1. Introduction
Small interfering RNA (siRNA) is double stranded RNA that silences gene transcription in a sequence specific manner. siRNA can specifically and efficiently silence oncogenic genes, providing a promising alternative for cancer therapy [
1]. However, in vivo applications of siRNA face many challenges including enzymatic degradation, aggregation with serum proteins, poor penetration of tissue and crossing biological membranes, non-specific binding, and inefficient endosomal escape [
2]. To overcome these challenges, siRNA is commonly formulated with a delivery vector [
2,
3], which is branched polyethylenimine (bPEI) in the present study. The cationic polymer bPEI, as a nucleic acid vector, has been reported to mediate efficient cellular uptake and endosomal escape both in vitro and in vivo [
4]. Polymers modified with receptor specific ligands have been used to efficiently and selectively deliver siRNA to tumor cells [
5]. Transferrin receptor (TfR) is a membrane receptor which regulates endocytosis of iron transport protein (Tf), and is expressed at a low level on most cells. However, TfR is overexpressed in many kinds of cancers due to their increased consumption of iron. The upregulation of TfR in cancer cells makes it a promising target for the selective delivery of siRNA for cancer therapies [
6]. Several strategies have been explored to target TfR, including the use of holo-Tf or monoclonal antibodies raised against TfR [
7,
8] which were coupled to polymers [
9], and liposomes [
10]. However, high concentrations of endogenous Tf in blood plasma (25 µM) may competitively inhibit the uptake mediated by the holo-Tf or the anti-TfR antibody [
11]. Furthermore, holo-Tf (molecular weight (MW) 80 kDa) and antibodies against TfR (e.g., the anti-human TfR antibody 5E9, MW = 30 kDa [
7]) are relatively large, and therefore, may introduce steric hindrance when the drug delivery system is synthesized. The stability of these proteins will also need to be taken into consideration in terms of synthesis conditions, storage, formulation, and expected shelf-life. A viable alternative to Tf and TfR specific antibodies is the use of TfR binding peptides. Two peptide sequences, HAIYPRH and THRPPMWSPVWP, discovered by phage display, were reported to be able to non-competitively bind to human TfR with high affinity and consequently trigger TfR internalization [
12]. The chemico-physical properties of these peptides are more stable and their MWs are smaller than those of holo-Tf and antibodies.
The aim of this study was to develop a siRNA delivery system to target TfR overexpressing tumor cells. In the present work, two TfR targeting peptides and bPEI were successfully coupled via two different crosslinkers, namely PEGylated succinimidyl 3-(2-pyridyldithio) propionate (PEG4-SPDP) and sulfosuccinimidyl 4-(N-maleimidomethyl) cyclohexane-1-carboxylate (sulfo-SMCC). The siRNA and bPEI or peptide-bPEI polyplexes were characterized regarding size, zeta-potential, and siRNA condensation efficiency. Selective delivery of siRNA using peptide-bPEI was investigated in the TfR overexpressing cell line H1299 and low TfR expressing cell lines A549 or H460. Gene knockdown efficiency of siRNA/peptide-bPEI polyplexes was measured both at the mRNA and protein level in H1299 cells.
3. Discussion
The selective and efficient delivery of siRNA to tumors is hindered by many biological barriers [
2]. Numerous modifications of polymers have been discovered to target tumor cells including folic acid [
14,
15,
16], transferrin [
17], epidermal growth factor (EGF) [
18,
19] and the tripeptide arginine-glycine-aspartate RGD [
20]. In this study, two human TfR binding peptides, HAIYPRH (HAI peptide) and THRPPMWSPVWP (THR peptide), were tested for gene delivery in TfR positive cells. Both peptides contain a distal cysteine to introduce an additional thiol group for further modification. The THR peptide was first evaluated since it demonstrated a high affinity to TfR (1.5 × 10
−8 M) compared to the affinity of native ligand Tf to TfR of 2.8 × 10
−9 M; in contrast, the affinity constant of the HAI peptide was 4.4 × 10
−4 M [
12]. The THR peptide was successfully coupled to 5 kDa bPEI via linker SPDP as shown in
Figure S1. Despite the fact that the THR peptide, which was fluorescently labeled with Alexa Fluor 488 (THR-AF488), did preferentially bind to TfR overexpressing H1299 cells compared to TfR low expressing A549 cells (
Figure S2a), the cellular uptake mediated by THR-bPEI polyplexes in H1299 cells was lower than that of bPEI polyplexes and showed no difference compared with A549 cells (
Figure S2b). It has been reported that the THR peptide conjugated with a chelator for radiolabeling demonstrated negligible uptake in a TfR positive cell line [
21] and that THR modified gold nanoparticles did not efficiently cross the blood brain barrier without the addition of a hydrophobic peptide to facilitate membrane binding [
22]. Therefore, it is possible that the binding cavity of the THR peptide to TfR is sterically very strict and only allows limited modification in the peptide. Consequently, THR modified with a small fluorescent probe, AF488 (MW = 720.66 Da), still demonstrated preferential binding to H1299 but THR conjugated to a large polymer, bPEI (MW = 5000 Da), cannot mediate significantly higher cellular uptake in H1299 cells compared with A549 cells. Another possible reason of low selective cellular uptake could be low receptor internalization efficiency. Confocal imaging revealed that THR-AF488 can bind to the membrane of H1299 but little internalization was observed (
Figure S3), and the co-localization of THR-AF488 and Tf was absent. Thus, THR may not be an optimal candidate for the TfR targeting of polyplexes.
