Three New Butenolides from the Fungus Aspergillus sp. CBS-P-2
Abstract
:1. Introduction
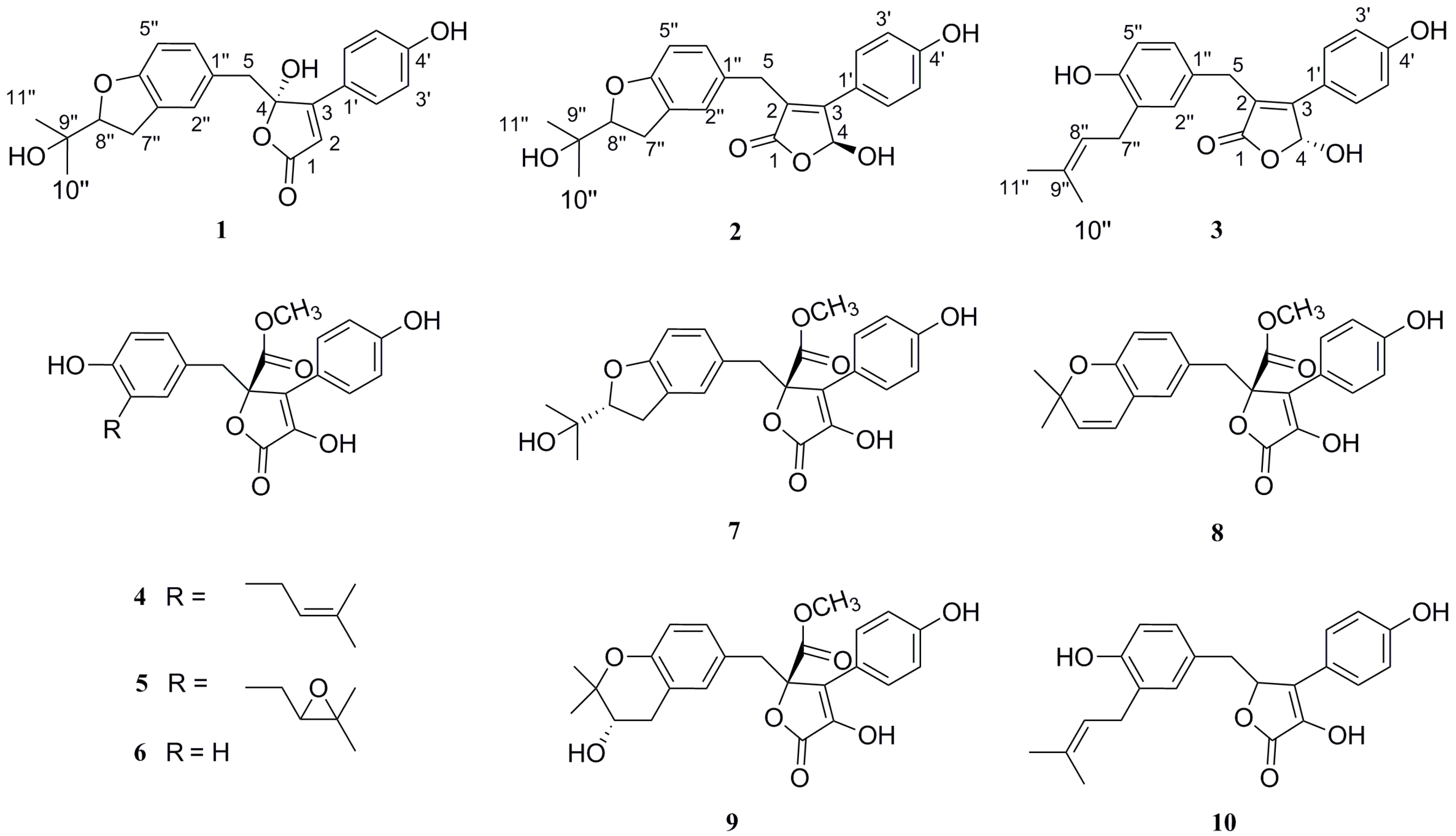
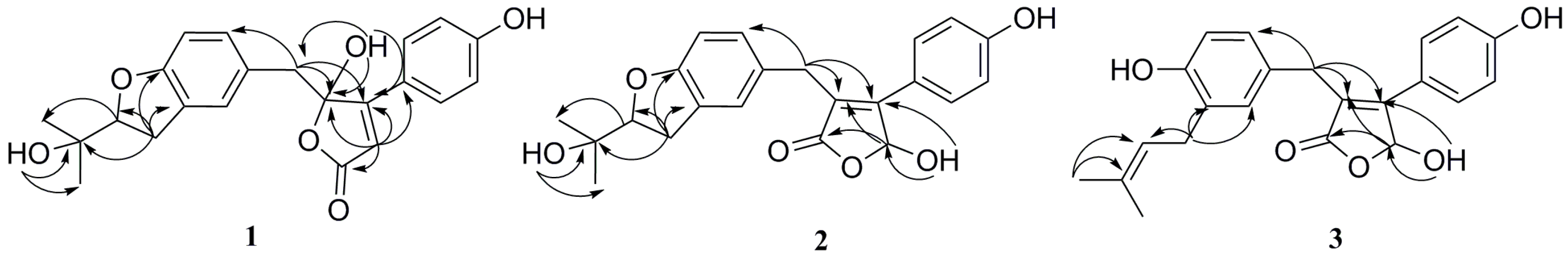
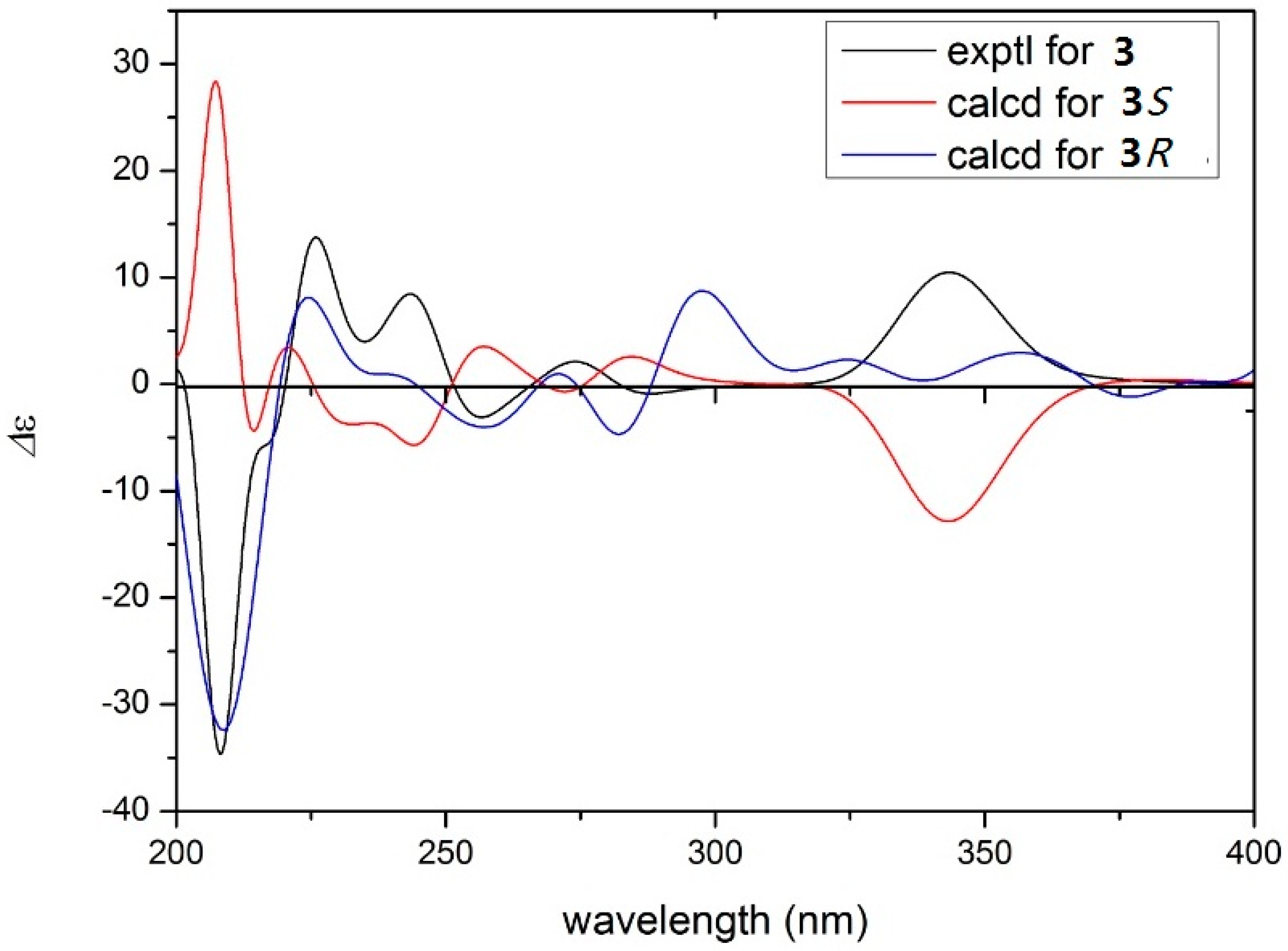
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Microorganism
3.3. Extraction and Isolation
3.4. Spectroscopic Data
3.5. Biological Activity
3.5.1. Cytotoxicity Assay
3.5.2 Antioxidant Activity
1-Diphenyl-2-picrylhydrazyl Free Radical (DPPH·) Scavenging Assay
2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonate) Free Radical (ABTS•+) Scavenging
3.5.3. Antimicrobial Bioassay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Che, Y.S. Discovery of new bioactive natural products from fungi of unique ecological niches. J. Int. Pharm. Res. 2011, 38, 12–27. [Google Scholar]
- Schulz, B.; Boyle, C.; Draeger, S.; Römmert, A.K.; Krohn, K. Endophytic fungi: A source of novel biologically active secondary metabolites. Mycol. Res. 2002, 106, 996–1004. [Google Scholar] [CrossRef]
- Omura, S.; Oiwa, R. The Search for Bioactive Compounds from Microorganisms; Springer-Verlag: New York, NY, USA, 1992. [Google Scholar]
- An, X.; Feng, B.M.; Chen, G.; Chen, S.F.; Wang, H.F.; Pei, Y.H. Two new asterriquinols from Aspergillus sp. CBS-P-2 with anti-inflammatory activity. J. Asian Nat. Prod. Res. 2016, 18, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.W.; Zhang, J.; Hu, S.; Zhang, Z.J.; Zhu, C.J.; Ng, S.W.; Tan, R.X. Ardeemins and cytochalasins from Aspergillus terreus residing in Artemisia annua. Planta Med. 2010, 76, 1616–1621. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Y.; Li, B.G.; Yang, T.; Yin, J.H.; Qi, H.Y.; Liu, G.Y.; Zhang, G.L. Sesterterpenoids, terretonins A−D, and an alkaloid, asterrelenin, from Aspergillus terreus. J. Nat. Prod. 2005, 68, 1243–1246. [Google Scholar] [CrossRef] [PubMed]
