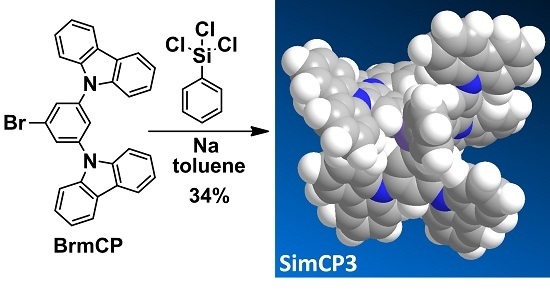
SimCP3—An Advanced Homologue of SimCP2 as a Solution-Processed Small Molecular Host Material for Blue Phosphorescence Organic Light-Emitting Diodes
Abstract
:1. Introduction
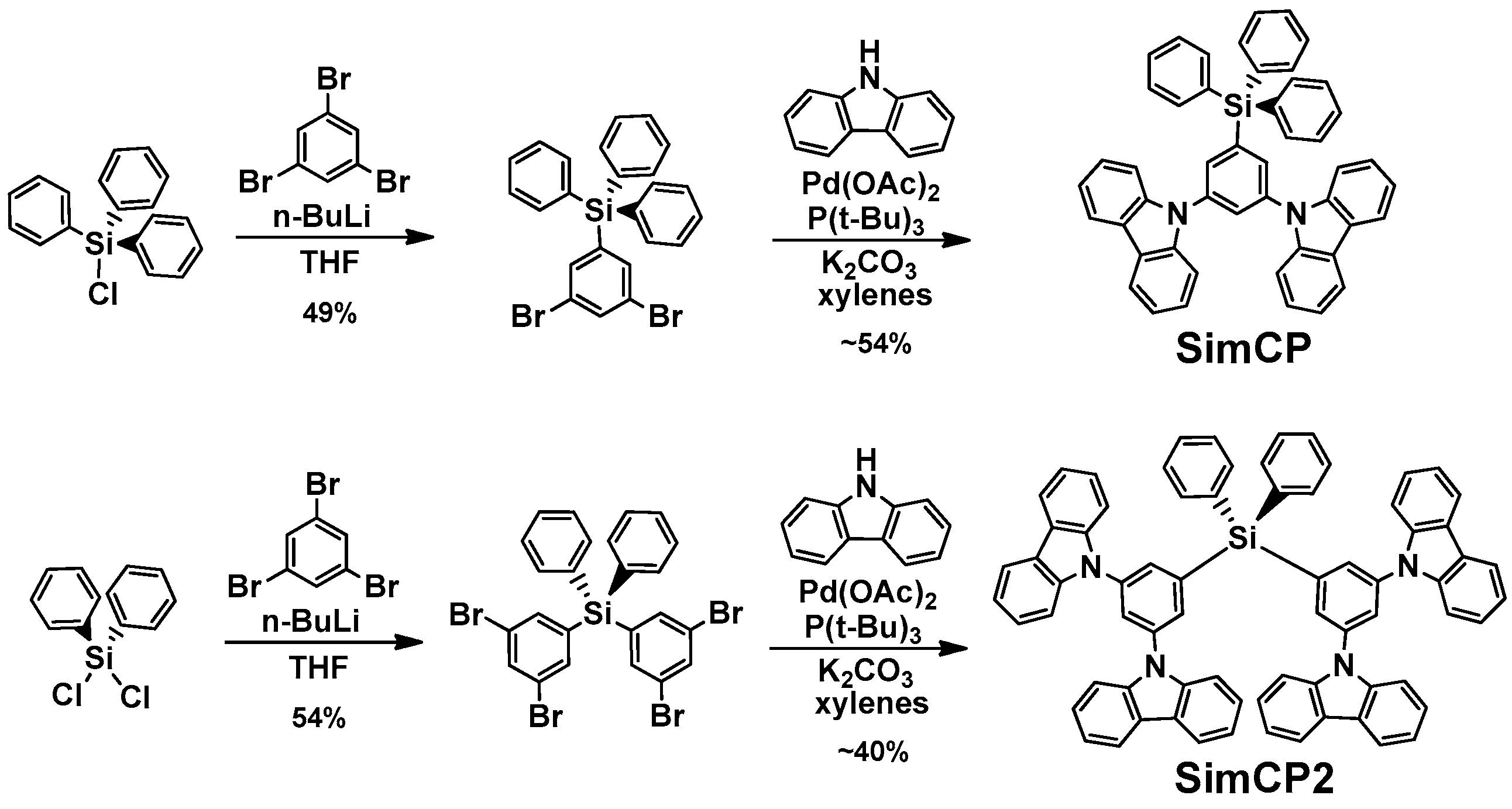
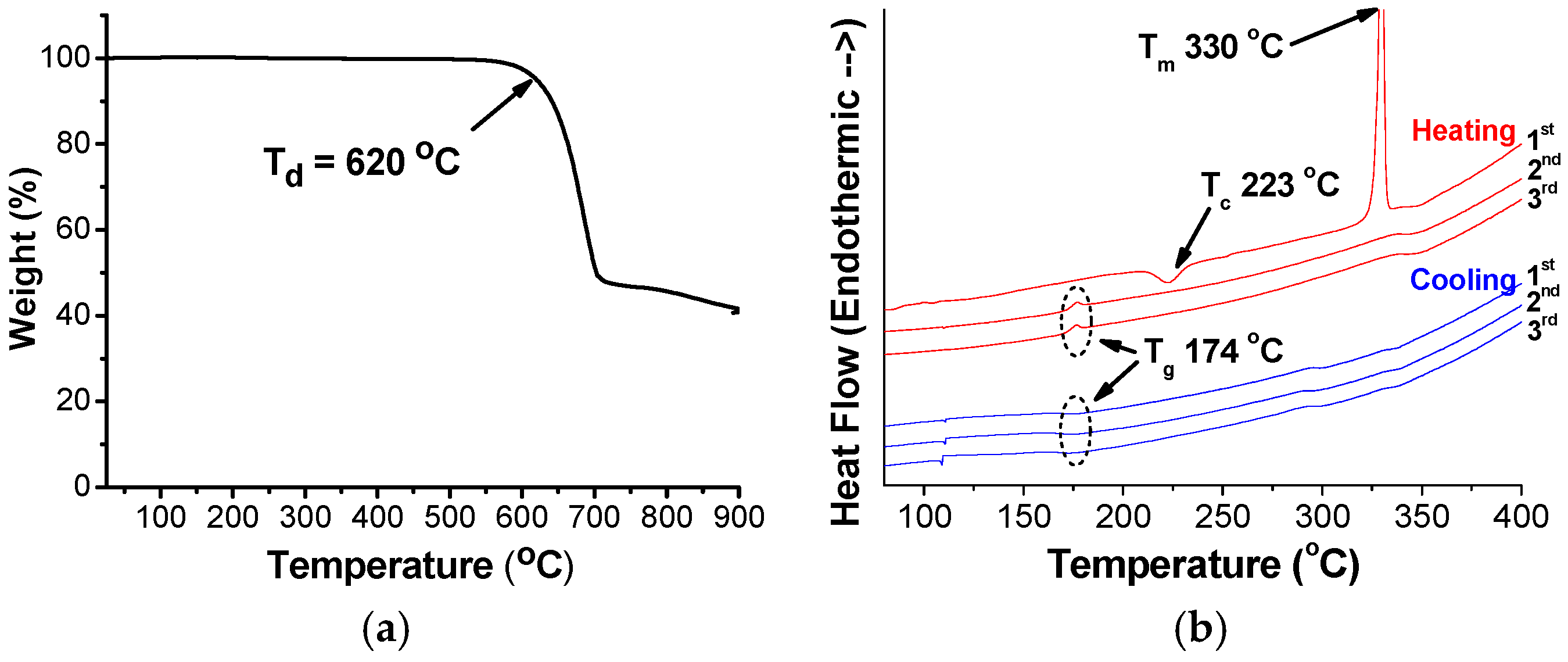
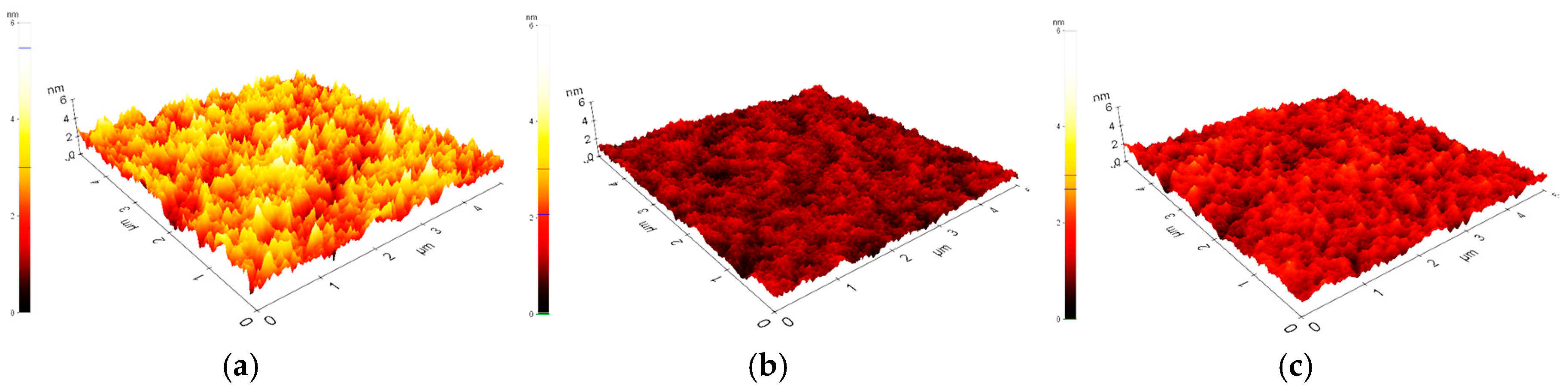
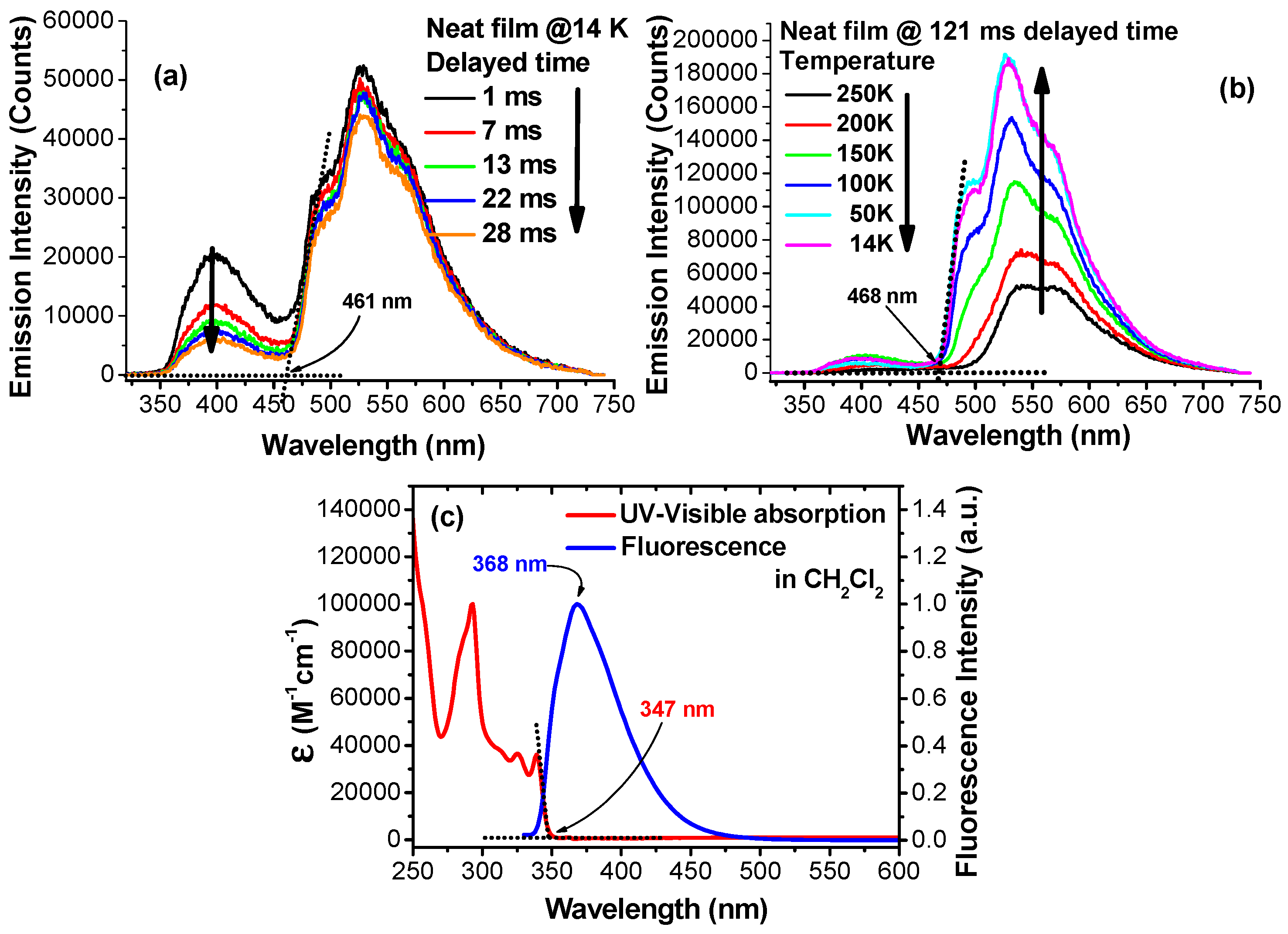
2. Results and Discussion
3. Materials and Methods
3.1. General Instrumentation
3.2. Device Fabrication and Performance Measurements
3.3. Materials Preparation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Romain, M.; Tondelier, D.; Geffroy, B.; Shirinskaya, A.; Jeannin, O.; Rault-Berthelot, J.; Poriel, C. Spiro-configured phenyl acridine thioxanthene dioxide as a host for efficient PhOLEDs. Chem. Commun. 2015, 51, 1313–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romain, M.; Tondelier, D.; Jeannin, O.; Geffroy, B.; Rault-Berthelot, J.; Poriel, C. Properties modulation of organic semi-conductors based on a donor-spiro-acceptor (d-spiro-A) molecular design: New host materials for efficient sky-blue PhOLEDs. J. Mater. Chem. C 2015, 3, 9701–9714. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.-S.; Xie, Y.-M.; Wang, Y.-K.; Zhong, C.; Deng, Y.-L.; Liu, X.-Y.; Jiang, Z.-Q.; Liao, L.