Effect of Raw Material, Pressing and Glycosidase on the Volatile Compound Composition of Wine Made From Goji Berries
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Parameters of Goji Wine
2.2. Volatile Composition in Wine Made of Dried and Fresh Goji Berry
2.2.1. Higher Alcohols
2.2.2. Aldehydes and Ketones
2.2.3. Volatile Acids
2.2.4. Esters
2.2.5. Benzenes and Volatile Phenols
2.2.6. Terpenes and Norisoprenoids
2.3. Effect of Pressing and Glycosidase on Volatile Composition in Goji Berry Wine
2.3.1. Effect on Dried Goji Berry Fermented Wine
2.3.2. Effect on Fresh Goji Berry Fermented Wine
2.4. Principal Component Analysis (PCA)
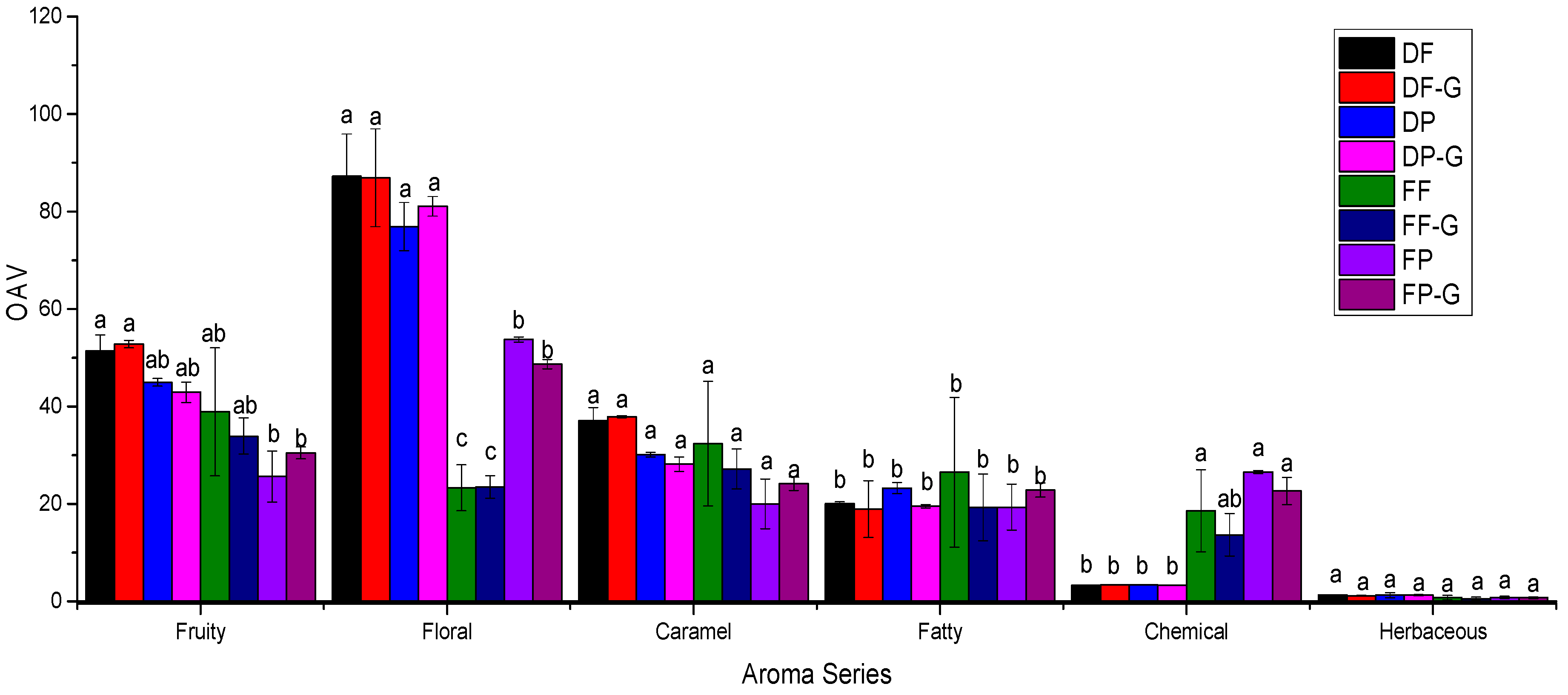
2.5. Aroma Profile of Goji Berry Fermented Wine
3. Materials and Methods
3.1. Samples and Reagents
3.2. Wine-Making Process
3.2.1. Wine Made of Dried Goji Berry
3.2.2. Wine Made of Fresh Goji Berry
3.3. Volatile Compound Analyses
3.4. Odor Activity Value and Aroma Series
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dong, J.Z.; Wang, Y.; Wang, S.H.; Yin, L.P.; Xu, G.J.; Zheng, C.; Lei, C.; Zhang, M.Z. Selenium increases chlorogenic acid, chlorophyll and carotenoids of lycium chinense leaves. J. Sci. Food. Agric. 2013, 93, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Inbaraj, B.S.; Lu, H.; Hung, C.F.; Wu, W.B.; Lin, C.L.; Chen, B.H. Determination of carotenoids and their esters in fruits of lycium barbarum linnaeus by hplc-dad-apci-ms. J. Pharmaceut. Biomed. 2008, 47, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Amagase, H.; Nance, D.M. Lycium barbarum increases caloric expenditure and decreases waist circumference in healthy overweight men and women: Pilot study. J. Am Coll. Nurt. 2011, 30, 304–309. [Google Scholar] [CrossRef]
- Donno, D.; Mellano, M.; Raimondo, E.; Cerutti, A.; Prgomet, Z.; Beccaro, G. Influence of applied drying methods on phytochemical composition in fresh and dried goji fruits by hplc fingerprint. Eur Food Res. Technol. 2016, 1–14. [Google Scholar] [CrossRef]
- Tang, W.-M.; Chan, E.; Kwok, C.-Y.; Lee, Y.-K.; Wu, J.-H.; Wan, C.-W.; Chan, R.Y.-K.; Yu, P.H.-F.; Chan, S.-W. A review of the anticancer and immunomodulatory effects of Lycium barbarum fruit. Inflammopharmacology 2012, 20, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Shahidi, F.; Naczk, M. Phytochemicals and health benefits of goji berries. In Dried Fruits: Phytochemicals and Health Effects; Alasalvar, C., Shahidi, F., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2013; pp. 133–144. [Google Scholar]
- Antalick, G.; Šuklje, K.; Blackman, J.W.; Meeks, C.; Deloire, A.; Schmidtke, L.M. Influence of grape composition on red wine ester profile: Comparison between cabernet sauvignon and shiraz cultivars from australian warm climate. J. Agric. Food Chem. 2015, 63, 4664–4672. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.F.; Kaczmarek, A. Handbook of enology, the chemistry of wine stabilization and treatments, 2nd ed.; Ribéreau-gayon, P., Glories, Y., Maujean, A., Dubourdieu, D., Eds.; John wiley & sons, Ltd.: New York, NY, USA, 2006; Volume 2. [Google Scholar]
- Cabaroglu, T.; Selli, S.; Canbas, A.; Lepoutre, J.-P.; Günata, Z. Wine flavor enhancement through the use of exogenous fungal glycosidases. Enzyme. Microb Tech. 2003, 33, 581–587. [Google Scholar] [CrossRef]
- Selli, S.; Bagatar, B.; Sen, K.; Kelebek, H. Evaluation of differences in the aroma composition of free-run and pressed neutral grape juices obtained from emir (vitis vinifera L.). Chem. Biodivers. 2011, 8, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Fundira, M.; Blom, M.; Pretorius, I.; Rensburg, P. Comparison of commercial enzymes for the processing of marula pulp, wine, and spirits. J. Food Sci. 2002, 67, 2346–2351. [Google Scholar] [CrossRef]
- Palomo, E.S.; Hidalgo, M.C.D.-M.; Gonzalez-Vinas, M.; Pérez-Coello, M. Aroma enhancement in wines from different grape varieties using exogenous glycosidases. Food Chem. 2005, 92, 627–635. [Google Scholar] [CrossRef]
- Li, X.; Lim, S.L.; Yu, B.; Curran, P.; Liu, S.Q. Impact of pulp on the chemical profile of mango wine. S. Afr. J. Enol. Vitic. 2013, 34, 119–128. [Google Scholar]
- Patel, P.; Herbst-Johnstone, M.; Lee, S.A.; Gardner, R.C.; Weaver, R.; Nicolau, L.; Kilmartin, P.A. Influence of juice pressing conditions on polyphenols, antioxidants, and varietal aroma of sauvignon blanc microferments. J. Agric. Food Chem. 2010, 58, 7280–7288. [Google Scholar] [CrossRef] [PubMed]
- Amagase, H.; Farnsworth, N.R. A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (goji). Food Res. Int. 2011, 44, 1702–1717. [Google Scholar] [CrossRef]
- Guadalupe, Z.; Ayestarán, B.; Williams, P.; Doco, T. Determination of must and wine polysaccharides by gas chromatography-mass spectrometry (gc-ms) and size-exclusion chromatography (sec). In Polysaccharides: Bioactivity and Biotechnology; Ramawat, G.K., Mérillon, J.-M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1265–1297. [Google Scholar]
- Cliff, M.A.; Pickering, G.J. Determination of odour detection thresholds for acetic acid and ethyl acetate in ice wine. J. Wine Res. 2006, 17, 45–52. [Google Scholar] [CrossRef]
- Panceri, C.P.; De Gois, J.S.; Borges, D.L.G.; Bordignon-Luiz, M.T. Effect of grape dehydration under controlled conditions on chemical composition and sensory characteristics of cabernet sauvignon and merlot wines. LWT—Food Sci. Technol. 2015, 63, 228–235. [Google Scholar] [CrossRef]
- Ni, Z.-J.; He, L.; Min, C. Changes of the main carotenoid pigment contents during the drying processes of the different harvest stage fruits of Lycium barbarum L. Agric. Sci. China 2008, 7, 363–369. [Google Scholar]
- Wang, D.; Cai, J.; Zhu, B.-Q.; Wu, G.-F.; Duan, C.-Q.; Chen, G.; Shi, Y. Study of free and glycosidically bound volatile compounds in air-dried raisins from three seedless grape varieties using hs-spme with gc-ms. Food Chem. 2015, 177, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.-B.; Qian, X.; Yang, Z.-J.; Xiang, X.-F.; Yang, W.-X.; Liu, T.; Zhu, B.-Q.; Pan, Q.-H.; Duan, C.-Q. Striking changes in volatile profiles at sub-zero temperatures during over-ripening of ’beibinghong’ grapes in northeastern china. Food Chem. 2016, 212, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Guiné, R.P.; Martín-Belloso, O.; Mínguez-Mosquera, M.I.; Paliyath, G.; Pessoa, F.L.; Le Quéré, J.-L.; Sidhu, J.S.; Sinha, N.; Peggy Stanfield, R.; Hui, Y. Handbook of Fruit and Vegetable Flavors; John Wiley and Sons: New York, NY, USA, 2010. [Google Scholar]
