O-trans-caffeoyl)-d-glucopyranose | Boschniakia rossica | [98] |
| 225 | Lavandulifolioside | leonurus glaucescens | [83] |
| 226 | β-(3,4-Dihydroxyphenyl)-ethyl-O-α-L-arabinopyranosyl-(1→2)-α-L-rhamnopyranosyl-(l →3)-4-O-feruloyl-β-D-glucopyranoside or leonosides A | leonurus glaucescens | [83] |
| 227 | β-(3-Hydroxy,4-methoxyphenyl)-ethyl-O-α-l-arabinopyranosyl-(l→2)-α-l-rhamnopyranosyl-(1→3)-4-O-feruloyl-β-d-glucopyranoside or leonoside B | leonurus glaucescens | [83] |
| 228 | 2R-Galactosyl-acteoside or lamalboside | Lamium album | [100] |
| 229 | 3,4-Dihydroxy-β-phenylethoxy-O-β-d-gluco-pyranosyl-(1→2)-α-l-rhamnopyranosyl-(1→3)-4-O-caffeoyl-β-d-glucopyranoside or phlinoside A | Phlomis linearis | [84] |
| 230 | 3,4-Dihydroxy-β-phenylethoxy-O-α-l-lyxo-pyranosyl-(1→2)-α-l-rhamnopyranosyl-(1→3)-4-O-caffeoyl-β-d-glucopyranoside or teucrioside | Teucrium chamaedrys | [101] |
| 231 | 3,4-Dihydroxy-β-phenylethoxy-O-β-d-xylo-pyranosyl-(1→2)-α-L-rhamnopyranosyl-(1→3)-4-O-caffeoyl-β-d-glucopyranoside or phlinoside B | Phlomis linearis | [84] |
| 232 | 3,4-Dihydroxy-β-phenylethoxy-O-β-d-xylo-pyranosyl-(1→2)-α-l-rhamnopyranosyl-(1→3)-4-O-feruloyl-β-d-glucopyranoside or phlinoside D | Phlomis lineuris | [102] |
| 233 | 2-(4-Hydroxyphenyl)-ethyl-[3-O-α-l-rhamno-pyranosyl(1→4)-α-l-rhamnopyranosyl][6-O-p-coumaroyl]-O-β-d-glucopyranoside or ligupurpuroside C | Ligustrum purpurascens | [24] |
| 234 | 2-(4-Hydroxyphenyl)-ethyl-[3-O-α-l-rhamno-pyranosyl(1→4)-α-l-rhamnopyranosyl][6-O-(E)-caffeoyl]-O-β-d-glucopyranoside or ligupurpuroside D | Ligustrum purpurascens | [24] |
| 235 | 3-O-[α-l-Rhamnopyranosyl(1→4)-α-l-rhamno-pyranosyl]-4-O-(E)-caffeoyl-d-glucopyranose or ligupurpuroside F | Ligustrum purpurascens | [24] |
| 236 | Ligupurpuroside B | Ligustrum purpurascens | [24] |
| 237 | Ligurobustosides N | Ligustrum purpurascens | [24] |
| 238 | Ligupurpuroside A | Ligustrum purpurascens | [24] |
| 239 | 3,4-Dihydroxy-β-phenylethoxy-O-α-l-rhamno-pyranosyl-(l→2)-α-l-rhamnopyranosyl-(1→3)-4-O-caffeoyl-β-d-glucopyranoside or phlinoside C | Phlomis linearis | [84] |
| 240 | 3,4-Dihydroxy-β-phenylethoxy-O-α-l-rhamno-pyranosyl-(l→2)-α-l-rhamnopyranosyl-(l→3)-4-O-feruloyl-β-d-glucopyranoside or phlinoside E | Phlomis lineuris | [102] |
| 241 | Myricoside | Clerodendrum serratum | [103] |
| 242 | 3-Hydroxy-4-methoxy-β-phenethyl-O-β-d-apio-furanosyl-(1→3)-α-l-rhamnopyranosyl-(1→3)-4-O-feruloyl-β-d-glucopyranoside or serratumoside A | Clerodendrum serratum | [103] |
| 243 | Aragoside | Aragoa cundinamarcensis | [96] |
| 244 | Persicoside | Aragoa cundinamarcensis | [96] |
| 245 | 1′-O-β-d-(3-Hydroxy-4-methoxy-β-phenyl)-ethyl-4′-O-feruloyl-β-d-glucopyranosyl-(1→3)-α-l-rhamnosyl-(1→6′)-glucopyranoside or artselaeroside B | Pedicularis artselaeri | [66] |