The other peptide discovered in the same study, the HAI peptide, demonstrated different properties during evaluation for polyplex targeting. Firstly, the HAI peptide was successfully conjugated to 25 kDa bPEI via two different linkers: sulfo-SMCC and PEG
4-SPDP. The coupling degrees were similar in both approaches and were around 18 HAI peptides per bPEI. It has been reported that protein/peptide modifications in polymer may result in less nucleic acid condensation efficiency compared to the non-modified polymer due to the introduction of steric hindrance [
23,
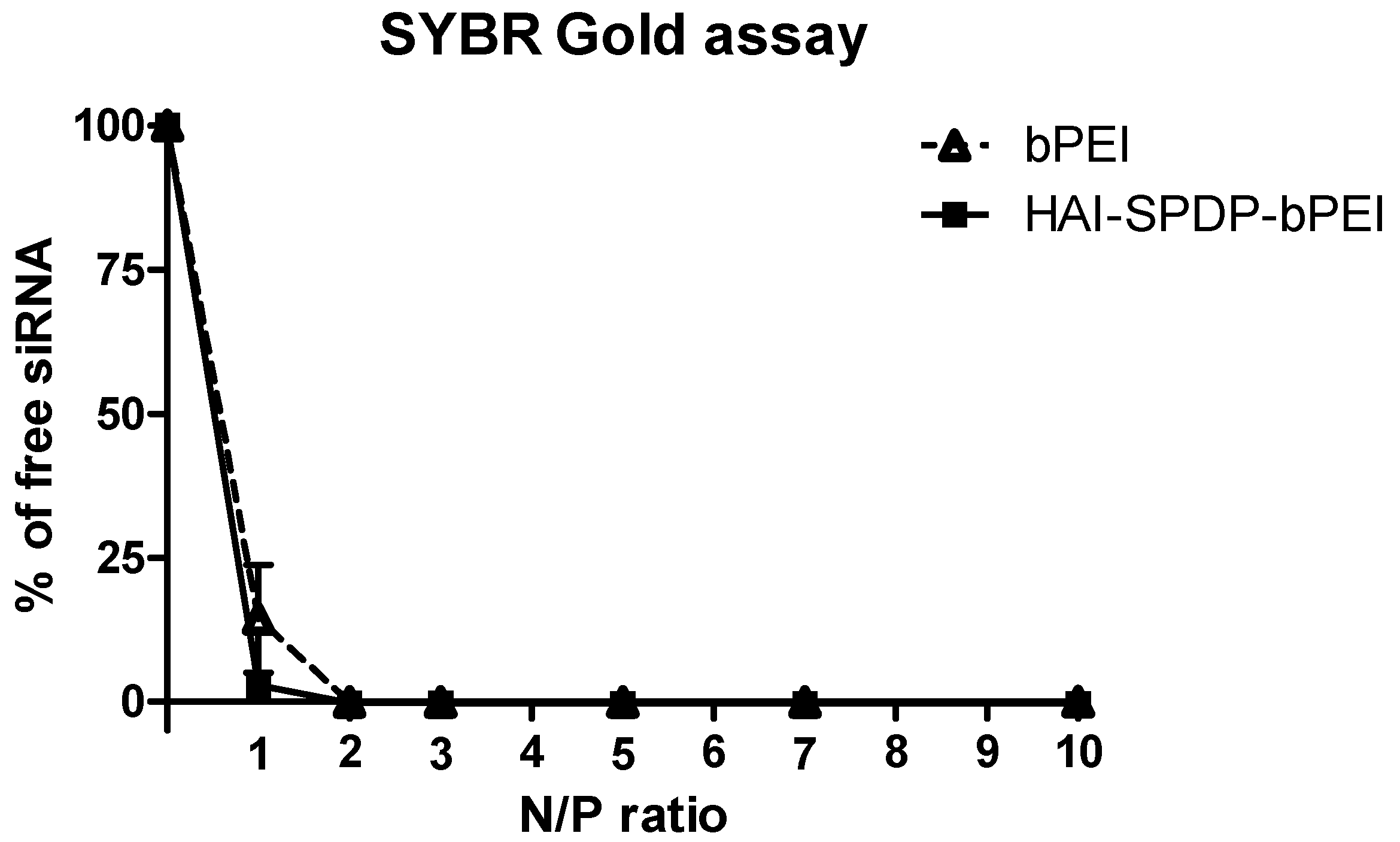
24]. The siRNA condensation efficiency of bPEI and HAI-SPDP-bPEI was therefore determined by a SYBR Gold assay, and the results demonstrated that HAI-peptide modification did not have any negative impact on bPEI’s condensation of siRNA (
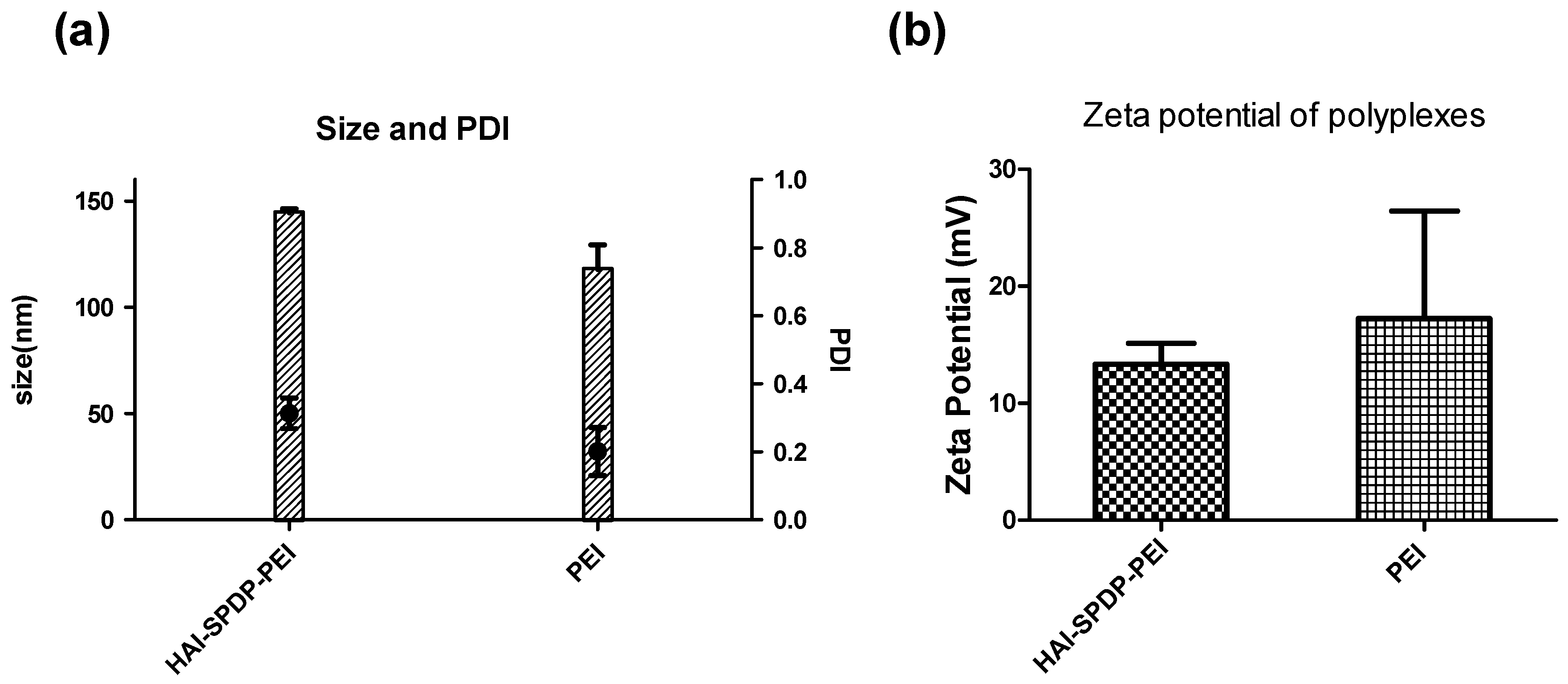
Figure 2). Chemico-physical properties of polyplexes are very important for successful and efficient siRNA delivery. Therefore, bPEI and HAI-SPDP-bPEI polyplexes (N/P = 5) were fully characterized in terms of the hydrodynamic diameter, PDI and zeta-potential. As shown in
Figure 3a, HAI-SPDP-bPEI and bPEI polyplexes had narrow size ranges with PDIs of around 0.3, indicating that uniform polyplexes were formed with little aggregation. HAI-SPDP-bPEI polyplexes were slightly larger than bPEI polyplexes which may be due to steric hindrance of the peptide during polyplexes formation. However, both polyplexes resulted in very small sizes (100–150 nm). These results suggested that both polyplexes may readily be taken up by cells [
25], avoid rapid clearance from macrophages in the liver and spleen after systemic administration, and could potentially easily accumulate in a tumor through the EPR effect [
26]. Both polyplexes demonstrated slightly positive surface charge (<30 mV) (
Figure 3b). Since HAI-SPDP-bPEI and bPEI can completely condense siRNA at N/P = 2 (
Figure 2), it was expected that at N/P = 5, an excess amount of cationic polymer formulated with siRNA would result in positive surface charges. HAI-SPDP-bPEI polyplexes exhibited a less positive charge than bPEI polyplexes which may be due to the assumption that the conjugated HAI peptide locates on the surface of the polyplexes and can shield some positive charge from bPEI. Another possible reason for the reduced surface charge of HAI-SPDP-PEI is that the SPDP coupling reaction consumed some of the primary amines in bPEI. A slightly positive surface charge could facilitate the binding between polyplexes and cellular membranes. Moreover, the HAI peptide located on the surface is expected to increase the possibility to interact with TfR, suggesting that efficient cellular uptake may be achieved by this formulation.