- de Lerma, N.L.; Moreno, J.; Peinado, R.A. Determination of the optimum sun-drying time for Vitis vinifera L. cv. Tempranillo grapes by e-nose analysis and characterization of their volatile composition. Food. Bioprocess Tech. 2014, 7, 732–740. [Google Scholar] [CrossRef]
- Romano, P. Metabolic characteristics of wine strains during spontaneous and inoculated fermentation. Food Technol. Biotech. 1997, 35, 255–260. [Google Scholar]
- Ruiz, M.J.; Zea, L.; Moyano, L.; Medina, M. Aroma active compounds during the drying of grapes cv. Pedro ximenez destined to the production of sweet sherry wine. Eur. Food Res. Technol. 2010, 230, 429–435. [Google Scholar] [CrossRef]
- Chung, T.-Y.; Hayase, F.; Kato, H. Volatile components of ripe tomatoes and their juices, purees and pastes. Agric. Biol. Chem. 1983, 47, 343–351. [Google Scholar]
- Ho, C.-T.; Chen, Q. Lipids in food flavors: An overview. In Proceedings of 205th National Meeting of the American Chemical Society, Denver, Colorado, 28 March–2 April 1993; American Chemical Society: Washington, DC, USA, 1994. [Google Scholar]
- Cai, J.; Zhu, B.-Q.; Wang, Y.-H.; Lu, L.; Lan, Y.-B.; Reeves, M.J.; Duan, C.-Q. Influence of pre-fermentation cold maceration treatment on aroma compounds of cabernet sauvignon wines fermented in different industrial scale fermenters. Food Chem. 2014, 154, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Swiegers, J.H.; Pretorius, I.S. Yeast modulation of wine flavor. Adv. Appl. Microbiol. 2005, 57, 131–175. [Google Scholar] [PubMed]
- Sánchez-Palomo, E.; Gómez García-Carpintero, E.; Alonso-Villegas, R.; González-Viñas, M. Characterization of aroma compounds of verdejo white wines from the la mancha region by odour activity values. Flavour Frag. J. 2010, 25, 456–462. [Google Scholar] [CrossRef]
- Noguerol-Pato, R.; González-Álvarez, M.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Aroma profile of garnacha tintorera-based sweet wines by chromatographic and sensorial analyses. Food Chem. 2012, 134, 2313–2325. [Google Scholar] [CrossRef] [PubMed]
- Noguerol-Pato, R.; González-Álvarez, M.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Evolution of the aromatic profile in garnacha tintorera grapes during raisining and comparison with that of the naturally sweet wine obtained. Food Chem. 2013, 139, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Microbial modulation of aromatic esters in wine: Current knowledge and future prospects. Food Chem. 2010, 121, 1–16. [Google Scholar] [CrossRef]
- Coghe, S.; Benoot, K.; Delvaux, F.; Vanderhaegen, B.; Delvaux, F.R. Ferulic acid release and 4-vinylguaiacol formation during brewing and fermentation: Indications for feruloyl esterase activity in saccharomyces cerevisiae. J. Agric. Food Chem. 2004, 52, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Dias, L.; Dias, S.; Sancho, T.; Stender, H.; Querol, A.; Malfeito-Ferreira, M.; Loureiro, V. Identification of yeasts isolated from wine-related environments and capable of producing 4-ethylphenol. Food Microbiol. 2003, 20, 567–574. [Google Scholar] [CrossRef]
- Kanasawud, P.; Crouzet, J.C. Mechanism of formation of volatile compounds by thermal degradation of carotenoids in aqueous medium. 1.. Beta.-carotene degradation. J. Agric. Food Chem. 1990, 38, 237–243. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, B.; Tu, C.; Duan, C.; Pan, Q. Generation of volatile compounds in litchi wine during winemaking and short-term bottle storage. J. Agric. Food Chem. 2011, 59, 4923–4931. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhu, B.; Li, Y.; Zhang, Y.; Zhang, B.; Fan, J. Acidic electrolyzed water efficiently improves the flavour of persimmon (diospyros kaki l. Cv. Mopan) wine. Food Chem. 2016, 197(Part A), 141–149. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulou, A.; Flouros, A.; Demertzis, P.; Akrida-Demertzi, K. Differences in concentration of principal volatile constituents in traditional greek distillates. Food Control. 2005, 16, 157–164. [Google Scholar] [CrossRef]
- Satora, P.; Tuszyński, T. Influence of indigenous yeasts on the fermentation and volatile profile of plum brandies. Food Microbiol. 2010, 27, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Baek, H.; Cadwallader, K. Contribution of free and glycosidically bound volatile compounds to the aroma of muscadine grape juice. J. Food Sci. 1999, 64, 441–444. [Google Scholar] [CrossRef]
- Chatonnet, P.; Dubourdie, D.; Boidron, J.; Pons, M. The origin of ethylphenols in wines. J. Sci. Food. Agric. 1992, 60, 165–178. [Google Scholar] [CrossRef]
- Towey, J.P.; Waterhouse, A.L. The extraction of volatile compounds from french and american oak barrels in chardonnay during three successive vintages. Am. J. Enol. Viticult. 1996, 47, 163–172. [Google Scholar]
- Pollnitz, A.P.; Pardon, K.H.; Sefton, M.A. Quantitative analysis of 4-ethylphenol and 4-ethylguaiacol in red wine. J. Chromatogr. A 2000, 874, 101–109. [Google Scholar] [CrossRef]
- Sun, S.Y.; Gong, H.S.; Jiang, X.M.; Zhao, Y.P. Selected non-saccharomyces wine yeasts in controlled multistarter fermentations with saccharomyces cerevisiae on alcoholic fermentation behaviour and wine aroma of cherry wines. Food Microbiol. 2014, 44, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Noble, A.C.; Arnold, R.A.; Buechsenstein, J.; Leach, E.J.; Schmidt, J.; Stern, P.M. Modification of a standardized system of wine aroma terminology. Am. J. Enol. Viticult. 1987, 38, 143–146. [Google Scholar]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food. Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Pino, J.A.; Queris, O. Analysis of volatile compounds of mango wine. Food Chem. 2011, 125, 1141–1146. [Google Scholar] [CrossRef]
- Guth, H. Quantitation and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Zhao, N.; Wen, Y.; Pan, Q. Determination of dry red wine polysaccharide by phenol-sulfuric acid method. Sino-Overseas Grapevine Wine 2011, 5, 003. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, Q.; Duan, C.; Qu, W.; Wu, Y. Comparative study of aromatic compounds in young red wines from cabernet sauvignon, cabernet franc, and cabernet gernischet varieties in china. J. Food Sci. 2007, 72, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Sinelnikov, I.V.; Han, B.; Wishart, D.S. Metaboanalyst 3.0—Making metabolomics more meaningful. Nucleic Acids Res. 2015, 43, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Some of the frozen wine samples are available from the authors.