-S. Pure Hydrocarbon Hosts for ≅100% exciton harvesting in both phosphorescent and fluorescent light-emitting devices. Adv. Mater. 2015, 27, 4213–4217. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Dong, S.-C.; Jiang, Z.-Q.; Chen, H.; Liao, L.-S. Orthogonal molecular structure for better host material in blue phosphorescence and larger OLED white lighting panel. Adv. Funct. Mater. 2015, 25, 645–650. [Google Scholar] [CrossRef]
- Lai, C.-C.; Huang, M.-J.; Cou, H.-H.; Liao, C.-Y.; Rajamalli, P.; Cheng, C.-H. m-Indolocarbazole derivative as a universal host material for RGB and white phosphorescent OLEDs. Adv. Funct. Mater. 2015, 25, 5548–5556. [Google Scholar] [CrossRef]
- Huang, J.-J.; Hung, Y.-H.; Ting, P.-L.; Tsai, Y.-N.; Gao, H.-J.; Chiu, T.-L.; Lee, J.-H.; Chen, C.-L.; Chou, P.-T.; Leung, M.-K. Orthogonally substituted benzimidazole-carbazole benzene as universal hosts for phosphorescent organic light-emitting diodes. Org. Lett. 2016, 18, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Thiery, S.; Tondelier, D.; Geffroy, B.; Jeannin, O.; Rault-Berthelot, J.; Poriel, C. Modulation of the Physicochemical Properties of Donor–Spiro–Acceptor Derivatives through Donor Unit Planarisation: Phenylacridine versus Indoloacridine—New Hosts for Green and Blue Phosphorescent Organic Light-Emitting Diodes (PhOLEDs). Chem. Eur. J. 2016, 22, 10136–10149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, L.; Hou, L.; Lee, T.