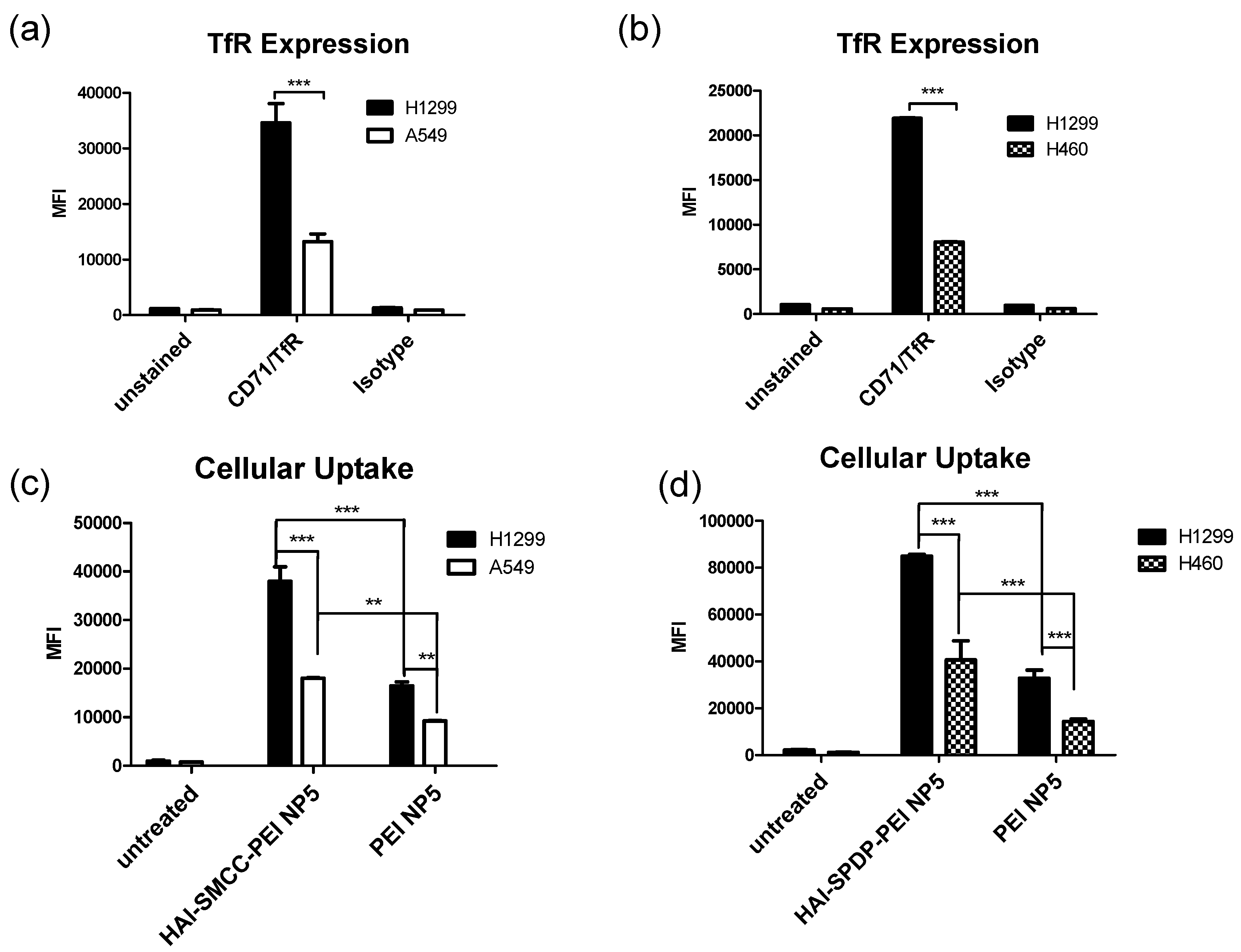
The TfR expression status of three different cell lines—H1299, A549 and H460—was determined by flow cytometry after immunostaining with the anti-CD71 (TfR) antibody. Based on the results (
Figure 4a,b), H1299 cells served as a TfR overexpressing cell model, and A549 and H460 served as TfR low expressing cell models for later studies. The peptide mediated TfR selectivity was investigated by confocal microscopy. HAI-SMCC-bPEI and bPEI were fluorescently labeled with FITC (HAI-SMCC-bPEI-FITC, bPEI-FITC respectively), and polyplexes were formed with non-fluorescently labeled siRNA. H1299 cells were incubated with Tf-Texas Red and the aforementioned polyplexes. As shown in
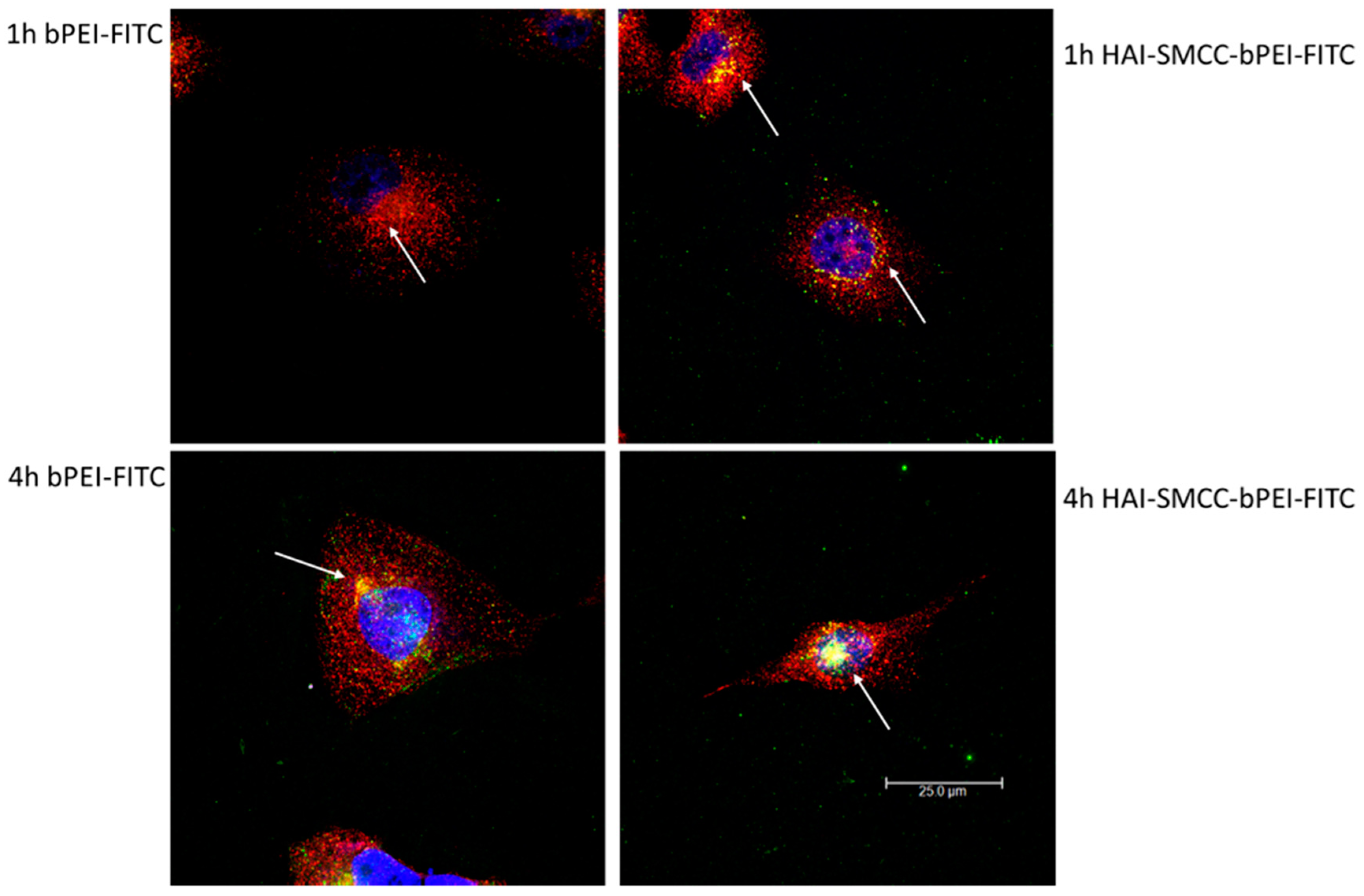
Figure 6, HAI-SMCC-bPEI polyplexes showed more binding (green dots) on the cell surface and more co-localization (yellow dots) with Tf-Texas Red (red dots) than bPEI polyplexes after 1 and 4 h of the co-incubation period, indicating that the HAI peptide can mediate selective binding to TfR overexpressing cells. Furthermore, HAI-SMCC-bPEI can achieve more co-localization with Tf, suggesting that HAI-SMCC-bPEI polyplexes may enter cells via a TfR mediated pathway in a non-competitive manner as reported before [
12]. The subcellular distribution of both polyplexes over time was observed under the confocal microscope (
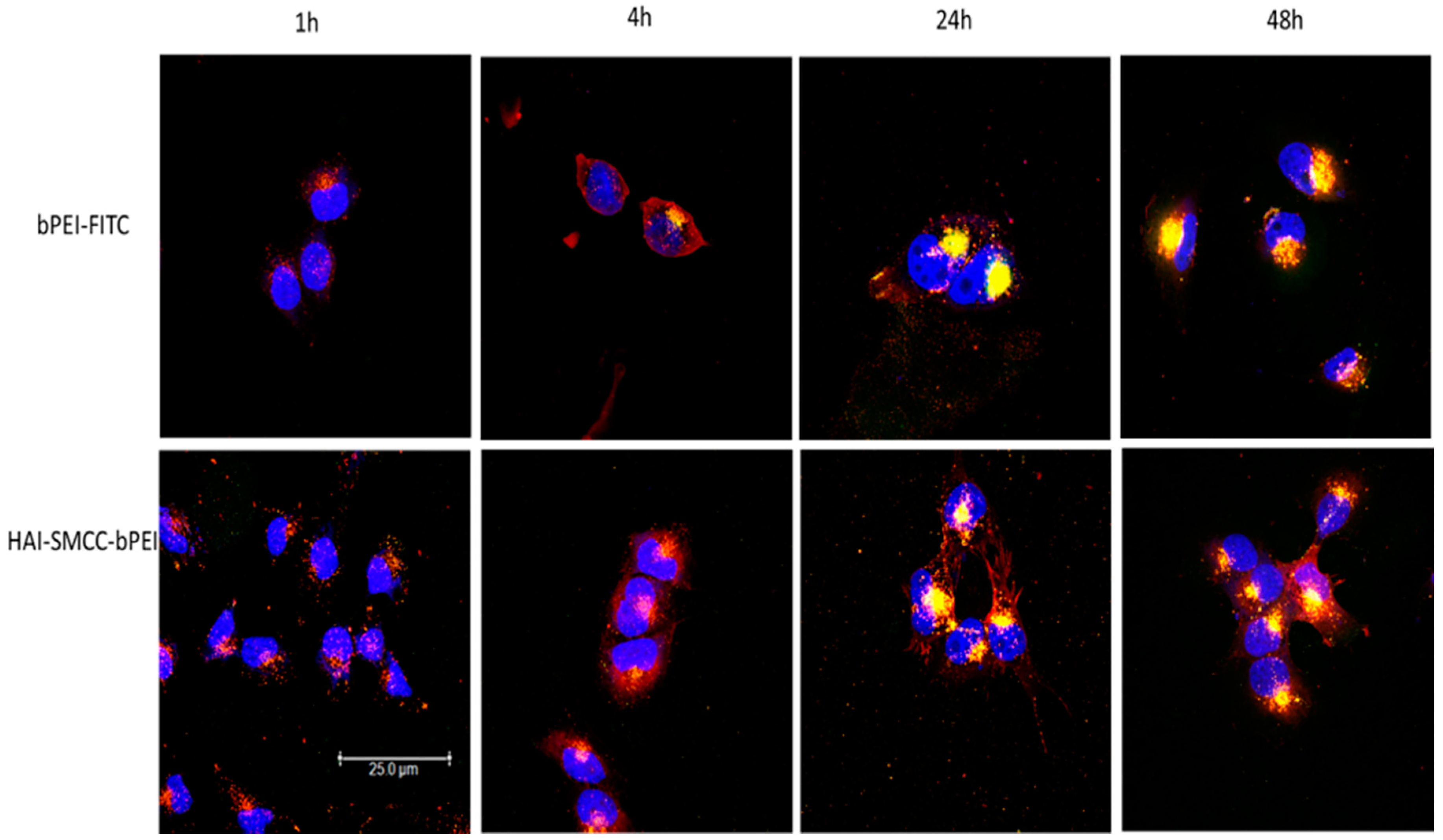