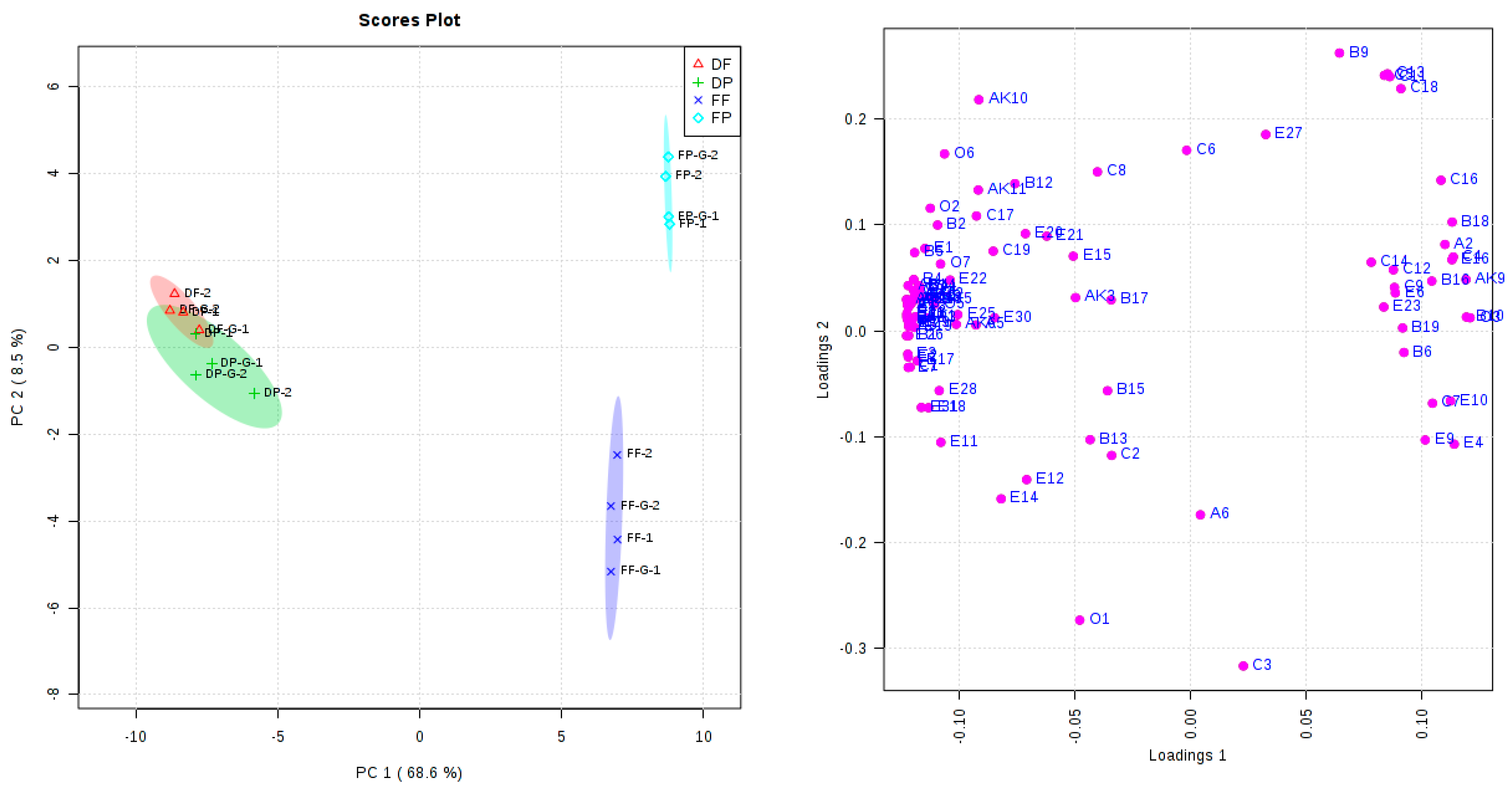
| Oenological Parameters | DF | DF-G | DP | DP-G | FF | FF-G | FP | FP-G |
|---|---|---|---|---|---|---|---|---|
| Alcohol (%, v/v) | 13.0 ± 0.0 a | 13.00 ± 0.0 a | 12.5 ± 0.2 bcd | 12.6 ± 0.0 bc | 12.4 ± 0.1 cd | 12.7 ± 0.0 b | 12.4 ± 0.0 d | 12.3 ± 0.0 d |
| Volatile acidity (g/L) | 1.2 ± 0.0 b | 1.2 ± 0.0 bc | 1.1 ± 0.0 e | 0.8 ± 0.0 g | 1.2 ± 0.0 cd | 1.0 ± 0.0 f | 1.1 ± 0.0 de | 2.1 ± 0.0 a |
| Titratable acidity a (g/L) | 12.2 ± 0.1 ab | 11.9 ± 0.1 bc | 11.8 ± 0.0 c | 12.4 ± 0.1 a | 7.9 ± 0.0 e | 7.7 ± 0.0 e | 8.2 ± 0.1 e | 7.8 ± 0.0 d |
| Residual sugar (g/L) | 9.3 ± 0.4 a | 10.0 ± 0.4 a | 10.1 ± 0.7 a | 9.1 ± 0.0 a | 7.8 ± 0.1 b | 6.6 ± 0.0 b | 8.4 ± 0.1 b | 7.6 ± 0.1 b |
| Polysaccharide b (g/L) | 1.6 ± 0.1 b | 1.5 ± 0.0 bc | 3.0 ± 0.1a | 2.9 ±0.1 a | 1.4 ± 0.0 cd | 1.2 ± 0.0 df | 1.3 ± 0.1 d | 1.1 ± 0.1 f |
| Free SO2 (mg/L) | 3.4 ± 0.0 c | 1.7 ± 0.1 d | 3.4 ± 0.0 c | 1.7 ± 0.0 d | 13.4 ± 0.0 a | 7.3 ± 0.4 b | 13.4 ± 0.0 a | 6.7 ± 0.0 b |
| Total SO2 (mg/L) | 100.4 ± 0.1 f | 98.8 ± 0.2 g | 93.7 ± 0.0 h | 132.1 ± 0.0 d | 210.5 ± 0.2 b | 153.9 ± 0.1 c | 237.5 ± 0.1 a | 112.0 ± 0.0 e |
| pH | 4.0 ± 0.0 c | 3.9 ± 0.0 c | 4.0 ± 0.0 b | 4.0 ±0.0 b | 4.4 ± 0.0 a | 4.5 ±0.0 a | 4.4 ± 0.0 a | 4.5 ± 0.0 a |
| Volatile | CN a | RI | ID b | QI c | Standard | Quantitative Curve | R2 | Linearity Range (μg/L) |
|---|---|---|---|---|---|---|---|---|
| Volatile acids | A | |||||||
| DMECA d | A1 | 1367.6 | B | 95 | 4-Ethylphenol | y = 233.46x + 1.5085 | R2 = 0.9967 | 0.78–50.00 |
| Acetic acid | A2 | 1453.8 | A | 43 | Acetic acid | y = 53.61x − 29.07 | R2 = 0.9948 | 32.78–1049.00 |
| Butanoic acid | A3 | 1630.4 | A | 60 | Butanoic acid | y = 21574x + 7.5319 | R2 = 0.9999 | 126.88–1015.00 |
| α-Methylbutyric acid | A4 | 1671.7 | B | 74 | Butanoic acid | y = 21814x − 0.9852 | R2 = 0.9994 | 63.44–1015.00 |
| Octanoic acid | A5 | 2062.1 | A | 60 | Octanoic acid | y = 7754.7x + 1401.7 | R2 = 0.9982 | 2365.00–9460.00 |
| Decanoic acid | A6 | 2271.8 | A | 60 | Decanoic acid | y = 55699x − 252.24 | R2 = 0.9609 | 1147.50–9180.00 |
| Higher alcohols | C | |||||||
| 2-Methyl-1-propanol | C1 | 1104.0 | A | 43 | 2-Methyl-1-propanol | y = 17537x + 5926.7 | R2 = 0.9861 | 10250.00–82000.00 |
| 1-Butanol | C2 | 1158.1 | A | 56 | 1-Butanol | y = 17266x + 36.061 | R2 = 1 | 299.69–1198.75 |
| Isopentyl alcohol | C3 | 1216.0 | A | 55 | Isopentyl alcohol | y = 9481.2x − 6073.4 | R2 = 0.9985 | 35300.00–282400.00 |
| Pentyl alcohol | C4 | 1255.5 | B | 42 | 1-Hexanol | y = 1162.6x − 1.2723 | R2 = 0.9999 | 4.93–78.95 |
| 1-Hexanol | C5 | 1352.5 | A | 56 | 1-Hexanol | y = 1166.2x − 2.2718 | R2 = 0.9999 | 39.47–315.78 |
| (Z)-3-Hexen-1-ol | C6 | 1384.7 | A | 41 | (Z)-3-Hexen-1-ol | y = 4651.6x − 7.5812 | R2 = 0.9968 | 32.62–260.94 |
| 2-Heptanol | C7 | 1320.0 | A | 45 | 2-Heptanol | y = 210.83x + 0.0189 | R2 = 0.9944 | 2.44–9.75 |
| 3-Ethoxy-1-propanol | C8 | 1376.9 | B | 31 | 1-Hexanol | y = 1161.1x − 1.192 | R2 = 0.9998 | 9.87–78.95 |
| 1-Octen-3-ol | C9 | 1448.6 | A | 57 | 1-Octen-3-ol | y = 118.3x − 0.4557 | R2 = 0.9993 | 3.22–25.78 |
| Sulcatol | C10 | 1461.0 | A | 95 | Sulcatol | y = 514.36x − 60.573 | R2 = 0.9936 | 0.69–11.00 |
| 2-Ethyl-1-hexanol | C11 | 1488.8 | A | 27 | 2-Ethyl-1-hexanol | y = 89.473x − 0.447 | R2 = 0.9988 | 0.44–14.06 |
| [R-(R*,R*)]-2,3-Butanediol | C12 | 1538.9 | B | 45 | [S-(R*,R*)]-2,3-Butanediol | y = 82896x − 16783 | R2 = 0.9993 | 39781.25–636500.00 |
| 1-Octanol | C13 | 1557.0 | A | 56 | 1-Octanol | y = 182.89x + 0.2501 | R2 = 0.9996 | 6.45–51.56 |
| [S-(R*,R*)]-2,3-Butanediol | C14 | 1574.9 | A | 45 | [S-(R*,R*)]-2,3-Butanediol | y = 82896x − 16783 | R2 = 0.9993 | 39781.25–636500.00 |
| trans-2-Octenol | C15 | 1616.0 | A | 57 | trans-2-Octenol | y = 258.79x + 0.2617 | R2 = 0.9977 | 2.61–20.88 |
| 1-Nonanol | C16 | 1659.1 | B | 56 | Acetic acid | y = 10.169x − 0.0206 | R2 = 0.9856 | 0.13–0.51 |
| Methionol | C17 | 1720.6 | A | 106 | Methionol | y = 34890x − 113.09 | R2 = 0.9962 | 280.00–1120.00 |
| E-5-Decen-1-ol | C18 | 1795.2 | B | 67 | 1-Dodecanol | y = 300.63x + 0.7633 | R2 = 0.9962 | 2.52–20.16 |
| Phenylethyl alcohol | C19 | 1920.2 | A | 91 | Phenylethyl alcohol | y = 1833.3x − 518.58 | R2 = 0.9940 | 2537.50–40600.00 |
| Aldehydes/Ketones | AK | |||||||
| Aldehydes | ||||||||
| Nonanal | AK1 | 1399.2 | A | 57 | Nonanal | y = 97.464x − 2.3267 | R2 = 0.9929 | 0.44–28.44 |
| Furfural | AK2 | 1471.8 | A | 96 | Furfural | y = 1798.6x − 2.8178 | R2 = 0.9987 | 5.25–84.00 |
| Decanal | AK3 | 1504.4 | A | 43 | Decanal | y = 120.85x + 0.8893 | R2 = 0.9878 | 0.31–39.53 |
| Safranal | AK4 | 1656.8 | B | 107 | α-Terpineol | y = 34.957x + 0.2522 | R2 = 0.9950 | 0.42–3.38 |
| 2-Butyl-2-octenal | AK5 | 1675.4 | B | 41 | Ethyl caprate | y = 277.88x − 4.4049 | R2 = 0.9917 | 4.98–39.84 |
| Ketones | ||||||||
| Isobutyl ketone | AK6 | 1194.0 | B | 57 | 6M5H2NE | y = 243.64x − 5.3362 | R2 = 0.9960 | 0.04–92.00 |
| TMCHN e | AK7 | 1325.0 | B | 82 | 6M5H3NE | y = 275.14x − 6.922 | R2 = 0.9947 | 0.72–11.50 |
| 6M5H2NE f | AK8 | 1344.7 | A | 43 | 6M5H4NE | y = 278.06x − 7.1512 | R2 = 0.9971 | 0.36–23.00 |
| 2-Undecanone | AK9 | 1603.7 | B | 58 | Dihydropseudoionone | y = 53.532x + 0.0331 | R2 = 0.9995 | 0.93–7.44 |
| β-Cyclocitral | AK10 | 1630.9 | B | 137 | α-Terpineol | y = 179.5x + 0.7247 | R2 = 0.9993 | 1.91–7.63 |
| Dihydropseudoionone | AK11 | 1861.4 | A | 43 | Dihydropseudoionone | y = 50.565x + 0.0693 | R2 = 0.9981 | 0.23–14.88 |
| 2-Acetylpyrrole | AK12 | 1981.6 | B | 94 | Hexanal | y = 1093.5x − 17.772 | R2 = 0.9994 | 30.74–245.94 |
| TMHAAL g | AK13 | 2372.3 | B | 111 | 4-Ethylphenol | y = 312.57x + 0.2238 | R2 = 0.9959 | 0.10–12.50 |
| Benzenes/Phenols | B | |||||||
| Benzenes | ||||||||
| Isodurene | B1 | 1498.2 | B | 119 | p-Cymene | y = 54.679x − 0.1459 | R2 = 0.9956 | 0.41–3.28 |
| TEMLNE h | B2 | 1677.5 | B | 159 | Ethyl salicylate | y = 79.909x + 0.0671 | R2 = 0.9932 | 0.53–17.00 |
| DPPXL i | B3 | 1694.1 | B | 175 | Ethyl salicylate | y = 56.72x + 3.6823 | R2 = 0.9945 | 8.50–34.00 |
| Naphthalene | B4 | 1756.0 | A | 128 | Naphthalene | y = 10.996x + 0.5718 | R2 = 0.9915 | 0.95–7.56 |
| TMDHPE j | B5 | 1758.3 | B | 157 | Ethyl salicylate | y = 88.273x − 0.0789 | R2 = 0.9900 | 4.98–39.84 |
| M2PB k | B6 | 1790.2 | B | 120 | Ethyl phenacetate | y = 35.188x + 0.2362 | R2 = 0.9924 | 0.84–3.38 |