-W.; Qiao, J.; Zhang, D.; Dong, G.; Wang, L.; Qiu, Y. Solution processable small molecules for organic light-emitting diodes. J. Mater. Chem. 2010, 20, 6392–6407. [Google Scholar] [CrossRef]
- Zhong, C.; Duan, C.; Hung, F.; Wu, H.; Cao, Y. Materials and devices toward fully solution processable organic light-emitting diodes. Chem. Mater. 2011, 23, 326–340. [Google Scholar] [CrossRef]
- Xiao, L.; Chen, Z.; Qu, B.; Luo, J.; Kong, S.; Gong, Q.; Kido, J. Recent progresses on materials for Electrophosphorescent organic light-emitting devices. Adv. Mater. 2011, 23, 926–952. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Yang, C.; Qin, J. Organic host materials for phosphorescent organic light-emitting diodes. Chem. Soc. Rev. 2011, 40, 2943–2970. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-T.; Chang, Y.-T.; Lee, M.-T.; Chiang, P.-H.; Chen, C.-T.; Chen, C.-T. Solution-processed bipolar small molecular host materials for single-layer blue phosphorescent organic light-emitting diodes. J. Mater. Chem. C 2014, 2, 382–391. [Google Scholar] [CrossRef]
- Jiang, W.; Xu, H.; Ban, X.; Yuan, G.; Sun, Y.; Huang, B.; Duan, L.; Qiu, Y. Alcohol-soluble Electron-transport small molecule for fully solution-processed multilayer white Electrophosphorescent devices. Org. Lett. 2014, 16, 1140–1143. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, N.; Pu, Y.J.; Watanabe, M.; Chiba, T.; Ideta, K.; Toyota, N.; Igarashi, M.; Suzuri, Y.; Sasabe, H.; Kido, J. Solution-processed multilayer small-molecule light-emitting devices with high-efficiency white-light emission. Nat. Commun. 2014, 5, 5756. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, T.; Liu, S.-W.; Wu, M.-F.; Chen, C.-T. Spectroscopic and electrical characteristics of highly efficient tetraphenylsilane-carbazole organic compound as host material for blue organic light emitting diodes. Org. Electron. 2009, 10, 1372–1377. [Google Scholar] [CrossRef]
- Jou, J.-H.; Wang, W.-B.; Chen, S.-Z.; Shyue, J.-J.; Hsu, M.-F.; Lin, C.-W.; Shen, S.-M.; Wang, C.-J.; Liu, C.-P.; Chen, C.-T.; et al. High-efficiency blue organic light-emitting diodes using a 3,5-di(9H-carbazol-9-yl)tetraphenylsilane host via a solution-process. J. Mater. Chem. 2010, 20, 8411–8416. [Google Scholar] [CrossRef]
- Liu, S.-W.; Yuan, C.-H.; Yeh, S.-J.; Wu, M.-F.; Chen, C.-T.; Lee, C.-C. Efficiency enhancement of solution-processed single-layer blue-phosphorescence organic light-emitting devices having co-host materials of polymer (PVK) and small-molecule (SimCP2). J. Soc. Inform. Disp. 2011, 19, 346–352. [Google Scholar] [CrossRef]