Figure 5). H1299 cells were transfected with HAI-SMCC-bPEI-FITC or bPEI-FITC polyplexes (green dots) containing siRNA-Ty563 (red dots). HAI-SMCC-bPEI polyplexes clearly achieved more cellular binding on the membrane than bPEI polyplexes during the first 1 and 4 h, suggesting that HAI-SMCC-bPEI preferentially bound to TfR overexpressing cells compared with bPEI polyplexes. At later time points (24 and 48 h), both bPEI and HAI-SMCC-bPEI polyplexes formed bright yellow dots in vesicle like structures, indicating that many of both types of polyplexes had accumulated and were trapped in endosomes or lysosomes. However, it is worth nothing that more siRNA distributed in the cytoplasm in the HAI-SMCC-bPEI treated group than in bPEI transfected cells. According to the cellular uptake results (
Figure 4c), it is possible that HAI-SMCC-bPEI polyplexes mediated higher cellular uptake in H1299 cells than bPEI polyplexes, consequently more siRNA escaped into the cytoplasm. The TfR specific cellular uptake mediated by the HAI peptide was also confirmed by flow cytometry (
Figure 4c,d). Both HAI-SMCC-bPEI and HAI-SPDP-bPEI polyplexes showed specific binding to the TfR overexpressing cell line (H1299 cells) compared with TfR low expressing cells (A549 and H460). Furthermore, both HAI peptide modified bPEI polyplexes mediated significantly higher cellular uptake in H1299 cells compared with non-modified bPEI, suggesting a specific interaction between the HAI peptide in the polymer and TfR on the cell membrane.