| Phenethyl acetate | B7 | 1826.3 | A | 104 | Phenethyl acetate | y = 69.63x + 5.0169 | R2 = 0.9996 | 17.81–285.00 |
| β-Methylnaphthalene | B8 | 1870.0 | B | 142 | Ethyl salicylate | y = 60.837x + 1.9511 | R2 = 0.9906 | 2.13–34.00 |
| Benzyl alcohol | B9 | 1883.4 | A | 79 | Benzyl alcohol | y = 3642.2x + 19.022 | R2 = 0.9979 | 91.56–732.50 |
| Ethyl dihydrocinnamate | B10 | 1895.6 | B | 104 | Ethyl phenacetate | y = 29.931x + 0.4139 | R2 = 0.9944 | 0.42–6.75 |
| α-Methylnaphthalene | B11 | 1906.9 | B | 142 | Ethyl salicylate | y = 79.909x + 0.0671 | R2 = 0.9932 | 0.53–17.00 |
| 4-Tolylcarbinol | B12 | 1981.6 | B | 107 | 4-Ethylphenol | y = 391.78x − 0.0605 | R2 = 0.9863 | 0.20–3.13 |
| Ethyl cinnamate | B13 | 2145.3 | B | 131 | Ethyl phenacetate | y = 29.468x + 0.4874 | R2 = 0.9940 | 0.84–6.75 |
| 3P4HP l | B14 | 2249.8 | B | 197 | Ethyl phenacetate | y = 34.957x + 0.2522 | R2 = 0.9950 | 0.42–3.38 |
| DTBPH m | B15 | 2308.7 | B | 191 | 4-Ethylphenol | y = 166.76x + 11.394 | R2 = 0.9934 | 25.00–100.00 |
| Coumaran | B16 | 2403.1 | B | 120 | 4-Ethylphenol | y = 159.63x + 29.604 | R2 = 0.9862 | 12.50–800.00 |
| Phenols | ||||||||
| Ethyl salicylate | B17 | 1824.6 | A | 120 | Ethyl salicylate | y = 60.837x + 1.9511 | R2 = 0.9906 | 2.13–34.00 |
| 4-Ethylphenol | B18 | 2182.8 | A | 107 | 4-Ethylphenol | y = 162.82x + 18.57 | R2 = 0.9863 | 1.56–800.00 |
| 4-Vinylguaiacol | B19 | 2208.5 | B | 135 | 4-Ethylphenol | y = 141.15x + 101.45 | R2 = 0.9973 | 200.00–800.00 |
| Esters Ethyl esters | E | |||||||
| Ethyl Acetate | E1 | 764.8 | A | 43 | Ethyl Acetate | y = 6076.6x − 2518.1 | R2 = 0.9978 | 19187.50–153500.00 |
| Ethyl butanoate | E2 | 1037.5 | A | 71 | Ethyl butanoate | y = 613.77x + 1.0444 | R2 = 0.9999 | 5.41–692.50 |
| Ethyl hexanoate | E3 | 1244.4 | A | 88 | Ethyl hexanoate | y = 68.562x − 6.8083 | R2 = 1 | 40.63–650.00 |
| Ethyl 3-hexenoate | E4 | 1307.6 | A | 69 | Ethyl 3-hexenoate | y = 80.813x − 0.0169 | R2 = 0.9976 | 0.62–9.94 |
| Ethyl enanthate | E5 | 1337.6 | A | 88 | Ethyl enanthate | y = 35.894x + 0.1203 | R2 = 0.9997 | 0.91–3.63 |
| Ethyl lactate | E6 | 1346.8 | A | 45 | Ethyl lactate | y = 28150x − 92.569 | R2 = 0.9960 | 199.22–6375.00 |
| Ethyl octanoate | E7 | 1438.7 | A | 88 | Ethyl octanoate | y = 42.269x + 91.381 | R2 = 0.9998 | 318.75–1275.00 |
| Ethyl mesitylacetate | E8 | 1511.8 | B | 133 | Ethyl phenacetate | y = 38.305x + 0.1918 | R2 = 0.9779 | 0.42–1.69 |
| Ethyl nonanoate | E9 | 1539.5 | A | 88 | Ethyl nonanoate | y = 42.505x + 0.9106 | R2 = 0.9940 | 1.50–12.03 |
| E2H4MP n | E10 | 1546.9 | A | 69 | E2H4MP | y = 394.42x + 1.2915 | R2 = 0.9999 | 8.87–35.47 |
| Ethyl caprate | E11 | 1642.8 | A | 88 | Ethyl caprate | y = 78.134x + 132.78 | R2 = 0.9911 | 159.38–1275.00 |
| Ethyl benzoate | E12 | 1677.4 | B | 105 | Ethyl phenacetate | y = 35.188x + 0.2362 | R2 = 0.9924 | 0.84–3.38 |
| Ethyl 9-decenoate | E13 | 1694.3 | B | 55 | Ethyl laurate | y = 278.11x + 0.9429 | R2 = 0.9891 | 0.65–41.64 |
| Ethyl laurate | E14 | 1847.5 | A | 88 | Ethyl laurate | y = 97.913x + 53.207 | R2 = 0.9985 | 83.28–333.13 |
| Ethyl myristate | E15 | 2053.2 | B | 88 | Ethyl laurate | y = 255.61x + 3.5176 | R2 = 0.9965 | 10.41–41.64 |
| Ethyl hexadecanoate | E16 | 2256.1 | B | 88 | Ethyl laurate | y = 140.86x + 16.882 | R2 = 0.9696 | 10.41–166.56 |
| Acetates | ||||||||
| Isobutyl acetate | E17 | 1009.6 | A | 43 | Isobutyl acetate | y = 417.2x − 134.5 | R2 = 0.9996 | 17.81–570.00 |
| Isopentyl acetate | E18 | 1135.9 | A | 43 | Isopentyl acetate | y = 186.69x − 260.63 | R2 = 0.9983 | 631.88–10110.00 |
| Hexyl acetate | E19 | 1298.8 | A | 43 | Hexyl acetate | y = 44.969x − 0.3454 | R2 = 0.9999 | 0.29–75.47 |
| Heptyl acetate | E20 | 1377.4 | B | 43 | Hexyl acetate | y = 46.025x − 0.4832 | R2 = 0.9999 | 2.36–18.87 |
| 2-Ethyl-1-hexanol acetate | E21 | 1387.2 | B | 43 | 2-Heptanol | y = 224.82x − 0.2812 | R2 = 0.9985 | 1.22–19.50 |
| Octyl acetate | E22 | 1477 | B | 43 | 2-Heptanol | y = 224.82x − 0.2812 | R2 = 0.9985 | 1.22–19.50 |
| Trimethylene acetate | E23 | 1740.3 | B | 43 | Diethyl succinate | y = 749.61x + 12.249 | R2 = 0.9987 | 38.67–309.38 |
| Other esters | ||||||||
| Isobutyl hexanoate | E24 | 1357.7 | B | 99 | Styrene | y = 54.92x − 0.4452 | R2 = 0.9928 | 0.25–3.99 |
| Methyl octanoate | E25 | 1393.8 | A | 74 | Methyl octanoate | y = 23.885x + 0.7001 | R2 = 0.9991 | 0.76–48.75 |
| Isopentyl hexanoate | E26 | 1462.3 | A | 70 | Isopentyl hexanoate | y = 58.981x + 0.1264 | R2 = 0.9945 | 0.61–9.77 |
| Isobutyl octanoate | E27 | 1554.6 | B | 57 | Isoamyl caprylate | y = 33.7x + 0.8987 | R2 = 0.9878 | 0.63–10.13 |
| Methyl caprate | E28 | 1599.0 | B | 74 | Ethyl caprate | y = 277.88x − 4.4049 | R2 = 0.9917 | 4.98–39.84 |
| Isoamyl octanoate | E29 | 1662.7 | A | 70 | Isoamyl octanoate | y = 25.546x + 1.5448 | R2 = 0.9815 | 0.63–20.25 |
| Diethyl succinate | E30 | 1680.1 | A | 101 | Diethyl succinate | y = 709.47x + 17.935 | R2 = 0.9991 | 38.67–618.75 |
| Isoamyl decanoate | E31 | 1866.8 | B | 70 | Isoamyl caprylate | y = 32.527x + 1.1504 | R2 = 0.9920 | 1.27–10.13 |
| IMDMMP o | E32 | 1883.2 | B | 71 | Isoamyl caprylate | y = 25.382x + 1.5173 | R2 = 0.9829 | 0.63–20.25 |
| NA3MBE p | E33 | 1905.7 | B | 141 | Isoamyl caprylate | y = 32.527x + 1.1504 | R2 = 0.9920 | 1.27–10.13 |
| Others | O | |||||||
| Styrene | O1 | 1269.8 | A | 104 | Styrene | y = 37.916x + 1.5324 | R2 = 0.9989 | 31.94–127.75 |
| α-Ionene | O2 | 1489.7 | B | 159 | Ethyl salicylate | y = 60.837x + 1.9511 | R2 = 0.9906 | 2.13–34.00 |
| ODETM q | O3 | 1549.5 | B | 138 | Ethyl salicylate | y = 78.368x + 0.3169 | R2 = 0.9952 | 1.06–17.00 |
| α-Cedrene | O4 | 1625.9 | B | 119 | E-Nerolidol | y = 12.967x + 0.0836 | R2 = 0.9864 | 0.03–2.13 |
| α-Calacorene | O5 | 1931.9 | B | 157 | Ethyl salicylate | y = 76.261x + 0.6681 | R2 = 0.9980 | 2.13–17.00 |
| β-Ionone | O6 | 1951.7 | B | 177 | Ethyl salicylate | y = 78.368x + 0.3169 | R2 = 0.9952 | 1.06–17.00 |
| DMUDL r | O7 | 1955.5 | B | 109 | E-Nerolidol | y = 16.415x + 0.0339 | R2 = 0.9952 | 0.07–1.06 |
| Cedrol | O8 | 2137.5 | B | 95 | E-Nerolidol | y = 15.972x + 0.0556 | R2 = 0.9981 | 0.13–1.06 |
| Volatile (µg/L) | DF | DF-G | DP | DP-G | FF | FF-G | FP | FP-G |
|---|---|---|---|---|---|---|---|---|
| Volatile acids | ||||||||
| DMECAd | 40.8 ± 1.5 a * | 41.2 ± 1.0 a | 39.8 ± 0.7 a | 41.3 ± 1.4 a | 2.5 ± 0.0 b | 2.5 ± 0.1 b | 2.6 ± 0.2 b | 2.4 ± 0.1 b |