- Liu, S.-W.; Chang, Y.-T.; Lee, C.-C.; Yuan, C.-H.; Liu, L.-A.; Chen, Y.-S.; Lin, C.-F.; Wu, C.-I.; Chen, C.-T. Single-layer blue Electrophosphorescent organic light-emitting diodes based on small-molecule mixed hosts: Comparison between the solution and vacuum fabrication processes. Jpn. J. Appl. Phys. 2013, 52, 012101. [Google Scholar] [CrossRef]
- Chang, Y.-T.; Liu, S.-W.; Yuan, C.-H.; Ho, Y.-H.; Chen, K.-Y.; Lee, Y.-T.; Wu, M.-F.; Lee, C.-C.; Wei, P.-K.; Chen, C.-T.; et al. Comparison of light out-coupling enhancements in single-layer blue-phosphorescent organic light emitting diodes using small-molecule or polymer hosts. J. Appl. Phys. 2013, 114, 173106. [Google Scholar] [CrossRef]
- Chang, Y.-T.; Chang, J.-K.; Lee, Y.-T.; Wang, P.-S.; Wu, J.-L.; Hsu, C.-C.; Wu, I.-W.; Tseng, W.-H.; Pi, T.-W.; Chen, C.-T.; et al. High-efficiency small-molecule-based organic light emitting devices with solution processes and oxadiazole-based electron transport materials. ACS Appl. Mater. Interfaces 2013, 5, 10614–10622. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-W.; Lee, Y.-T.; Tseng, M.-R.; Jang, M.-H.; Yeh, H.-C.; Luo, F.-T.; Meng, H.-F.; Chen, C.-T.; Chi, Y.; Qiu, Y.; et al. General Application of blade coating to small-molecule hosts for organic light-emitting diode. Synth. Metals 2014, 196, 99–109. [Google Scholar] [CrossRef]