The gene knockdown efficiency of the HAI peptide, modified bPEI and non-modified bPEI was determined by RT-PCR, and the housekeeping gene GAPDH was targeted. Surprisingly, HAI-SMCC-bPEI polyplexes did not mediate efficient GAPDH gene knockdown, as shown in
Figure S4, which did not agree with the cellular uptake result (
Figure 4c) and the confocal microscopy result (
Figure 5 and
Figure 6). It is possible that the HAI peptide modification reduced the number of primary amines in bPEI and consequently reduced the buffering capacity of bPEI. Furthermore, TfR rapidly recycles back to the cell surface after internalization [
27], therefore, HAI-SMCC-bPEI polyplexes that are still bound to TfR are recycled back to the cell surface before siRNA can be released. To address this problem, HAI-SPDP-bPEI was synthesized using a linker PEG
4-SPDP. PEG
4-SPDP (25.7 Å) is longer than sulfo-SMCC (8.3 Å) which may make the HAI peptide more accessible to TfR and increase the binding efficiency. Moreover, it introduced a disulfide bond into the HAI-SPDP-bPEI conjugate which can be reduced in the endosomal compartment [
28] and may facilitate the release of bPEI/siRNA complexes from the HAI peptide/TfR complexes before TfR recycles back to the cell surface. The GAPDH gene knockdown efficiency of HAI-SPDP-bPEI polyplexes was determined in H1299 and H460 cells. In line with previous cellular uptake results (
Figure 4d), HAI-SPDP-bPEI polyplexes can achieve more efficient GAPDH gene knockdown in H1299 cells (
Figure 7a) compared with bPEI polyplexes but not in H460 cells (
Figure S5). These results revealed that the introduction of a long and reducible linker into target moiety-polymer conjugates may increase their transfection efficiency. Additionally, it was shown that the transfection efficiency of HAI-SPDP-bPEI polyplexes is highly dependent on the TfR expression status of the targeted cells.
Next, the gene silencing efficiency of HAI-SPDP-bPEI polyplexes at the protein level was investigated in H1299/eGFP cells. H1299/eGFP cells were treated with HAI-SPDP-bPEI and bPEI polyplexes for 48 h, and the eGFP expression was determined by flow cytometry. However, as shown in
Figure 7b, there was no significant difference among the polyplexes treated groups and the untreated group. Based on the confocal images (
Figure 5), it is possible that most polyplexes were trapped in the endosome and only a small amount of siRNA was released into the cytoplasm. Therefore, mRNA level silencing can be observed since mRNA is the direct target of siRNA. However, it is more difficult to achieve protein level silencing. A sufficient amount of siRNA needs to be released into the cytoplasm. Additionally, the silencing efficiency also depends on the half-life of the targeted protein, and wild type GFP has a relatively long half-life (around 26 h) [
29]. To increase the release of siRNA from endosomes, H1299/eGFP cells were transfected with HAI-SPDP-bPEI and bPEI polyplexes in media containing chloroquine, which is a chemical that can dramatically increase the transfection efficiency by disrupting the endosomal membrane [
13]. As shown in
Figure 7c, significantly higher eGFP knockdown can be achieved by siGFP/HAI-SPDP-bPEI polyplexes compared with siGFP/bPEI polyplexes in a dose dependent manner.
4. Materials and Methods
4.1. Materials
Branched 5k Da bPEI (Lupasol
® G100) and 25k Da bPEI (Lupasol
® HF) were obtained from BASF (Ludwigshafen, Germany). PEGylated succinimidyl 3-(2-pyridyldithio) propionate (PEG
4-SPDP) and sulfosuccinimidyl 4-(N-maleimidomethyl) cyclohexane-1-carboxylate (sulfo-SMCC) were purchased from Thermo Fisher (Waltham, MA, USA). Dulbecco’s phosphate buffered saline (PBS), 1,4-Dithio-DL-threitol (DTT), dimethyl sulfoxide (DMSO), HEPES, ethylenediaminetetraacetic acid disodium salt dehydrate (EDTA), picrylsulfonic acid solution (TNBS, 5%
w/v), sodium pyruvate, sodium bicarbonate (NaHCO
3) and glucose were bought from Sigma-Aldrich (St. Louis, MO, USA). Human holo-transferrin was obtained from EMD Millipore (Billerica, MA, USA). Cysteine modified TfR binding peptide HAIYPRH (Cys-His-Ala-Ile-Tyr-Pro-Arg-His) was synthesized by BACHEM Americas (Torrance, CA, USA) and THRPPMWSPVWP (Thr-His-Arg-Pro-Pro-Met-Trp-Ser-Pro-Val-Trp-Pro-Cys) was synthesized by the American Peptide Company (Sunnyvale, CA, USA). Enhanced green fluorescent protein (eGFP) siRNA, amine modified eGFP siRNA, siRNA fluorescently labeled with Ty563 (siRNA-Ty563), human glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) siRNA and scrambled siRNA were purchased from Integrated DNA Technologies (Coralville, IA, USA), the sequence of siRNA see
Table 1.