| 246 | 3,4-Dihydroxy-β-phenyl-ethyl-O-α-l-rhamno-pyranosyl-(1→2)-O-β-d-glucopyranosyl-(1→6)-3-O-caffeoyl-β-d-allopyranoside or magnoloside B | Magnolia obovata Thunb | [62] |
| 247 | α-l-Xylopyranosyl-(4′′→2′)-(3-O-β-d-gluco-pyranosyl)-1′-O-E-caffeoyl-β-d-glucopyranoside | Coussarea hydrangeifolia | [21] |
| 248 | 2-(3,4-Dihydroxyphenyl)-R,S-2-ethoxyethyl-O-β-d-glucopyranosyl(1→4)-α-l-rhamno-pyranosyl(1→3)(4-O-trans-caffeoyl)-β-d-gluco-pyranoside or rossicaside F | Boschniakia rossica | [97] |
| 249 | 4-Cinnamoyl desxylosylmussatioside | Mussatia | [88] |
| 250 | 4-p-Coumaroylmussatioside | Mussatia | [88] |
| 251 | 4-cis-p-Coumaroylmussatioside | Mursatia byacinthima | [104] |
| 252 | 4-p-Methoxycmnamoylmussatioslde ormussatloside III | Mussatia | [88] |
| 253 | 4-Feruloylmussatioside | Mussatia | [88] |
| 254 | 4-Dimethylcaffeoylmussatloside or mussatioside II | Mussatia | [88] |
| 255 | 3-O-[(E)-Sinapoyl]-β-d-fructofuranosyl-(2→1)-[β-d-glucopyranosyl-(1→4)-β-d-gluco-pyranosyl-(1→2)]-[6-O-(E)-sinapoyl]-α-d-gluco-pyranoside or tricornose G | Polygala tricornis | [37] |
| 256 | 3-O-(E)-Sinapoyl-[4-O-(E)-p-coumaroyl]-β-d-fructofuranosyl-(2→1)-[β-d-glucopyranosyl-(1→4)-β-d-glucopyranosyl-(1→2)]-[6-O-(E)-sinapoyl]-α-d-glucopyranoside or tricornose L | Polygala tricornis | [37] |
| 257 | 3-O-(E)-Sinapoyl-[4-O-(E)-feruloyl]-β-d-fructo-furanosyl-(2→1)-[β-d-glucopyranosyl-(1→4)-β-d-glucopyranosyl-(1→2)]-[6-O-(E)-sinapoyl] -α-d-glucopyranoside or tricornose K | Polygala tricornis | [37] |
| 258 | 3-O-(E)-sinapoyl-[4-O-(E)-sinapoyl]-β-d-fructo-furanosyl-(2→1)-[β-d-glucopyranosyl-(1→4)-β-d-glucopyranosyl-(1→2)]-[6-O-(E)-sinapoyl]-α-d-glucopyranoside or tricornose H | Polygala tricornis | [37] |
| 259 | 3-O-(E)-3,4,5-Trimethoxylcinnamoyl-[4-O-(E)-feruloyl]-β-d-fructofuranosyl-(2→1)-[β-d-gluco-pyranosyl-(1→4)-β-d-glucopyranosyl-(1→2)]-[6-O-(E)-sinapoyl]-α-d-glucopyranoside or tricornose J | Polygala tricornis | [37] |
| 260 | 3-O-(E)-3,4,5-Trimethoxylcinnamoyl-[4-O-(E)-sinapoyl]-β-d-fructofuranosyl-(2→1)-[β-d-glucopyranosyl-(1→4)-β-d-glucopyranosyl-(1→2)]-[6-O-(E)-sinapoyl]-α-d-glucopyranoside or tricornose I | Polygala tricornis | [37] |
| 261 | Senegose I | Polygala senega var. latifolia Torr. Et Gray | [105] |
| 262 | 1-O-(E)-p-Coumaroyl-(3-O-benzoyl)-β-d-fructo-furanosyl-(2→1)-[β-d-glucopyranosyl-(1→2)]-[6-O-acetyl-β-d-glucopyranoysl-(1→3)]-[4-O-(E)-feruloyl]-(6-d-acetyl)-α-d-glucopyranoside or glomeratose F | Polygala glomerata | [38] |
| 263 | 1-O-(E)-p-Coumaroyl-(3-O-benzoyl)-β-d-fructo-furanosyl-(2→l)-[β-d-glucopyranosyl-(1→2)]-[6-O-acetyl-β-d-glucopyranoside-(1→3)]-{4-O-[4-O-β-d-glucopyranosyl-(E)-feruloyl]}-[6-O-(E)-p-coumaroyl]-α-d-glucopyranosyl or glomeratose G | Polygala glomerata | [38] |