- Qiao, K.J.; Zhou, H.; Xu, W.; Zhang, W.J.; Garg, N.; Tang, Y.A. fungal nonribosomal peptide synthetase module that can synthesize thiopyrazines. Org. Lett. 2011, 13, 1758–1761. [Google Scholar] [CrossRef] [PubMed]
- Haritakun, R.; Rachtawee, P.; Chanthaket, R.; Boonyuen, N.; Isaka, M. Butyrolactones from the fungus Aspergillus terreus BCC 4651. Chem. Pharm. Bull. 2010, 58, 1545–1548. [Google Scholar] [CrossRef] [PubMed]
- Parvatkar, R.R.; D’Souza, C.; Tripathi, A.; Naik, C.G. Aspernolides A and B, butenolides from a marine-derived fungus Aspergillus terreus. Phytochemistry 2009, 70, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Hosaka, Y.; Matsushima, H.; Goto, T.; Kitamura, T.; Kawabe, K. Butyrolactone I induces cyclin B1 and causes G2/M arrest and skipping of mitosis in human prostate cell lines. Cancer Lett. 1999, 138, 121–130. [Google Scholar] [CrossRef]
- Dewi, R.T.; Tachibana, S.; Darmawan, A. Effect on α-glucosidase inhibition and antioxidant activities of butyrolactone derivatives from Aspergillus terreus MC751. Med. Chem. Res. 2014, 23, 454–460. [Google Scholar] [CrossRef]
- Shen, Y.; Zou, J.H.; Xie, D.; Ge, H.L.; Cao, X.P.; Dai, J.G. Butyrolactone and cycloheptanetrione from mangrove-associated fungus Aspergillus terreus. Chem. Pharm. Bull. 2012, 60, 1437–1441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, X.M.; Wang, J.N.; Li, X.; Wang, B.G. New butenolide derivatives from the marine-derived fungus Paecilomyces variotii with DPPH radical scavenging activity. Phytochem. Lett. 2015, 11, 85–88. [Google Scholar] [CrossRef]
- Gong, T.; Dong, S.H.; Zhu, P. Butyrolactone derivatives isolated from the marine fungus Aspergillus versicolor F62. Mycosystema 2014, 33, 706–712. [Google Scholar]