4.2. Synthesis of Peptide–bPEI Conjugate
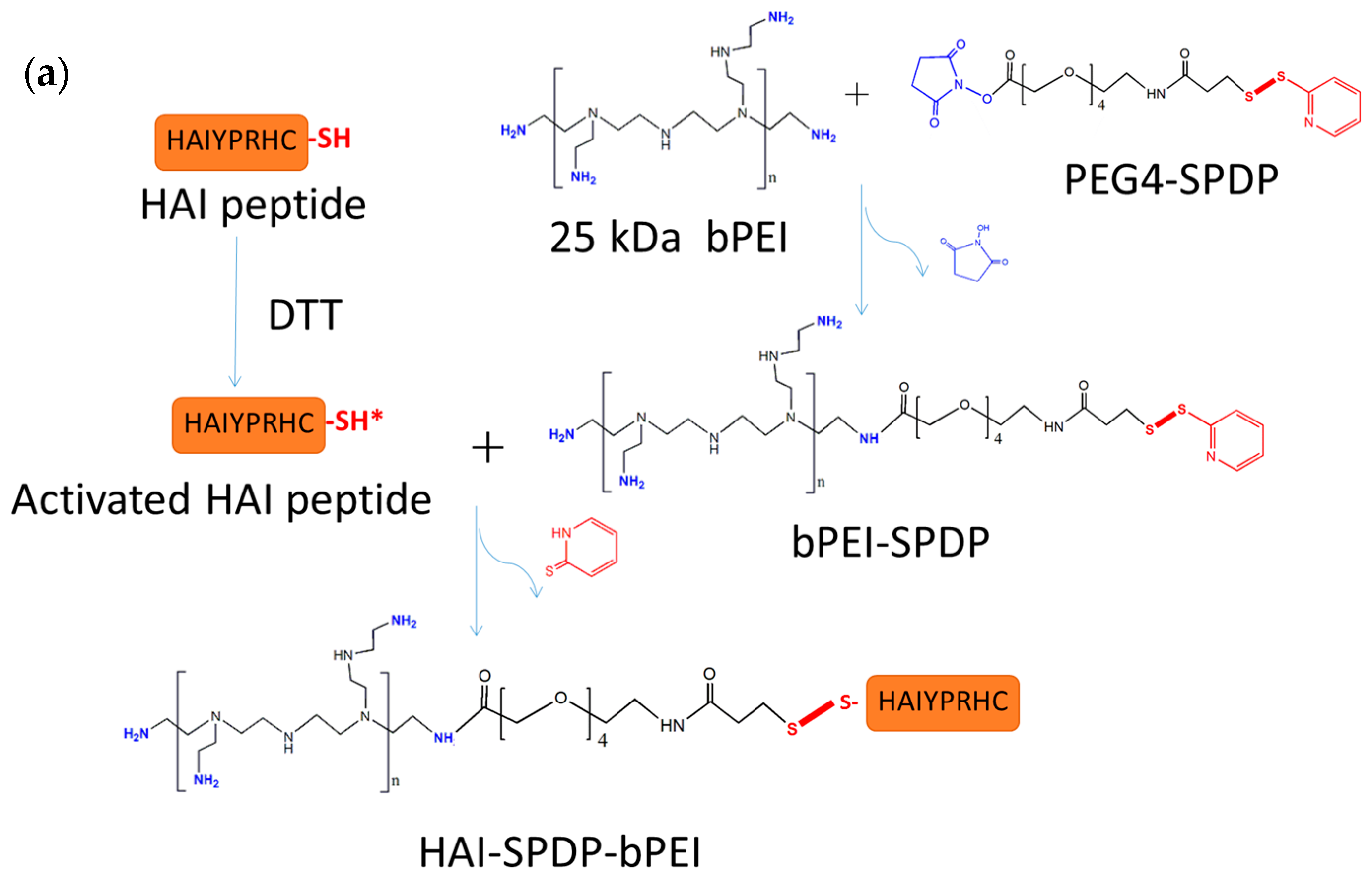
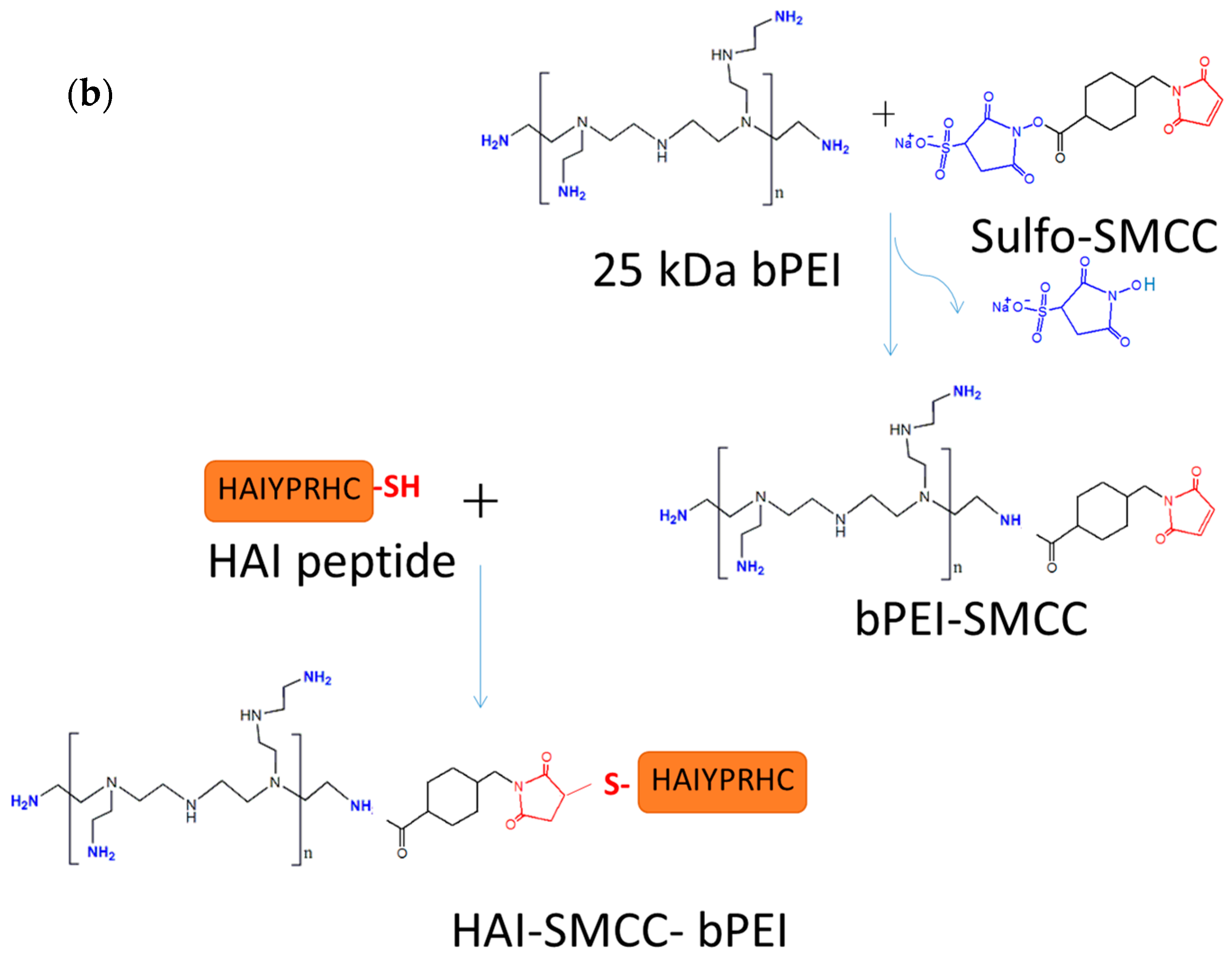
The HAIYPRH peptide (HAI) was coupled to bPEI using two different crosslinkers, namely sulfo-SMCC and PEG
4-SPDP. In the sulfo-SMCC crosslinking approach, as shown in
Figure 1b, 1 mg of 25k bPEI was dissolved in HEPES buffered saline (HBS) buffer (20 mM of HEPES and 150 mM of NaCl, pH = 7.2) at 1 mg/mL, and 1 mg of sulfo-SMCC was dissolved in water at 10 mg/mL. bPEI solution and sulfo-SMCC were mixed together and allowed to stir for 4 h at room temperature (RT). bPEI-SMCC was purified using 10,000 molecular weight cut off (MWCO) centrifugal filters (Millipore) with HBS containing 2 mM of EDTA buffer. The bPEI-SMCC solution was mixed with 1 mg of HAI peptide dissolved in HBS containing 2 mM of EDTA (5 mg/mL) and stirred overnight at RT. The next day, free HAI peptide was removed from HAI-bPEI using 10,000 MWCO centrifugal filters. In the PEG
4-SPDP crosslinking approach, as shown in
Figure 1a, 5 mg of 25k bPEI was dissolved in HBS buffer at 5 mg/mL, and 2 mg of PEG4-SPD was dissolved in DMSO at 20 mM. PEG
4-SPDP was added drop-wise into the bPEI solution and stirred overnight at RT. Meanwhile, 2 mg of HAI peptide was dissolved in HBS containing 2 mM of EDTA buffer at 4 mg/mL and reduced by 10 molar excess DTT for 2 h. Reduced HAI peptide solution was transferred to a 500–1000 MWCO dialysis tube (Spectrum laboratories, Rancho Dominguez, CA, USA) and dialysis was performed against HBS containing 2 mM of EDTA buffer overnight at 4 °C. The next day, bPEI-SPDP was purified using 10,000 MWCO centrifugal filters with HBS containing 2 mM of EDTA buffer and then mixed with the reduced HAI peptide. The mixture was stirred for 24 h at RT followed by purification using 10,000 MWCO centrifugal filters with HBS. The concentration of HAI peptide in the conjugate was measured spectrophotometrically at 280 nm. The concentration of bPEI in the conjugate was determined by a TNBS assay [
30]. Briefly, the according amount of HAI peptide was added into bPEI serial dilution to generate a standard curve in a clear 96-well plate (Corning, Corning, NY, USA). Then 30 µL of 3 mM of TNBS solution was added to 100 µL of bPEI/HAI solution and the absorbance at 405 nm was determined after 5 min.
4.3. Fluorescent Labeling of bPEI, Conjugate, Transferrin and siRNA
bPEI was fluorescently labeled with fluorescein-5-isothiocyanate (FITC) (Thermo Fisher). 15 mg of bPEI was dissolved in 0.1 M NaHCO
3 (pH = 9) at 2.5 mg/mL, and 2 mg of FITC was dissolved in 200 µL of DMSO. The FITC solution was added drop-wise to the bPEI solution and allowed to stir for 3 h at RT. Free FITC was removed using 10,000 MWCO centrifugal filters, and bPEI-FITC was stored in water. The bPEI concentration was determined by a TNBS assay as described above, and the presence of FITC showed no interference with the results, as reported before [
20]. For the conjugates, 0.2 mg of FITC (1 mg/mL) in DMSO was added dropwise into 2 mg of HAI-bPEI synthesized via the sulfo-SMCC approach and allowed to react for 3 h with stirring. The concentration of bPEI in the conjugate was determined by a TNBS assay with a bPEI-FITC standard curve. Holo human Transferrin was fluorescently labeled with Texas Red modified with succinimidyl ester (Thermo Fisher) following the manufacturer’s protocol. Amine modified siRNA was fluorescently labeled with Alexa Fluor 488 (Thermo Fisher) following the manufacturer’s protocol, followed by purification using ethanol precipitation and silica spin column binding as reported before [