| Acetic acid | 172.6 ± 53.0 bc | 199.8 ± 96.6 bc | 135.5 ± 81.7 c | 177.3 ± 32.0 bc | 469.4 ± 122.5 abc | 417.6 ± 86.1 abc | 639.3 ± 121.1 a | 525.3 ± 101.0 ab |
| Butanoic acid | 1506.8 ± 135.7 a | 1489.4 ± 359.1 a | 1488.7 ± 213.3 a | 1503.4 ± 69.9 a | 414.3 ± 99.4 b | 412.4 ± 13.0 b | 418.2 ± 71.9 b | 373.7 ± 5.8 b |
| α-Methylbutyric acid | 1532.2 ± 42.6 a | 1450.2 ± 188.3 a | 1544.0 ± 210.31 a | 1519.5 ± 14.8 a | 531.2 ± 104.0 b | 488.5 ± 6.4 b | 539.4 ± 9.3 b | 473.4 ± 5.3 b |
| Octanoic acid | 1018.7 ± 49.8 a | 819.8 ± 297.4 a | 1045.5 ± 122.6 a | 914.2 ± 10.2 a | 740.8 ± 318.8 a | 598.5 ± 172.9 a | 610.5 ± 39.9 a | 563.6 ± 38.0 a |
| Decanoic acid | 2712.4 ± 146.2 a | 2186.8 ± 995.4 a | 6053.6 ± 1901.4 a | 3325.7 ± 203.0 a | 5047.2 ± 1410.4 a | 4215.8 ± 1303.4 a | 2570.7 ± 28.2 a | 2752.2 ± 385.4 a |
| Total | 6983.5 ± 340.5 ab | 6187.3 ± 1936.0 ab | 10306.8 ± 1274.1 a | 7481.4 ± 98.0 ab | 7205.3 ± 2055.1 ab | 6135.4 ± 1568.9 ab | 4780.6 ± 195.3 b | 4690.6 ± 235.5 b |
| Higher alcohols | ||||||||
| 2-Methyl-1-propanol | 35548.7 ± 1625.3 a | 35130.6 ± 589.8 a | 36260.1 ± 1605.77 a | 35318.8 ± 1043.7 a | 23463.5 ± 907.0 b | 23601.0 ± 687.37 b | 20073.4 ± 98.2 b | 20012.0 ± 418.5 b |
| 1-Butanol | 967.2 ± 87.1 a | 955.0 ± 104.7 a | 948.5 ± 42.7 a | 953.4 ± 10.5 a | 948.0 ± 33.48 a | 978.0 ± 61.4 a | 929.3 ± 103.8 a | 866.2 ± 16.9 a |
| Isopentyl alcohol | 187579.3 ± 2742.2 b | 184874.0 ± 6446.52 b | 188753.9 ± 5009.5 b | 183698.5 ± 816.3 b | 217389.5 ± 7670.1 a | 214993.6 ± 267.8 a | 173630.3 ± 1822.6 b | 173019.9 ± 48.9 b |
| Pentyl alcohol | 17.0 ± 3.19 c | 21.4 ± 3.1 bc | 17.9 ± 3.3 c | 15.7 ± 0.3 c | 28.1 ± 1.1 ab | 27.5 ± 3.0 ab | 32.1 ± 0.2 a | 34.3 ± 1.8 a |
| 1-Hexanol | 91.5 ± 3.0 b | 99.3 ± 5.3 b | 92.8 ± 6.5 b | 90.5 ± 5.1 b | 94.5 ± 2.7 b | 101.5 ± 1.7 b | 160.7 ± 1.0 a | 169.1 ± 2.7 a |
| (Z)-3-Hexen-1-ol | 123.8 ± 21.6 a | 132.7 ± 5.2 a | 134.0 ± 4.58 a | 115.6 ± 8.4 a | 100.3 ± 0.4 a | 122.4 ± 21.7 a | 129.8 ± 32.3 a | 144.4 ± 29.2 a |
| 2-Heptanol | 5.6 ± 0.1 c | 5.7 ± 0.3 c | 6.3 ± 0.2 bc | 6.3 ± 0.1 bc | 7.8 ± 0.3 a | 7.3 ± 0.1 ab | 6.7 ± 0.1 abc | 7.8 ± 0.6 a |
| 3-Ethoxy-1-propanol | 39.9 ± 1.6 a | 35.1 ± 15.8 a | 33.7 ± 9.1 a | 32.6 ± 1.1 a | 32.5 ± 3.1 a | 26.4 ± 6.1 a | 34.9 ± 2.9 a | 32.4 ± 3.8 a |
| 1-Octen-3-ol | 6.8 ± 0.6 a | 7.6 ± 0.1 a | 6.9 ± 0.3 a | 8.0 ± 0.56 a | 9.4 ± 3.8 a | 13.6 ± 0.1 a | 13.9 ± 0.5 a | 13.2 ± 8.1 a |
| Sulcatol | TR ** | TR | TR | TR | TR | TR | TR | TR |
| 2-Ethyl-1-hexanol | 12.2 ± 0.2 f | 13.0 ± 0.4 ef | 16.2 ± 1.2 cd | 15.4 ± 0.3 de | 19.4 ± 1.4 b | 18.4 ± 0.2 bc | 75.5 ± 0.5 a | 76.0 ± 0.2 a |
| [R-(R*,R*)]-2,3-Butanediol | 375898.6 ± 82505.1 a | 363649.8 ± 241292.74 a | 348050.8 ± 196950.2 a | 279674.0 ± 61987.2 a | 1766887.6 ± 1645173.9 a | 979257.2 ± 660565.6 a | 1313477.4 ± 699634.8 a | 1735789.3 ± 233651.6 a |
| 1-Octanol | 10.9 ± 2.9 b | 11.1 ± 2.0 b | 9.3 ± 2.1 b | 10.2 ± 1.2 b | 12.3 ± 0.8 b | 11.8 ± 0.5 b | 31.9 ± 0.7 a | 33.1 ± 0.5 a |
| [S-(R*,R*)]-2,3-Butanediol | 117488.4 ± 16482.5 a | 123910.7 ± 67104.1 a | 124577.5 ± 48004.6 a | 86202.4 ± 13306.7 a | 305149.2 ± 252625.20 a | 190949.9 ± 107619.4 a | 205124.8 ± 82211.3 a | 338514.7 ± 20924.2 a |
| trans-2-Octenol | 9.5 ± 0.7 b | 10.3 ± 0.4 b | 9.5 ± 0.3 b | 10.0 ± 1.21 b | 5.3 ± 0.4 a | 5.0 ± 0.1 a | 4.6 ± 0.0 a | 5.9 ± 0.5 a |
| 1-Nonanol | 0.1 ± 0.0 c | 0.1 ± 0.0 c | 0.1 ± 0.0 c | 0.1 ± 0.0 c | 0.2 ± 0.0 b | 0.2 ± 0.0 bc | 0.3 ± 0.0 a | 0.3 ± 0.0 a |
| Methionol | 1316.9 ± 37.0 a | 1162.2 ± 63.3 a | 1276.4 ± 540.2 a | 1280.3 ± 143.6 a | 775.5 ± 483.1 a | 544.1 ± 340.8 a | 816.2 ± 254.5 a | 770.5 ± 168.4 a |
| E-5-Decen-1-ol | ND *** | ND | ND | ND | 8.9 ± 1.7 b | 7.7± 0.8 b | 61.7 ± 1.2 a | 58.8 ± 1.1 a |
| Phenylethyl Alcohol | 20978.7 ± 1150.9 a | 20111.8 ± 5434.5 a | 19807.4 ± 7958.7 a | 20117.5 ± 489.0 a | 15526.1 ± 8671.7 a | 11607.3 ± 4687.1 a | 14267.9 ± 1231.0 a | 13133.9 ± 2106.6 a |
| Total | 740054.1 ± 98959.9 a | 730092.8 ± 320914.5 a | 719960.1 ± 246869.2 a | 607507.9 ± 77776.8 a | 2330406.2 ± 1915574.3 a | 1422220.4 ± 773718.3 a | 1728818.3 ± 781475.9 a | 2282631.4 ± 257372.4 a |
| Aldehyde/Ketones | ||||||||
| Nonanal | 14.2 ± 0.3 a | 15.7 ± 1.2 a | 10.7 ± 0.7 a | 12.8 ± 0.0 a | 5.8 ± 0.3 a | 5.1 ± 0.3 a | 4.5 ± 0.3 a | 4.5 ± 0.9 a |
| Furfural | 492.2 ± 0.8 a | 484.6 ± 32.0 a | 436.9 ± 38.4 a | 465.3 ± 4.0 a | 11.7 ± 2.9 b | 11.7 ± 3.3 b | 12.8 ± 1.6 b | 11.8 ± 0.3 b |
| Decanal | 3.7 ± 0.7 a | 4.9 ± 1.0 a | 2.5 ± 0.0 a | 4.5 ± 0.6 a | 4.0 ± 0.90 a | 2.4 ± 0.3 a | 2.4 ± 0.6 a | 3.7 ± 1.2 a |
| Safranal | 2.3 ± 0.0 a | 2.4 ± 0.2 a | 2.2 ± 0.1 a | 2.2 ± 0.1 a | 0.5 ± 0.0 b | 0.5 ± 0.0 b | 0.6 ± 0.0 b | 0.6 ± 0.0 b |
| 2-Butyl-2-octenal | 16.7 ± 1.3 a | 17.1 ± 3.1 a | 17.4 ± 3.4 a | 18.1 ± 0.4 a | 11.9 ± 2.4 a | 10.6 ± 1.4 a | 11.0 ± 1.1 a | 10.9 ± 1.3 a |
| Isobutyl ketone | 22.0 ± 5.0 ab | 24.0 ± 8.0 ab | 7.58 ± 5.93 ab | 33.1 ± 13.2 a | TR | 2.48 ± 0.37 b | TR | 0.09 ± 0.53 b |
| TMCHN | TR | TR | TR | TR | TR | TR | TR | TR |
| 6M5H2NE | 11.0 ± 1.15 a | 11.9 ± 4.1 a | 8.2 ± 1.1 a | 8.0 ± 0.7 a | TR | TR | TR | TR |
| 2-Undecanone | 1.9 ± 0.0 b | 2.1 ± 0.1 b | 2.0 ± 0.1 a | 2.0 ± 0.07 a | 3.5 ± 0.5 c | 3.2 ± 0.1 c | 3.6 ± 0.0 d | 3.9 ± 0.2 cd |
| β-Cyclocitral | 6.5 ± 0.1 a | 6.6 ± 0.4 a | 5.6 ± 0.0 b | 5.6 ± 0.1 b | 3.0 ± 0.2 c | 3.4 ± 0.3 c | 5.3 ± 0.1 b | 4.9 ± 0.1 b |
| Dihydropseudoionone | 4.4 ± 0.4 ab | 4.5 ± 0.1 ab | 5.5 ± 2.3 a | 3.7 ± 0.1 abc | 1.3 ± 0.3 cd | 0.9 ± 0.1 c | 2.7 ± 0.0 abc | 2.8 ± 0.1 abc |
| 2-Acetylpyrrole | 121.6 ± 8.1 a | 116.16 ± 43.2 a | 114.3 ± 53.6 a | 111.7 ± 0.5 a | ND | ND | ND | ND |
| TMHAAL | 6.8 ± 0.34 a | 6.5 ± 1.6 a | 7.1 ± 1.6 a | 6.0 ± 0.45 a | 0.9 ± 0.1 b | 1.6 ± 0.5 b | 1.4 ± 0.1 b | 1.1 ± 0.1 b |
| Total | 702.1 ± 13.8 a | 696.3 ± 70.2 a | 618.8 ± 100.2 b | 671.7 ± 8.3 ab | 37.4 ± 7.0 c | 37.4 ± 6.0 c | 42.3 ± 0.0 c | 43.4 ± 1.9 c |
| Benzene | ||||||||
| Isodurene | 4.3 ± 0.1 a | 4.1 ± 0.2 a | 4.6 ± 0.2 a | 4.6 ± 0.4 a | 1.3 ± 0.0 b | 1.3 ± 0.1 b | 1.0 ± 0.1 b | 0.9 ± 0.0 b |
| TEMLNE | 7.2 ± 0.1 a | 5.8 ± 1.4 abc | 5.8 ± 2.1 abc | 6.9 ± 0.7 ab | 2.8 ± 0.6 c | 2.2 ± 0.2 c | 3.8 ± 0.1 abc | 3.1 ± 0.7 bc |
| DPPXL | 31.1 ± 0.9 a | 29.2 ± 1.3 ab | 24.3 ± 7.7 abc | 28.4 ± 2.0 ab | 18.4 ± 1.5 bc | 18.2 ± 0.1 bc | 16.7 ± 0.6 c | 16.5 ± 0.6 c |
| Naphthalene | 4.5 ± 0.2 a | 5.3 ± 0.4 a | 4.5 ± 0.3 a | 4.7 ± 0.1 a | 2.2 ± 0.3 b | 2.0 ± 0.1 b | 2.3 ± 0.0 b | 2.3 ± 0.0 b |
| TMDHPE | 6.2 ± 0.2 a | 5.9 ± 0.1 a | 5.3 ± 1.1 a | 5.8 ± 0.5 a | 1.9 ± 0.2 b | 1.6 ± 0.0 b | 2.4 ± 0.2 b | 2.4 ± 0.3 b |