- Rao, K.V.; Sadhukhan, A.K.; Veerender, M.; Ravikumar, V.; Mohan, E.V.S.; Dhanvantri, S.D.; Sitaramkumar, M.; Moses Babu, J.; Vyas, K.; Reddy, O. Butyrolactones from Aspergillus terreus. Chem. Pharm. Bull. 2000, 48, 559–562. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Bao, J.; Zhang, X.Y.; Tu, Z.C.; Shi, Y.M.; Qi, S.H. Asperterrestide A, a cytotoxic cyclic tetrapeptide from the marine-derived fungus Aspergillus terreus SCSGAF0162. J. Nat. Prod. 2013, 76, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Morishima, H.; Fujita, K.; Nakano, M.; Atsumi, S.; Ookubo, M.; Kitagawa, M.; Matsumoto, H.; Okuyama, A.; Okabe, T. Preparation, Antitumor Activity, and Formulations of Dihydrofuran Compounds. Japanese Kokai Tokkyo Koho JP 06100445, 1994. [Google Scholar]
- Frelek, J.; Szczepek, W.J. [Rh2(OCOCF3)4] as an auxiliary chromophore in chiroptical studies on steroidal alcohols. Tetrahedron Asymmetry 1999, 10, 1507–1520. [Google Scholar] [CrossRef]
- Gerards, M.; Snatzke, G. Circular dichroism, XCIII1 determination of the absolute configuration of alcohols, olefins, epoxides, and ethers from the CD of their “in situ” complexes with [Rh2(O2CCF3)4]. Tetrahedron Asymmetry 1990, 1, 221–236. [Google Scholar] [CrossRef]
- Uchida, I.; Kuriyama, K. The π-π* circular dichroism of αβ-unsaturated γ-lactones. Tetrahedron Lett. 1974, 15, 3761–3764. [Google Scholar] [CrossRef]
- Gawronski, J.K.; Oeveren, A.V.; Deen, H.V.D.; Leung, C.W.; Feringa, B.L. Simple circular dichroic method for the determination of absolute configuration of 5-substituted 2(5H)-furanones. J. Org. Chem. 1996, 61, 1513–1515. [Google Scholar] [CrossRef]
- Beecham, A.F. The CD of αβ-unsaturated lactones. Tetrahedron 1972, 28, 5543–5554. [Google Scholar] [CrossRef]