| 264 | 1-O-p-coumaroyl-(3-O-benzoyl)-β-d-fructo-furanosyl-(2→1)-[β-d-glucopyranosyl-(1→2)]-[6-O-acetyl-β-d-glucopyranosyl-(1→3)]-(4-O-p-coumaroyl)-α-d-glucopyranoside or fallaxose C | Polyyala fallax | [92] |
| 265 | Reiniose G | Polygala glomerata; Polygala reinii Fr. et Sav | [38,46] |
| 266 | Dalmaisiose H | Polygala dalmaisiana | [93] |
| 267 | 1-O-p-Coumaroyl-(3-O-benzoyl)-β-d-fructo-furanosyl-(2→1)-[β-d-glucopyranosyl-(1→2)]-[6-O-acetyl-β-d-glucopyranosyl-(1→3)]-(4-O-feruloyl)-α-d-glucopyranoside or fallaxose D | Polyyala fallax | [92] |
| 268 | Dalmaisiose J | Polygala dalmaisiana | [93] |
| 269 | Dalmaisiose L | Polygala dalmaisiana | [93] |
| 270 | Dalmaisiose M | Polygala dalmaisiana | [93] |
| 271 | Reiniose H | Polygala reinii Fr. et Sav | [46] |
| 272 | Senegose G | Polyyala fallax; Polygala senega var. latifolia Torr. Et Gray | [92,105] |
| 273 | Senegose H | Polygala senega var. latifolia Torr. Et Gray | [105] |
| 274 | Senegose F | Polygala reinii Fr. et Sav; Polygala senega var. latifolia Torr. Et Gray | [46,105] |
| 275 | 3-O-β-D-Glucopyranosylpresenegenin 28-O-β-D-xylopyranosyl-(1→4)-α-L-rhamnopyranosyl-(1→2)-{4-O-[(E)-3,4-dimethoxycinnamoyl]}-β-D-fucopyranosyl ester or Polygalasaponin XLII | Polygala glomerata Lour | [106] |
| 276 | 3,4-Dihydroxy-β-phenylethyl-O-α-l-rhamno-pyranosyl-(1→2)-O-[O-β-d-glucopyranosyl-(1→4)-β-d-glucopyranosyl-(1→6)]-3-O-caffeoyl-β-d-allopyranoside or magnoloside C | Magnolia obovata Thunb | [62] |
| 278 | 3-O-{4-O-[β-d-Glucopyranosyl-(1→3)-(2-O-acetyl)-α-l-rhamnopyranosyl]-feruloyl}-β-d-fructo-furanosyl-(2→1)-(4,6-di-O-benzoyl)-α-d-gluco-pyranoside or fallaxose B | Polyyala fallax | [92] |
| 279 | 2-(3,4-Dihydroxyphenyl)ethyl O-β-apio-furanosyl-(1→6)-O-[O-β-apiofuranosyl-(1→4)-α-rhamnopyranosyl-(1→3)]-4-O-(E)-caffeoyl-β-glucopyranoside or lunariifolioside | Phlomis lunariifolia | [106] |
| 280 | Tenuifoliose K | Polygala tenuifolia Willd | [11] |
| 281 | Tenuifoliose J | Polygala tenuifolia Willd | [11] |
| 282 | tenuifoliose I | Polygala tenuifolia Willd | [11] |
| 283 | Tenuifoliose H | Polygala tenuifolia Willd | [11] |
| 284 | Tenuifoliose C | Polygala tenuifolia Willd; Polyyala fallax | [12,92] |
| 285 | Tenuifoliose B | Polygala tenuifolia Willd | [12] |
| 286 | Tenuifoliose D | Polygala tenuifolia Willd; Polygala reinii Fr. et Sav | [12,46] |
| 287 | Tenuifoliose E | Polygala tenuifolia Willd | [12] |
| 288 | Tenuifoliose A | Polygala tenuifolia Willd | [11,12] |
| 289 | Tenuifoliose P | Polygala tenuifolia Willd | [11] |
| 290 | Tenuifoliose O | Polygala tenuifolia Willd | [11] |