31].
4.4. Preparation of Polyplexes
To prepare the siRNA and polymer polyplexes, polymer was diluted with 5% glucose to different concentrations. An equal volume of polymer solution was added to a predetermined amount of siRNA solution to yield different amine to phosphate ratios (N/P ratios) and allowed to incubate for 15 min at RT before measurement or transfection. The calculation to prepare polyplexes is based on the following equation: mpolymer (pg) = nsiRNA (pmol) × MWprotonable (g/mol) × N/P × Nnucleotide (MWprotonable of bPEI = 43.1 g/mol, Nnucleotide of siRNA = 52).
4.5. Measurement of Hydrodynamic Size and Zeta-Potential
Polyplexes were prepared with bPEI or HAI-SPDP-bPEI and 50 pmol of siRNA at N/P 5 in 100 µL 5% glucose buffer as described above and were transferred to a disposable micro cuvette (Brand GMBH, Wertheim, Germany). Size measurement was performed in three runs using a Zetasizer Nano ZS (Malvern Instruments Inc., Westborough, MA, USA). Results were collected and analyzed with the Zetasizer Software (Malvern) with settings of 173° backscatter angle, 0.88 mPa*s for viscosity and 1.33 for refractive index. Subsequently, the same polyplex solutions were diluted to 1 mL with water and transferred to a folded capillary cell (Malvern). The zeta-potentials of polyplexes were determined in three runs using a Zetasizer Nano ZS (Malvern).
4.6. siRNA Condensation Measured by a SYBR Gold Assay
SYBR Gold is a fluorescent dye which is used to stain nucleic acids. It intercalates into siRNA and produces fluorescence. When siRNA is condensed by polymer and becomes inaccessible for SYBR Gold, the fluorescence will decrease. Polyplexes were prepared with bPEI or HAI-SPDP-bPEI and 50 pmol of siRNA at different N/P ratios (1, 2, 3, 5, 7, 10) in 100 µL per well in a FluoroNunc 96 well white plate (Thermo Fisher). Subsequently, 30 µL of 4 × SYBR Gold was added to each well and incubated for 10 min in the dark. The fluorescence of SYBR Gold was determined by a Synergy 2 multi-mode microplate reader (BioTek Instrument, Winooski, VT, USA) with an excitation filter 485/20 nm and an emission filter 520/20 nm. The fluorescence of free siRNA (N/P = 0) represented 100% free siRNA. The experiment was performed in triplicate.
4.7. Cell Culture
NCI-H1299 cells (human non-small cell lung carcinoma) and A549 cells (human non-small cell lung carcinoma) were purchased from ATCC (Manassas, VA, USA). NCI-H460 cells (human large cells lung carcinoma) were a kind gift from Dr. Larry H. Matherly (School of medicine, Wayne State University, Detroit, MI, USA). H1299/eGFP is a H1299 cell line stably expressing the reporter gene eGFP. H1299 and H460 cells were cultured in RPMI-1640 media (GE healthcare, Buckinghamshire, UK) supplemented with 10% heat inactivated fetal bovine serum (v/v) (Sigma), 1× penicillin/streptomycin (Corning), 1× GlutaMax (Thermo Fisher), 10 mM of HEPES, 1.5 g/L of NaHCO3, 4.5 g/L of glucose and 1 mM of sodium pyruvate. A549 cells were cultured in DMEM media (Corning) supplemented with 10% fetal bovine serum (FBS) and 1× pen/strep. All cells were maintained in a humidified atmosphere and 5% CO2 at 37 °C.