- Lee, C.L.; Chang, F.R.; Hsieh, P.W.; Chiang, M.Y.; Wu, C.C.; Huang, Z.Y.; Lan, Y.H.; Chen, M.; Lee, K.H.; Yen, H.F. Cytotoxic ent-abietane diterpenes from Gelonium aequoreum. Phytochemistry 2008, 69, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Hamerski, L.; Bomm, M.D.; Silva, D.H.S.; Young, M.C.M.; Furlan, M.; Eberlin, M.N.; Castro-Gamboa, I.; Cavalheiro, A.J.; Bolzani, V.D.S. Phenylpropanoid glucosides from leaves of Coussarea hydrangeifolia (Rubiaceae). Phytochemistry 2005, 66, 1927–1932. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.X.; Bai, M.; Zhou, L.; Lou, L.L.; Liu, Q.B.; Zhang, Y.; Li, L.Z.; Song, S.J. Food Byproducts as a New and Cheap Source of Bioactive Compounds: Lignans with Antioxidant and Anti-infl amatory Properties from Crataegus pinnatifi da Seeds. J. Agric. Food. Chem. 2015, 63, 7252–7260. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Hua, H.M.; Pei, Y.H.; Chen, D.; Jing, Y.K. Triterpenoids from the resin of Styrax tonkinensis and their antiproliferative and differentiation effects in human leukemia HL-60 Cells. J. Nat. Prod. 2006, 69, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Shi, X.D.; Chen, J.G.; Mao, X.; Zhu, L.; Yu, L.; Shi, J.Y. Alkaloids from Toddalia asiatica and their cytotoxic, antimicrobial and antifungal activities. Food Chem. 2014, 148, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.B.; Di, Y.T.; Bao, Y.; Yuan, C.M.; Chen, G.; Li, D.H.; Bai, J.; He, H.P.; Hao, X.J.; Pei, Y.H.; et al. Peganumine A, a β‑Carboline dimer with a new octacyclic scaffold from Peganum harmala. Org. Lett. 2014, 16, 4028–4031. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.C.; Reeves, J.C.; Dage, R.C.; Schnettler, R.A. Antioxidant properties of 2-imidazolones and 2-imidazolthiones. Biochem. Pharmacol. 1987, 36, 1457–1460. [Google Scholar] [CrossRef]