| M2PB | 1.5 ± 0.1 ab | 1.6 ± 0.0 ab | 1.5 ± 0.1 ab | 1.3 ± 0.0 b | 1.9 ± 0.3 a | 1.7 ± 0.0 ab | 1.7 ± 0.0 ab | 1.7 ± 0.1 ab |
| Phenethyl acetate | 144.7 ± 10.9 a | 141.0 ± 17.4 a | 114.9 ± 15.3 a | 122.2 ± 1.0 a | 43.6 ± 7.4 b | 37.5 ± 2.1 b | 28.1 ± 0.7 b | 26.4 ± 0.2 b |
| β-Methylnaphthalene | 17.2 ± 0.4 a | 16.0 ± 1.6 a | 16.0 ± 1.8 a | 17.0 ± 0.44 a | 5.6 ± 0.7 b | 5.4 ± 0.1 b | 4.7 ± 0.1 b | 4.7 ± 0.1 b |
| Benzyl alcohol | 308.3 ± 2.0 a | 297.9 ± 60.4 a | 305.6 ± 111.7 a | 314.6 ± 7.7 a | 314.5 ± 140.8 a | 289.1 ± 75.4 a | 527.1 ± 23.0 a | 514.1 ± 80.8 a |
| Ethyl dihydrocinnamate | 1.2 ± 0.0 b | 1.3 ± 0.1 b | 1.2 ± 0.0 b | 1.3 ± 0.0 b | 3.2 ± 0.6 a | 2.7 ± 0.2 a | 3.2 ± 0.1 a | 3.1 ± 0.1 a |
| α-Methylnaphthalene | 11.4 ± 0.2 a | 10.8 ± 0.7 a | 11.1 ± 1.1 a | 11.5 ± 0.3 a | 2.7 ± 0.5 b | 2.1 ± 0.1 b | 2.0 ± 0.1 b | 1.9 ± 0.1 b |
| 4-Tolylcarbinol | 1.4 ± 0.1 a | 1.3 ± 0.3 a | 1.4 ± 0.1 a | 1.4 ± 0.1 a | 0.9 ± 0.9 | 0.5 ± 0.1 | 1.0 ± 0.06 | 1.0 ± 0.0 |
| Ethyl cinnamate | 1.9 ± 0.2 a | 2.5 ± 0.5 a | 2.4 ± 0.4 a | 2.9 ± 0.1 a | 2.1 ± 0.4 a | 2.5 ± 0.3 a | 1.8 ± 0.0 a | 2.2 ± 0.1 a |
| 3P4HP | 1.9 ± 0.0 a | 1.9 ± 0.1 a | 1.1 ± 0.1 b | 1.2 ± 0.1 b | ND | ND | ND | ND |
| DTBPHm | 41.5 ± 5.0 a | 46.0 ± 0.9 a | 44.0 ± 0.7 a | 47.0 ± 1.0 a | 49.2 ± 9.7 a | 38.7 ± 4.8 a | 39.2 ± 2.6 a | 40.8 ± 1.3 a |
| Coumaran | 202.5 ± 4.54 a | 196.6 ± 43.0 a | 240.6 ± 66.7 a | 219.0 ± 3.9 a | 1096.6 ± 651.1 a | 622.4 ± 312.0 a | 970.0 ± 132.30 a | 981.9 ± 22.0 a |
| Ethyl salicylate | 6.1 ± 0.97 b | 11.8 ± 3.6 a | 4.3 ± 0.3 b | 4.8 ± 0.3 b | 5.8 ± 0.7 b | 5.3 ± 0.3 b | 5.3 ± 0.1 b | 5.6 ± 0.0 b |
| 4-Ethylphenol | 1466.5 ± 3.1 b | 1480.8 ± 5.3 b | 1482.8 ± 14.6 b | 1464.6 ± 0.9 b | 8167.3 ± 3706.2 a | 6012.7 ± 1923.9 ab | 11677.1 ± 140.4 a | 9968.4 ± 1214.6 a |
| 4-Vinylguaiacol | 329.1 ± 9.4 a | 310.5 ± 56.6 a | 372.2 ± 93.1 a | 371.6 ± 4.6 a | 791.7 ± 394.5 a | 548.7 ± 211.3 a | 656.6 ± 15.3 a | 617.2 ± 60.1 a |
| Total | 2588.3 ± 23.0 b | 2570.2 ± 185.4 b | 2643.6 ± 285.7 b | 2630.6 ± 23.2 b | 10511.8 ± 4916.4 ab | 7594.7 ± 2530.2 ab | 13943.7 ± 312.4 a | 12194.2 ± 1375.9 a |
| Esters | ||||||||
| Ethyl Acetate | 74687.2 ± 1416.4 a | 78072.4 ± 2474.7 a | 75663.3 ± 6509.8 a | 72540.3 ± 646.6 a | 47864.3 ± 1550.9 b | 49750.7 ± 3003.3 b | 52201.6 ± 1608.2 b | 57245.4 ± 1761.9 b |
| Ethyl butanoate | 306.4 ± 15.3 a | 315.7 ± 17.8 a | 306.2 ± 23.2 a | 294.7 ± 11.6 a | 87.4 ± 0.1 b | 93.1 ± 7.2 b | 39.8 ± 0.7 b | 42.4 ± 0.7 b |
| Ethyl hexanoate | 395.7 ± 21.7 a | 402.1 ± 20.1 a | 378.9 ± 12.10 a | 366.3 ± 15.6 a | 147.0 ± 0.8 bc | 154.5 ± 14.4 b | 95.2 ± 3.4 c | 99.4 ± 3.0 c |
| Ethyl 3-hexenoate | 2.4 ± 0.2 d | 3.0 ± 0.4 d | 1.6 ± 1.1 d | 2.6 ± 0.0 d | 8.5 ± 0.0 ab | 9.1 ± 0.3 a | 6.8 ± 0.3 c | 7.0 ± 0.2 bc |
| Ethyl enanthate | 3.1 ± 0.1 a | 3.1 ± 0.1 a | 3.0 ± 0.0 a | 2.8 ± 0.1 a | 1.6 ± 0.0 b | 1.6 ± 0.1 b | 1.5 ± 0.1 b | 1.5 ± 0.1 b |
| Ethyl lactate | 3460.5 ± 645.5 a | 3190.9 ± 907.9 a | 3265.6 ± 604.4 a | 3387.4 ± 131.4 a | 4251.8 ± 507.7 a | 4276.0 ± 520.7 a | 4818.9 ± 994.4 a | 4173.8 ± 365.8 a |
| Ethyl octanoate | 931.8 ± 18.3 ab | 955.1 ± 6.3 a | 858.0 ± 39.6 b | 898.5 ± 43.4 ab | 506.8 ± 18.8 c | 527.3 ± 2.3 c | 370.5 ± 1.0 d | 403.9 ± 4.5 d |
| Ethyl mesitylacetate | 1.1 ± 0.1 a | 1.0 ± 0.0 a | 1.0 ± 0.0 a | 1.0 ± 0.0 a | 0.5 ± 0.0 b | 0.5 ± 0.1 b | 0.4 ± 0.0 b | 0.4 ± 0.0 b |
| Ethyl nonanoate | 4.0 ± 0.2 a | 4.3 ± 0.1 a | 4.2 ± 0.7 a | 3.7 ± 1.1 a | 7.5 ± 0.6 a | 7.9 ± 2.4 a | 7.0 ± 0.6 a | 6.1 ± 1.5 a |
| E2H4MP | 16.0 ± 0.59 c | 16.1 ± 0.8 c | 18.3 ± 2.1 bc | 18.1 ± 0.2 bc | 27.4 ± 2.1 a | 25.0 ± 2.3 a | 24.6 ± 1.3 a | 24.0 ± 1.3 ab |
| Ethyl caprate | 738.6 ± 0.1 b | 746.7 ± 14.1 b | 888.1 ± 54.1 a | 922.8 ± 46.2 a | 620.0 ± 58.9 bc | 601.1 ± 4.4 c | 426.7 ± 4.2 d | 462.0 ± 1.6 d |
| Ethyl benzoate | 1.7 ± 0.1 a | 1.8 ± 0.1 a | 1.7 ± 0.1 a | 1.8 ± 0.1 a | 1.7 ± 0.2 a | 1.7 ± 0.1 a | 1.5 ± 0.1 a | 1.6 ± 0.0 a |
| Ethyl 9-decenoate | 17.1 ± 1.1 a | 18.4 ± 1.7 a | 16.2 ± 2.3 a | 18.9 ± 1.3 a | 3.9 ± 0.0 b | 3.5 ± 0.0 b | 3.4 ± 0.1 b | 3.5 ± 0.0 b |
| Ethyl laurate | 135.3 ± 2.6 bcd | 137.3 ± 4.6 abcd | 173.2 ± 13.4 bc | 182.6 ± 11.6 a | 153.5 ± 26.7 abc | 119.4 ± 2.7 cd | 96.8 ± 0.0 d | 99.3 ± 2.9 d |
| Ethyl myristate | 24.0 ± 0.5 a | 23.3 ± 5.7 a | 22.9 ± 4.2 a | 27.5 ± 1.1 a | 22.3 ± 3.9 a | 20.8 ± 1.6 a | 19.4 ± 2.1 a | 24.8 ± 3.9 a |
| Ethyl hexadecanoate | 38.7 ± 1.0 bc | 33.6 ± 4.8 c | 39.9 ± 1.1 bc | 40.0 ± 2.2 bc | 60.9 ± 11.8 a | 54.9 ± 5.3 ab | 61.4 ± 3.3 a | 70.4 ± 1.0 a |
| Isobutyl acetate | 206.8 ± 13.2 a | 216.3 ± 17.0 a | 158.9 ± 14.6 b | 154.6 ± 0.4 b | 61.8 ± 8.4 c | 68.0 ± 12.2 c | 17.2 ± 2.2 d | 33.4 ± 6.7 cd |
| Isopentyl acetate | 4878.4 ± 338.0 a | 5027.3 ± 383.3 a | 3790.5 ± 325.2 b | 3603.7 ± 160.7 b | 2382.1 ± 5.8 c | 2515.1 ± 158.2 c | 1110.7 ± 11.2 d | 1182.4 ± 48.9 d |
| Hexyl acetate | 9.10 ± 0.5 a | 9.3 ± 0.3 a | 6.8 ± 0.4 b | 6.5 ± 0.1 b | 1.3 ± 0.1 cd | 2.3 ± 0.0 c | 1.2 ± 0.7 cd | 0.7 ± 0.5 d |
| Heptyl acetate | 0.5 ± 0.3 a | 0.4 ± 0.2 a | 0.3 ± 0.1 a | 0.2 ± 0.0 a | 0.2 ± 0.1 a | 0.1 ± 0.1 a | 0.1 ± 0.2 a | 0.2 ± 0.1 a |
| 2-Ethyl-1-hexanol acetate | 13.1 ± 1.3 a | 13.2 ± 0.5 a | 13.0 ± 1.3 a | 8.1 ± 6.8 a | 8.6 ± 0.3 a | 8.3 ± 0.4 a | 9.2 ± 0.2 a | 9.9 ± 0.7 a |
| Octyl acetate | 7.5 ± 0.5 ab | 7.8 ± 0.4 ab | 7.2 ± 0.9 ab | 8.3 ± 0.9 a | 4.5 ± 1.2 b | 5.7 ± 0.3 ab | 5.4 ± 1.6 ab | 5.3 ± 0.6 ab |
| Trimethylene acetate | 129.3 ± 33.4 a | 135.0 ± 39.4 a | 122.4 ± 33.8 a | 105.1 ± 14.0 a | 435.3 ± 324.9 a | 243.1 ± 123.2 a | 265.8 ± 116.0 a | 381.8 ± 9.4 a |
| Isobutyl hexanoate | 0.6 ± 0.2 ab | 0.5 ± 0.0 a | 0.4 ± 0.0 c | 0.4 ± 0.0 bc | ND | ND | ND | ND |
| Methyl octanoate | 5.2 ± 0.1 a | 5.5 ± 0.1 a | 5.1 ± 0.2 a | 3.4 ± 2.4 a | 2.6 ± 0.1 a | 2.7 ± 0.0 a | 2.3 ± 0.0 a | 2.4 ± 0.0 a |
| Isopentyl hexanoate | 7.0 ± 0.1 a | 7.4 ± 0.3 a | 6.4 ± 0.6 a | 7.0 ± 0.4 a | 2.3 ± 0.0 b | 2.3 ± 0.1 b | 1.5 ± 0.0 b | 1.7 ± 0.0 b |
| Isobutyl octanoate | 2.7 ± 0.5 a | 2.6 ± 0.6 a | 2.3 ± 0.4 a | 2.4 ± 0.4 a | 3.0 ± 0.4 a | 1.6 ± 0.1 a | 2.8 ± 1.9 a | 4.3 ± 0.1 a |
| Methyl caprate | 12.2 ± 0.2 a | 12.1 ± 0.1 a | 18.3 ± 1.4 a | 18.9 ± 1.2 a | 6.8 ± 1.1 a | 6.7 ± 0.1 a | 3.4 ± 0.1 a | 4.5 ± 0.3 a |
| Isoamyl octanoate | 9.4 ± 0.3 ab | 9.7 ± 0.4 a | 7.7 ± 0.7 b | 8.3 ± 0.8 ab | 4.5 ± 0.2 c | 4.6 ± 0.0 c | 3.7 ± 0.1 c | 4.2 ± 0.1 c |
| Diethyl succinate | 125.6 ± 2.5 b | 126.4 ± 13.2 b | 216.0 ± 45.4 a | 227.6 ± 5.7 a | 100.5 ± 30.0 | 87.5 ± 13.5 b | 108.4 ± 3.5 b | 92.0 ± 9.5 b |