| 291 | Reiniose I | Polygala reinii Fr. et Sav | [46] |
| 292 | Tenuifoliose N | Polygala tenuifolia Willd | [11] |
| 293 | Reiniose J | Polygala reinii Fr. et Sav | [46] |
| 294 | 1-O-Feruloyl-(3-O-benzoyl)-β-d-fructofuranosyl-(2→1)-[β-d-glucopyranosyl-(1→2)]-[β-d-gluco-pyranosyl-(1→3)-(6-o-acetyl)-β-d-gluco-pyranosyl-(1→3)]-(6-o-feruloyl)-α-d-glucopyranoside or fallaxose E | Polyyala fallax | [92] |
| 295 | Senegose K | Polygala senega L. | [107] |
| 296 | Senegose J | Polygala senega L. | [107] |
| 297 | Senegose N | Polygala senega L. | [107] |
| 298 | Senegose O | Polygala senega L. | [107] |
| 299 | Senegose M | Polygala senega L. | [107] |
| 300 | Senegose L | Polygala senega L. | [107] |
| 301 | Senegose D | Polygala senega var. latifolia Torr. Et Gray | [108] |
| 302 | Senegose C | Polygala senega var. latifolia Torr. Et Gray | [108] |
| 303 | Senegose B | Polygala senega var. latifolia Torr. Et Gray | [108] |
| 304 | Senegose A | Polygala senega var. latifolia Torr. Et Gray | [108] |
| 305 | Senegose E | Polygala senega var. latifolia Torr. Et Gray | [108] |
| 306 | Dalmaisiose D | Polygala dalmaisiana | [93] |
| 307 | Dalmaisiose B | Polygala dalmaisiana | [93] |
| 308 | Dalmaisiose E | Polygala dalmaisiana | [93] |
| 309 | Dalmaisiose I | Polygala dalmaisiana | [93] |
| 310 | Dalmaisiose N | Polygala dalmaisiana | [93] |
| 311 | Dalmaisiose F | Polygala dalmaisiana | [93] |
| 3312 | Dalmaisiose P | Polygala dalmaisiana | [93] |
| 313 | Dalmaisiose G | Polygala dalmaisiana | [93] |
| 314 | Dalmaisiose C | Polygala dalmaisiana | [93] |
| 315 | Dalmaisiose K | Polygala dalmaisiana | [93] |
| 316 | Dalmaisiose O | Polygala dalmaisiana | [93] |
| 317 | E-Senegasaponin b | Polygala senega L.var. latifolia Torrey et Gray | [109] |
| 318 | Z-Senegasaponin b | Polygala senega L.var. latifolia Torrey et Gray | [109] |
| 319 | Senegin II | Polygala glomerata Lour | [106] |
| 320 | (Z)-Senegin II | Polygala glomerata Lour | [106] |
| 321 | Tenuifoliose M | Polygala tenuifolia Willd | [11] |
| 322 | Tenuifoliose L | Polygala tenuifolia Willd | [11] |
| 323 | Tenuifoliose G | Polygala tenuifolia Willd | [11] |
| 324 | Tenuifoliose F | Polygala tenuifolia Willd | [11,12] |
| 325 | 3-O-β-d-Glucopyranosylpresenegenin 28-O-β-d-galactopyranosyl-(1→4)-β-d-xylo-pyranosyl-(1→4)-α-l-rhamnopyranosyl-(1→2)[β-d-glucopyranosyl-(1→3)]-(4-O-[(E)-3,4-dimethoxycinnamoyl]}-8-O-fucopyranosyl ester or polygalasaponin XLIV | Polygala glomerata Lour | [106] |
| 326 | 3-O-β-d-Glucopyranosylpresenegenin 28-O-β-d-galactopyranosyl-(1→4)-β-d-xylopyranosyl-(1→4)-α-l-rhamnopyranosyl-(1→2)-[6-O-acetyl-β-d-glucopyranosyl-(1→3)]-{4-O-[(E)-3,4-dimethoxycinnamoyl])-β-d-fucopyranosyl ester or polygalasaponin XLV | Polygala glomerata Lour | [106] |