- Wu, S.B.; Dastmalchi, K.; Long, C.L.; Kennelly, E.J. Metabolite profiling of Jaboticaba (Myrciaria cauliflora) and other dark-colored fruit juices. J. Agric. Org. Lett. 2012, 60, 7513–7525. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 2–7 and 9–10 are available from the authors.
| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | 170.3 | 172.9 | 172.4 | |||
| 2 | 6.23 (s) | 112.9 | 124.1 | 124.3 | ||
| 3 | 164.3 | 156.4 | 156.6 | |||
| 4 | 108 | 6.54 (d, 8.6) | 97.8 | 6.52 (d, 8.4) | 97.7 | |
| 5 | 3.12 (d, 13.5) | 43.8 | 3.65 (d, 15.4) | 29.2 | 3.57 (d, 15.6) | 29 |
| 3.25 (d, 13.5) | 3.74 (d, 15.4) | 3.68 (d, 15.6) | ||||
| 1′ | 121.2 | 122 | 122.1 | |||
| 2′ | 7.74 (d, 8.4) | 130.8 | 7.45 (d, 8.8) | 130.9 | 7.44 (d, 8.8) | 130.8 |
| 3′ | 6.89 (d, 8.4) | 116.3 | 6.84 (d, 8.8) | 116.2 | 6.82 (d, 8.8) | 116 |
| 4′ | 160.6 | 158.9 | 159.8 | |||
| 5′ | 6.89 (d, 8.4) | 116.3 | 6.84 (d, 8.8) | 116.2 | 6.82 (d, 8.8) | 116 |
| 6′ | 7.74 (d, 8.4) | 130.8 | 7.45 (d, 8.8) | 130.9 | 7.44 (d, 8.8) | 130.8 |
| 1′′ | 126.1 | 129.4 | 128.1 | |||
| 2′′ | 6.48 (brs) | 129.6 | 7.02 (brs) | 124.8 | 6.88 (d, 2.0) | 129.5 |
| 3′′ | 127.3 | 128.3 | 128.8 | |||
| 4′′ | 159.1 | 159 | 154.1 | |||
| 5′′ | 6.46 (d, 8.4) | 116.1 | 6.64 (d, 8.4) | 109 | 6.67 (d, 8.0) | 115.3 |
| 6′′ | 6.54 (brd, 8.4) | 129.7 | 6.89 (brd, 8.4) | 127.4 | 6.80 (dd, 8.8, 2.0) | 126.3 |
| 7′′ | 2.98 (dd, 15.2, 8.8) | 30.2 | 3.07 (2H, m) | 30.4 | 3.14 (2H, d, 7.2) | 28.4 |
| 2.92 (dd, 15.2, 10.0) | ||||||
| 8′′ | 4.44 (t, 8.8) | 89.4 | 4.49 (t, 9.0) | 89.5 | 5.21 (t, 7.2) | 123.2 |
| 9′′ | 70.4 | 70 | 131.7 | |||
| 10′′ | 1.06 (3H,s) | 25.3 | 1.12 (3H, s) | 25.4 | 1.62 (3H, s) | 18 |