4.8. Immunofluorescent Staining
H1299, H460 and A549 cells were harvested from flasks and washed with PBS. Each sample contained 100,000 cells and was resuspended in 10 µL of a 50-fold diluted human Fc receptor binding inhibitor (eBioscience, San Diego, CA, USA) for 5 min on ice. Subsequently, 20 µL of a 20-fold diluted phycoerythrin (PE) labeled human CD71 antibody (OKT9, eBioscience) or 20 µL of a 20-fold diluted PE labeled mouse IgG1 isotype control antibody (P3.6.2.8.1, eBioscience) were added to the samples and incubated for 30 min in the dark at 4 °C. Samples were washed with PBS/ 2 mM of EDTA three times and were resuspended in 400 µL of PBS/ 2 mM of EDTA. The median fluorescent intensity (MFI) of samples was quantified using an Attune® Cytometer (Life Technologies, Waltham, MA, USA) with an excitation laser 488 nm and emission filter 574/26 nm. Cell populations were gated according to the forward and side scattering channel, and 10,000 events were collected. Data analysis was performed using the Attune® cytometer software (Life Technologies). Experiments were conducted in duplicate.
4.9. Quantification of Cellular Uptake
For cellular uptake experiments, H1299, H460 and A549 cells were seeded at the density of 50,000 cells/well in 24-well plates (Corning). After 24 h of incubation, polyplexes were prepared with 50 pmol of siRNA-AF488 and bPEI, HAI-SMCC-bPEI or HAI-SPDP-bPEI at N/P = 5. The cells were transfected with polyplexes at the siRNA concentration of 125 nM for 4 h. To reduce the potential cytotoxicity induced by concentrated polyplexes, fresh media were added to dilute siRNA concentration to 50 nM followed by an additional 20 h of incubation. The cells were harvested and washed three times with PBS/2 mM of EDTA. All samples were resuspended in 400 µL of PBS/2 mM of EDTA, and MFIs of samples were determined via an Attune® Cytometer (Life Technologies) with an excitation laser 488 nm and emission filter 530/30 nm. In each sample, 10,000 events were collected and data analysis was performed using the Attune® cytometer software (Life Technologies). Experiments were conducted in triplicate.
4.10. Confocal Laser Scanning Microscopy
H1299 cells were seeded at the density of 50,000 cells/well in 24-well plates with a 12 mm circle cover glass (Fisherbrand, 12CIR-1) in each well and were incubated for 24 h. To determine intracellular distribution of polyplexes, fluorescently labeled polymer bPEI-FITC and HAI-SMCC-bPEI-FITC were formulated with 50 pmol of siRNA-Ty563 at N/P = 5. Cells were transfected with polyplexes at a siRNA concentration of 125 nM for the first 4 h and 50 nM for an additional 44 h. Cells were washed with PBS and fixed with 4% paraformaldehyde (PFA) solution in PBS (Affymetrix, Thermo Fisher) for 20 min at RT at different transfection time points (1, 4, 24, 48 h). To investigate the co-localization of the HAI peptide binding part with TfR, cells were incubated with 2 µg/mL of Tf-Texas Red and polyplexes formulated with bPEI-FITC or HAI—SMCC-bPEI-FITC and 50 pmol of siRNA at N/P = 5 for 1 h or 4 h. Slides were washed with PBS and fixed with 4% PFA. After fixation, the nuclei were stained with 5 µM of DRAQ5 (Invitrogen, Thermo Fisher) for 5 min and rinsed with PBS. Slides were mounted with a Fluoromount mounting medium (Southern Biotech, Birmingham, AL, USA). Slides were imaged by a Leica TCS SPE-II laser scanning confocal microscope (Leica, Wetzlar, Germany), and the images were exported from the Leica Image Analysis Suite (Leica).
4.11. In Vitro GAPDH Gene Knockdown
To determine mRNA level knockdown efficiency of polyplexes, silencing of housekeeping gene GAPDH in H1299 and H460 was determined by real time PCR (RT-PCR). Cells were seeded at the density of 50,000 cells/well in 24-well plates for 24 h. bPEI or HAI-bPEI were formulated with 50 pmol of siRNA against GAPDH or scrambled siRNA at N/P = 5. Cells were transfected with polyplexes at a siRNA concentration of 125 nM for the first 4 h and 50 nM for an additional 20 h. Total mRNA was isolated from cells using the PureLink® RNA mini kit (Life Technologies) following the manufacturer’s protocol in the addition of DNase I digestion (Sigma). The brilliant III ultra-fast SYBR® green QRT-PCR master mix kit (Agilent Technologies, Santa Clara, CA, USA) was used to reverse transcribe 100 ng of mRNA to cDNA and perform RT-PCR. RT-PCR was conducted in a Stratagene Mx 3005p qPCR system (Agilent Technologies) and cycle threshold (Ct) values were exported from MxPro software (Agilent Technologies). Hs_GAPDH_2_SG primers for GAPDH and Hs_ACTB_2-SG primers for β-actin (Qiagen, Valencia, CA, USA) were used in this experiment. GAPDH gene expression was normalized to β-actin gene expression for quantification and comparison, and the untreated group represented 100% GAPDH expression. Experiments were conducted in triplicate.
4.12. In Vitro eGFP Knockdown
To determine the protein level knockdown efficiency of polyplexes, silencing of reporter gene eGFP was determined by flow cytometry. H1299/eGFP cells were seeded at the density of 50,000 cells/well in 24 well-plates for 24 h. On the day of transfection, old media were replaced with 350 µL of fresh media with or without 115 µM of chloroquine. Cells were transfected with 50 pmol of siRNA against eGFP formulated with bPEI or HAI-bPEI at N/P = 5, 10 in total volume of polyplexes of 50 µL. bPEI and HAI-bPEI polyplexes containing scrambled siRNA at N/P = 10 were included as the negative control. Cells were transfected with 50 µL of polyplexes and incubated for 4 h with or without 100 µM of chloroquine. To dilute the concentration of chloroquine and polyplexes, 600 µL of fresh media were added, and cells were incubated for an additional 44 h. Afterward, cells were harvested and washed three times with PBS/2 mM of EDTA. Samples were resuspended in 400 µL of PBS/2 mM of EDTA, and the MFIs were determined by an Attune® Cytometer (Life Technologies) with an excitation laser 488 nm and emission filter 530/30 nm. In each sample, 10,000 events were collected, and data analysis was performed using Attune® cytometer software (Life Technologies). Experiments were conducted in triplicate.
4.13. Statistics
Results were represented as mean ± standard deviation (SD). All statistical analysis used One-way ANOVA with Newman–Keuls multiple comparison post-test in GraphPad Prism software (Graph Pad Software, La Jolla, CA, USA).