| 327 | 3-O-β-d-Glucopyranosylpresenegenin 28-O-β-d-galactopyranosyl-(1→4)-β-O-xylopyranosyl-(1→4)-α-l-rhamnopyranosyl-(1→2)-[6-O-acetyl-β-d-glucopyranosyl-(1→3)]-{4-O-[(Z)-3,4-dimethoxycinnamoyl]}-β-d-fucopyranosyl ester or polygalasaponin XLVI | Polygala glomerata Lour | [106] |
| 328 | 3-O-β-d-Glucopyranosylpresenegenin, 28-O-β-d-galactopyransyl(1→4)-β-d-xylopyranosyl -(1→4)-α-l-rhamnopyranosyl-(1→2)-{4-O-p-methoxycinnamoyl]}-[β-d-glucopyranosy l(1→3)]-β-d-fucopyranosyl ester or polygalasaponin X X X | Polygala japonica Houtt. | [56] |
| 329 | 3-O-β-d-Glucopyranosylpresenegenin 28-O-β-d-galactopyranosyl-(1→4)-β-d-xylo-pyranosyl-(1→4)-α-l-rhamnopyranosyl-(1→2)-[α-l-arabinopyranosyl-(1→3)]-[4-O-(E)-p-methoxycinnamoyl]-β-d-fucopyranosyl ester or polygalasaponin XLIII | Polygala glomerata Lour | [106] |
| 330 | 3-O-β-d-Glucopyranosylpresenegenin 28-O-α-l-arabinopyransyl (1→4)-β-d-xylopyranosyl-(1→4)-[β-d-apiofuranosyl-(1→3)]-α-l-rhamnopyranosyl-(1→2)-[4-O-3,4,5-trimethoxy-cinnamoyl]-β-d-fucopyranosyl ester or polygalasaponin XXXI | Polygala japonica Houtt. | [56] |
| 331 | E-Senegasaponin a | Polygala senega L.var. latifolia Torrey et Gray | [109] |
| 332 | Z-Senegasaponin a | Polygala senega L.var. latifolia Torrey et Gray | [109] |
| 333 | 1-O-(E)-p-Coumaroyl-(3-O-benzoyl)-β-d-fructo-furanosyl-(2→1)-[6-O-(E)-feruloyl-β-d-gluco-pyranosyl-(1→2)]-[6-O-acetyl-β-d-gluco-pyranosyl-(1→3)-(4-O-acetyl)-β-d-glucopyranosyl-(1→3)]-4-O-[4-O-α-l-rhamnopyranosyl-(E)-p-coumaroyl]-α-d-glucopyranoside or polygalajaponicose I | Polygala japonica | [110] |
| 334 | 3-O-β-d-Glucopyranosylpresenegenin 28-O-α-L-arabinopyransyl-(1→4)-β-d-xylo-pyranosyl-(1→4)-[β-d-apiofuranosyl-(1→3)]-α-l-rhamnopyranosyl-(1→2)-[4-O-p-methoxy-cinnamoyl]-[α-l-rhamnopyranosyl(1→3)]-β-d-fucopyranosyl ester or polygalasaponin XXXII | Polygala japonica Houtt. | [56] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Liu, W.; Lu, Y.; Wang, Y.; Chen, X.; Bai, S.; Zhao, Y.; He, T.; Lao, F.; Shang, Y.; et al. Naturally Occurring Cinnamic Acid Sugar Ester Derivatives. Molecules 2016, 21, 1402. https://doi.org/10.3390/molecules21101402
Tian Y, Liu W, Lu Y, Wang Y, Chen X, Bai S, Zhao Y, He T, Lao F, Shang Y, et al. Naturally Occurring Cinnamic Acid Sugar Ester Derivatives. Molecules. 2016; 21(10):1402. https://doi.org/10.3390/molecules21101402
Chicago/Turabian StyleTian, Yuxin, Weirui Liu, Yi Lu, Yan Wang, Xiaoyi Chen, Shaojuan Bai, Yicheng Zhao, Ting He, Fengxue Lao, Yinghui Shang, and et al. 2016. "Naturally Occurring Cinnamic Acid Sugar Ester Derivatives" Molecules 21, no. 10: 1402. https://doi.org/10.3390/molecules21101402