| 11′′ | 1.07 (3H,s) | 26.5 | 1.12 (3H, s) | 26.6 | 1.66 (3H, s) | 25.9 |
| 4-OH | 7.91 (brs) | 7.76 (d, 8.8) | 7.77 (d, 8.4) | |||
| 4′-OH | 10.26 (brs) | 10.07 (brs) | 10.06 (brs) | |||
| 4′′-OH | 9.15 (s) | |||||
| Compounds | Antioxidant Activities | |
|---|---|---|
| DPPH | ABTS | |
| 1 | >100 | 33.1 ± 1.12 |
| 2 | >100 | 9.1 ± 1.21 |
| 3 | >100 | 29.5 ± 1.11 |
| 4 | 34.1 ± 0.3 | 2.8 ± 0.11 |
| 5 | 25.6 ± 0.36 | 5.0 ± 0.7 |
| 6 | 30.0 ± 1.25 | 6.6 ± 0.6 |
| 7 | 15.9 ± 0.56 | 5.1 ± 0.3 |
| 8 | 25.6 ± 1.8 | 6.2 ± 0.3 |
| 9 | 20.7 ± 3.65 | 3.7 ± 0.08 |
| 10 | 34.3 ± 0.03 | 5.3 ± 0.05 |
| Ascorbic acid a | 25.1 ± 0.18 | 12.5 ± 0.02 |
| Compounds | IC50 (μM) | |||
|---|---|---|---|---|
| HL-60 | ASPC1 | PC-3 | HCT-116 | |
| 1 | >80 | >80 | >80 | >80 |
| 2 | 39.4 | >80 | >80 | >80 |
| 3 | >80 | >80 | >80 | >80 |
| 4 | 13.2 | >80 | 41.7 | >80 |
| 5 | >80 | >80 | >80 | >80 |
| 6 | >80 | >80 | >80 | >80 |
| 7 | 41.6 | >80 | >80 | >80 |
| 8 | >80 | >80 | 58.3 | 66.9 |
| 9 | >80 | >80 | >80 | >80 |
| 10 | 16.3 | >80 | >80 | >80 |
| 5-Fluorouracil a | 6.38 | 2.70 | 7.77 | 15.6 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, X.; Pei, Y.; Chen, S.; Li, S.; Hu, X.; Chen, G.; Lin, B.; Wang, H. Three New Butenolides from the Fungus Aspergillus sp. CBS-P-2. Molecules 2016, 21, 1361. https://doi.org/10.3390/molecules21101361
An X, Pei Y, Chen S, Li S, Hu X, Chen G, Lin B, Wang H. Three New Butenolides from the Fungus Aspergillus sp. CBS-P-2. Molecules. 2016; 21(10):1361. https://doi.org/10.3390/molecules21101361
Chicago/Turabian StyleAn, Xiao, Yuehu Pei, Shaofei Chen, Shengge Li, Xiaolong Hu, Gang Chen, Bin Lin, and Haifeng Wang. 2016. "Three New Butenolides from the Fungus Aspergillus sp. CBS-P-2" Molecules 21, no. 10: 1361. https://doi.org/10.3390/molecules21101361