| Isoamyl decanoate | 4.6 ± 0.1 a | 4.6 ± 0.4 a | 5.2 ± 0.0 a | 5.5 ± 0.4 a | 3.2 ± 0.3 b | 2.9 ± 0.0 bc | 2.0 ± 0.0 d | 2.2 ± 0.0 cd |
| IMDMMPo | 19.7 ± 1.5 a | 19.4 ± 1.3 a | 12.5 ± 1.0 b | 13.3 ± 0.4 b | 3.1 ± 0.7 c | 2.5 ± 0.9 c | 2.0 ± 0.3 c | 2.3 ± 0.8 c |
| NA3MBE | 5.2 ± 0.1 a | 5.0 ± 0.2 a | 5.0 ± 0.4 a | 5.3 ± 0.2 a | 2.1 ± 0.2 b | 1.9 ± 0.0 b | 1.9 ± 0.0 b | 1.8 ± 0.0 b |
| Total | 86200.3 ± 1208.5 a | 89527.3 ± 1927.2 a | 86020.1 ± 6080.8 a | 82887.4 ± 822.9 a | 56786.7 ± 2501.9 b | 58602.3 ± 2531.9 b | 59713.0 ± 716.2 b | 64394.2 ± 1452.0 b |
| Others | ||||||||
| Styrene | 97.1 ± 1.7 ab | 97.8 ± 2.1 ab | 83.3 ± 3.5 b | 86.5 ± 2.7 b | 93.5 ± 3.5 ab | 105.7 ± 7.5 a | 65.5 ± 4.2 c | 67.3 ± 1.9 c |
| α-Ionene | 7.8 ± 0.2 a | 6.9 ± 0.2 a | 6.4 ± 2.2 a | 7.2 ± 0.7 a | 2.6 ± 0.4 a | 2.2 ± 0.1 a | 3.8 ± 0.2 a | 3.8 ± 0.3 a |
| ODETMq | 2.7 ± 0.1 c | 2.8 ± 0.1 c | 2.6 ± 0.0 cd | 2.7 ± 0.2 c | 6.0 ± 0.2 b | 6.7 ± 0.0 ab | 6.6 ± 0.1 b | 7.5 ± 0.5 a |
| α-Cedrene | 2.1 ± 0.0 a | 2.0 ± 0.1 a | 1.2 ± 0.2 a | 1.4 ± 0.2 a | 0.2 ± 0.0 b | 0.1 ± 0.0 b | 0.1 ± 0.0 b | 0.1 ± 0.0 b |
| α-Calacorene | 6.2 ± 0.1 a | 5.8 ± 0.2 ab | 5.2 ± 1.4 ab | 5.9 ± 0.5 ab | 4.4 ± 0.6 ab | 3.9 ± 0.0 b | 4.0 ± 0.0 ab | 3.9 ± 0.2 b |
| β-Ionone | 7.4 ± 0.8 a | 7.3 ± 0.8 a | 6.5 ± 0.4 a | 6.8 ± 0.2 a | 1.7 ± 0.3 c | 1.8 ± 0.2 c | 4.5 ± 0.0 b | 4.1 ± 0.0 b |
| DMUDL | 0.4 ± 0.1 ab | 0.4 ± 0.1 ab | 0.5 ± 0.0 a | 0.5 ± 0.0 a | 0.2 ± 0.1 c | 0.2 ± 0.0 c | 0.3 ± 0.0 bc | 0.3 ± 0.0 bc |
| Cedrol | 0.5 ± 0.1 a | 0.4 ± 0.0 a | 0.3 ± 0.0 b | 0.3 ± 0.0 b | ND | ND | ND | ND |
| Total | 124.1 ± 1.0 a | 123.5 ± 0.8 a | 106.0 ± 7.77 a | 111.2 ± 4.5 a | 108.7 ± 1.9 a | 120.6 ± 7.2 a | 84.8 ± 4.0 b | 86.9 ± 2.8 b |
| Volatile | T-test between DW and FW | Dried Goji Berry Fermented Wine | Fresh Goji Berry Fermented Wine | ||||
|---|---|---|---|---|---|---|---|
| p Value | p Value | ||||||
| Pressing | Glycosidase | Interaction | Pressing | Glycosidase | Interaction | ||
| Volatile acids | |||||||
| DMECA | 16.35 *** | 0.639 | 0.316 | 0.584 | 0.758 | 0.386 | 0.427 |
| Acetic acid | 0.33 *** | 0.582 | 0.527 | 0.890 | 0.145 | 0.341 | 0.706 |
| Butanoic acid | 3.70 *** | 0.990 | 0.994 | 0.924 | 0.710 | 0.624 | 0.651 |
| α-Methylbutyric acid | 3.47 *** | 0.599 | 0.537 | 0.66 | 0.567 | 0.367 | 0.489 |
| Octanoic acid | 1.51 ** | 0.626 | 0.225 | 0.784 | 0.559 | 0.506 | 0.732 |
| Decanoic acid | 0.98 | 0.043 * | 0.100 | 0.223 | 0.047 * | 0.663 | 0.505 |
| Total | 1.37 * | 0.052 | 0.097 | 0.313 | 0.107 | 0.546 | 0.687 |
| Higher alcohols | |||||||
| 2-Methyl-1-propanol | 1.63 *** | 0.648 | 0.498 | 0.789 | 0.001 ** | 0.934 | 0.829 |
| Isopentyl alcohol | 0.96 | 1.000 | 0.273 | 0.720 | 0.0001 *** | 0.619 | 0.765 |
| Pentyl alcohol | 0.59 *** | 0.278 | 0.598 | 0.164 | 0.014 * | 0.565 | 0.350 |
| 1-Hexanol | 0.71 * | 0.362 | 0.487 | 0.235 | 0.000 *** | 0.007 ** | 0.673 |
| 2-Heptanol | 0.81 *** | 0.014 * | 0.707 | 0.608 | 0.314 | 0.317 | 0.029 * |
| 1-Octen-3-ol | 0.58 ** | 0.478 | 0.042 * | 0.777 | 0.557 | 0.617 | 0.475 |
| 2-Ethyl-1-hexanol | 0.30 ** | 0.002 ** | 0.949 | 0.146 | 0.000 *** | 0.633 | 0.261 |
| [R-(R*,R*)]-2,3-Butanediol | 0.24 ** | 0.655 | 0.746 | 0.821 | 0.001 *** | 0.801 | 0.423 |
| 1-Octanol | 0.46 ** | 0.458 | 0.754 | 0.832 | 0.000 *** | 0.408 | 0.125 |
| [S-(R*,R*)]-2,3-Butanediol | 0.43 ** | 0.638 | 0.624 | 0.498 | 0.827 | 0.929 | 0.290 |
| trans-2-Octenol | 0.56 *** | 0.543 | 0.240 | 0.481 | 0.085 | 0.879 | 0.227 |
| 1-Nonanol | 0.43 *** | 0.010 ** | 0.000 *** | 0.040 * | 0.005 ** | 0.300 | 0.278 |
| Methionol | 1.73 *** | 0.855 | 0.724 | 0.711 | 0.601 | 0.587 | 0.713 |
| (E)-5-Decen-1-ol | 0.00 ** | 0 | 0 | 0 | 0.000 *** | 0.077 | 0.384 |
| Phenylethyl Alcohol | 1.49 ** | 0.874 | 0.939 | 0.872 | 0.972 | 0.52 | 0.718 |
| Total | 0.36 ** | 0.659 | 0.704 | 0.750 | 0.877 | 0.833 | 0.405 |
| Aldehyde/Ketones | |||||||
| Furfural | 39.11 *** | 0.103 | 0.59 | 0.369 | 0.733 | 0.782 | 0.801 |
| Decanal | 1.26 | 0.176 | 0.029 ** | 0.453 | 0.859 | 0.795 | 0.070 |
| Safranal | 3.85 *** | 0.111 | 0.728 | 0.810 | 0.000 *** | 0.176 | 0.270 |
| 2-Butyl-2-octenal | 1.25 *** | 0.649 | 0.761 | 0.963 | 0.811 | 0.598 | 0.639 |
| Isobutyl ketone | 16.21 ** | 0.682 | 0.088 | 0.126 | 0.003 ** | 0.003 ** | 0.378 |
| TMCHN | 0.00 * | 0 | 0 | 0 | 0.002 ** | 0.463 | 0.463 |
| 6M5H2NE | *** | 0.102 | 0.821 | 0.745 | 0 | 0 | 0 |
| 2-Undecanone | 2.86 *** | 0.001 *** | 0.774 | 0.598 | 0.002 ** | 0.281 | 0.222 |
| β-Cyclocitral | 1.46 *** | 0.003 ** | 0.977 | 0.855 | 0.000 *** | 0.836 | 0.055 |
| Dihydropseudoionone | 2.38 *** | 0.911 | 0.342 | 0.308 | 0.000 *** | 0.260 | 0.167 |
| 2-Acetylpyrrole | *** | 0.822 | 0.878 | 0.956 | 0 | 0 | 0 |
| TMHAAL | 5.22 *** | 0.916 | 0.426 | 0.619 | 0.851 | 0.396 | 0.043 * |
| Total | 16.03 *** | 0.065 | 0.191 | 0.182 | 0.879 | 0.812 | 0.259 |
| Benzene | |||||||
| Isodurene | 3.86 *** | 0.125 | 0.555 | 0.568 | 0.001 ** | 0.221 | 0.820 |
| TEMLNE | 2.16 *** | 0.858 | 0.839 | 0.238 | 0.052 | 0.158 | 0.896 |
| DPPXL | 1.62 *** | 0.258 | 0.711 | 0.352 | 0.044 * | 0.776 | 0.950 |
| Naphthalene | 2.20 *** | 0.193 | 0.063 | 0.154 | 0.137 | 0.416 | 0.439 |
| TMDHPE | 2.78 *** | 0.355 | 0.851 | 0.417 | 0.007 ** | 0.341 | 0.553 |
| M2PB | 0.83 *** | 0.019 ** | 0.842 | 0.024 ** | 0.629 | 0.522 | 0.420 |
| Phenethyl acetate | 3.86 *** | 0.055 | 0.857 | 0.577 | 0.008 ** | 0.228 | 0.461 |
| β-Methylnaphthalene | 3.25 *** | 0.882 | 0.903 | 0.269 | 0.029 * | 0.583 | 0.619 |
| Benzyl alcohol | 0.75 | 0.884 | 0.989 | 0.840 | 0.027 * | 0.778 | 0.926 |
| Ethyl dihydrocinnamate | 0.41 *** | 0.677 | 0.600 | 0.821 | 0.477 | 0.223 | 0.498 |
| α-Methylnaphthalene | 5.12 *** | 0.753 | 0.858 | 0.376 | 0.097 | 0.179 | 0.286 |
| 4-Tolylcarbinol | 1.59 ** | 0.650 | 0.549 | 0.884 | 0.441 | 0.580 | 0.504 |
| 3P4HP | *** | 0.001 ** | 0.179 | 0.498 | 0 | 0 | 0 |
| Coumaran | 0.23 *** | 0.342 | 0.651 | 0.793 | 0.677 | 0.424 | 0.402 |
| 4-Ethylphenol | 0.16 *** | 0.996 | 0.745 | 0.044 * | 0.072 | 0.278 | 0.892 |
| 4-Vinylguaiacol | 0.53 *** | 0.249 | 0.816 | 0.828 | 0.845 | 0.427 | 0.558 |
| Total | 0.25 *** | 0.738 | 0.900 | 0.980 | 0.117 | 0.312 | 0.787 |
| Esters | |||||||
| Ethyl Acetate | 1.45 *** | 0.418 | 0.961 | 0.267 | 0.016 * | 0.077 | 0.341 |
| Ethyl butanoate | 5.41 *** | 0.438 | 0.935 | 0.445 | 0.167 | 0.290 | 0.346 |
| Ethyl hexanoate | 3.11 *** | 0.104 | 0.818 | 0.494 | 0.001 *** | 0.335 | 0.768 |
| Ethyl 3-hexenoate | 0.31 *** | 0.196 | 0.133 | 0.654 | 0.000 ** | 0.059 | 0.330 |
| Ethyl enanthate | 1.94 *** | 0.058 | 0.156 | 0.148 | 0.013 ** | 0.772 | 0.402 |
| Ethyl lactate | 0.76 ** | 0.999 | 0.878 | 0.686 | 0.636 | 0.532 | 0.502 |
| Ethyl octanoate | 2.01 *** | 0.041 * | 0.218 | 0.714 | 0.000 *** | 0.017 ** | 0.404 |
| Ethyl mesitylacetate | 2.42 *** | 0.229 | 0.444 | 0.038* | 0.089 | 0.773 | 0.788 |
| Ethyl nonanoate | 0.56 *** | 0.682 | 0.810 | 0.441 | 0.337 | 0.800 | 0.576 |
| E2H4MP | 0.68 *** | 0.057 | 0.980 | 0.832 | 0.209 | 0.299 | 0.516 |
| Ethyl caprate | 1.56 *** | 0.003 ** | 0.451 | 0.632 | 0.001 ** | 0.717 | 0.266 |
| Ethyl benzoate | 1.08 * | 0.432 | 0.183 | 0.859 | 0.037 * | 0.509 | 0.491 |
| Ethyl 9-decenoate | 4.92 *** | 0.907 | 0.170 | 0.586 | 0.009 ** | 0.024 * | 0.004 ** |
| Ethyl laurate | 1.34 ** | 0.003 ** | 0.433 | 0.605 | 0.016 * | 0.172 | 0.128 |
| Ethyl hexadecanoate | 0.61 *** | 0.123 | 0.266 | 0.247 | 0.165 | 0.764 | 0.190 |
| Isobutyl acetate | 4.09 *** | 0.004 ** | 0.790 | 0.497 | 0.002 ** | 0.126 | 0.439 |
| Isopentyl acetate | 2.41 *** | 0.005 ** | 0.936 | 0.491 | 0.000 *** | 0.156 | 0.629 |
| Hexyl acetate | 5.82 *** | 0.000 *** | 0.829 | 0.342 | 0.054 | 0.466 | 0.058 |
| Heptyl acetate | 2.31 * | 0.283 | 0.487 | 0.989 | 0.658 | 0.983 | 0.454 |
| 2-Ethyl-1-hexanol acetate | 1.32 * | 0.351 | 0.379 | 0.370 | 0.023 ** | 0.596 | 0.155 |
| Octyl acetate | 1.47 *** | 0.831 | 0.236 | 0.540 | 0.739 | 0.534 | 0.416 |
| Trimethylene acetate | 0.37 ** | 0.457 | 0.811 | 0.634 | 0.911 | 0.783 | 0.300 |
| Isobutyl hexanoate | 7.32 *** | 0.004 ** | 0.095 | 0.639 | 0.000 *** | 0.476 | 0.476 |
| Methyl octanoate | 1.94 ** | 0.256 | 0.439 | 0.327 | 0.000 *** | 0.033 ** | 0.940 |
| Isopentyl hexanoate | 3.60 *** | 0.149 | 0.162 | 0.722 | 0.000 *** | 0.085 | 0.028 ** |
| Methyl caprate | 0.81 | 0.000 *** | 0.106 | 0.253 | 0.688 | 0.144 | 0.346 |
| Isoamyl octanoate | 2.06 *** | 0.022 * | 0.348 | 0.765 | 0.003 | 0.022 | 0.076 |
| Diethyl succinate | 1.79 ** | 0.005 ** | 0.729 | 0.764 | 0.635 | 0.292 | 0.892 |
| Isoamyl decanoate | 1.95 *** | 0.027 ** | 0.469 | 0.55 | 0.001 ** | 0.950 | 0.143 |
| IMDMMP | 6.60 *** | 0.001 ** | 0.798 | 0.499 | 0.287 | 0.868 | 0.406 |
| NA3MBE | 2.67 *** | 0.756 | 0.954 | 0.255 | 0.059 | 0.262 | 0.456 |
| Total | 1.44 *** | 0.222 | 0.965 | 0.234 | 0.034 | 0.086 | 0.387 |
| Others | |||||||
| Styrene | 1.10 | 0.002 ** | 0.355 | 0.527 | 0.000 *** | 0.105 | 0.198 |
| α-Ionene | 2.48 *** | 0.783 | 0.169 | 0.701 | 0.078 | 0.212 | 0.988 |
| ODETM | 0.40 *** | 0.407 | 0.185 | 0.865 | 0.023 * | 0.012 * | 0.622 |
| α-Cedrene | 1.92 *** | 0.772 | 0.277 | 0.772 | 0.446 | 0.333 | 0.142 |
| α-Calacorene | 1.42 *** | 0.435 | 0.787 | 0.372 | 0.418 | 0.232 | 0.454 |
| β-Ionone | 2.32 *** | 0.158 | 0.788 | 0.666 | 0.000 *** | 0.135 | 0.131 |
| DMUDL | 1.85 *** | 0.024 * | 0.915 | 0.928 | 0.152 | 0.442 | 0.702 |
| Cedrol | *** | 0.002 ** | 0.808 | 0.654 | 0 | 0 | 0 |
| Total | 1.18 * | 0.011 * | 0.414 | 0.465 | 0.001 ** | 0.123 | 0.288 |
| Volatile | Threshold (μg/L) | Aroma Descriptor | Aroma Series | DF | DF-G | DP | DP-G | FF | FF-G | FP | FP-G |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Butanoic acid | 173 [45] | Butter, cheese, stinky [45] | 1 [46] | 8.7 a | 8.6 a | 8.6 a | 8.7 a | 2.4 b | 2.4 b | 2.4 b | 2.2 b |
| Octanoic acid | 500 [37] | Rancid, cheese, fatty acid [28] | 1 [28] | 2.0 a | 1.6 a | 2.1 a | 1.8 a | 1.5 a | 1.2 a | 1.2 a | 1.1 a |
| Decanoic acid | 1000 [37] | Fatty, rancid [37] | 1 [46] | 2.7 a | 2.2 a | 6.1 a | 3.3 a | 5.0 a | 4.2 a | 2.6 a | 2.8 a |
| Isopentyl alcohol | 30000 [47] | Solvent, sweety, alcohol, polish [38] | 1,2 [38] | 6.3 b | 6.2 b | 6.3 b | 6.1 b | 7.2 a | 7.2 a | 5.8 b | 5.8 b |
| [R-(R*,R*)]-2,3-Butanediol | 150000 [28] | Fruit, sweet, butter [28] | 1,2,3 [28] | 2.5 b | 2.4 b | 2.3 b | 1.9 b | 11.8 a | 6.5 a | 8.8 a | 11.6 a |
| [S-(R*,R*)]-2,3-Butanediol | 150000 [28] | Fruit, sweet, butter [28] | 1,2,3 [28] | 0.8 a | 0.8 a | 0.8 a | 0.6 a | 2.0 a | 1.3 a | 1.4 a | 2.3 a |
| Methionol | 1000 [28] | Cabbage, cooked potato, garlic [28] | 4 [28] | 1.3 a | 1.2 a | 1.3 a | 1.3 a | 0.8 a | 0.5 a | 0.8 a | 0.8 a |
| Phenylethyl Alcohol | 14000 [28] | Roses, honey [28] | 5 [28] | 1.5 a | 1.4 a | 1.4 a | 1.4 a | 1.1 a | 0.8 a | 1.0 a | 0.9 a |
| Ethyl cinnamate | 1.1 [47] | Flowery, balsamic [48] | 5 [46] | 1.7 a | 2.3 a | 2.2 a | 2.7 a | 1.9 a | 2.3 a | 1.6 a | 2.0 a |
| 4-Ethylphenol | 440 [28] | Phenolic, leather [28] | 6 [28] | 3.3 b | 3.4 b | 3.4 b | 3.3 b | 18.6 a | 13.7 ab | 26.5 a | 22.7 a |
| Ethyl Acetate | 5000 [8,49] | Spicy, pineapple, fruity [38] | 3 [46] | 6.2 a | 6.5 a | 6.3 a | 6.0 a | 4.0 b | 4.1 b | 4.4 b | 4.8 b |
| Ethyl hexanoate | 14 [47] | Banana, green, apple [28] | 3 [28] | 4.9 a | 5.0 a | 4.7 a | 4.6 a | 1.8 bc | 1.9 b | 1.2 c | 1.2 c |
| Ethyl octanoate | 5 [28] | Sweet, floral, fruity, banana, pear [28] | 3,5 [46] | 1.6 ab | 1.6 a | 1.5 b | 1.5 ab | 0.9 c | 0.9 c | 0.6 d | 0.7 d |
| Ethyl caprate | 200 [37] | Brandy, fruity, grape [37] | 3 [37] | 3.7 b | 3.7 b | 4.4 a | 4.6 a | 3.1 bc | 3.0 c | 2.1 d | 2.3 d |
| Isopentyl acetate | 30 [47] | Sweety, fruity [38] | 2,3 [46] | 30.5 a | 31.4 a | 23.7 b | 22.5 b | 14.9 c | 15.7 c | 6.9 d | 7.4 d |
| β-Ionone | 0.09 [47] | Violet [32] | 5 [46] | 92.6 a | 91.6 a | 80.7 a | 84.8 | 21.8 c | 21.8 c | 56.8 b | 50.6 b |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, G.; Ren, J.; Ouyang, X.; Wang, L.; Wang, M.; Shen, X.; Zhang, B.; Zhu, B. Effect of Raw Material, Pressing and Glycosidase on the Volatile Compound Composition of Wine Made From Goji Berries. Molecules 2016, 21, 1324. https://doi.org/10.3390/molecules21101324
Yuan G, Ren J, Ouyang X, Wang L, Wang M, Shen X, Zhang B, Zhu B. Effect of Raw Material, Pressing and Glycosidase on the Volatile Compound Composition of Wine Made From Goji Berries. Molecules. 2016; 21(10):1324. https://doi.org/10.3390/molecules21101324
Chicago/Turabian StyleYuan, Guanshen, Jie Ren, Xiaoyu Ouyang, Liying Wang, Mengze Wang, Xiaodong Shen, Bolin Zhang, and Baoqing Zhu. 2016. "Effect of Raw Material, Pressing and Glycosidase on the Volatile Compound Composition of Wine Made From Goji Berries" Molecules 21, no. 10: 1324. https://doi.org/10.3390/molecules21101324
APA StyleYuan, G., Ren, J., Ouyang, X., Wang, L., Wang, M., Shen, X., Zhang, B., & Zhu, B. (2016). Effect of Raw Material, Pressing and Glycosidase on the Volatile Compound Composition of Wine Made From Goji Berries. Molecules, 21(10), 1324. https://doi.org/10.3390/molecules21101324