- Jou, J.-H.; Chen, S.-Z.; An, C.-C.; Peng, S.-H.; Ting, T.-Y.; Shyue, J.-J.; Chin, C.-L.; Chen, C.-T.; Wang, C.-W. A wet and dry processable phosphorescent green dye based organic light-emitting diodes. Dyes Pigments 2015, 113, 341–350. [Google Scholar] [CrossRef]
- Sun, D.; Zhou, X.; Li, H.; Sun, X.; Ren, Z.; Ma, D.; Yan, S. Multi-3,3′-bicarbazole-substituted arylsilane host materials with balanced charge transport for highly efficient solution-processed blue phosphorescent organic light-emitting diodes. ACS Appl. Mater. Interfaces 2015, 7, 17802–17810. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.-J.; Wu, M.-F.; Chen, C.-T.; Song, Y.-H.; Chi, Y.; Ho, M.-H.; Hsu, S.-F.; Chen, C.H. New dopant and host materials for blue-light-emitting phosphorescent organic electroluminescent devices. Adv. Mater. 2005, 17, 285–289. [Google Scholar] [CrossRef]
- Wu, M.-F.; Yeh, S.-J.; Chen, C.-T.; Murayama, H.; Tsuboi, T.; Li, W.-S.; Chao, I.; Liu, S.-W.; Wang, J.-K. The quest for high-performance host materials for Electrophosphorescent blue dopants. Adv. Funct. Mater. 2007, 17, 1887–1895. [Google Scholar] [CrossRef]
- Bassett, E.A.; Emblem, H.G.; Frankel, M.; Ridge, D. The use of the Würtz–Fittig reaction in the preparation of organo-substituted silanes. J. Soc. Chem. Ind. 1948, 67, 177–179. [Google Scholar] [CrossRef]
- Freiser, H.; Eagle, M.V.; Speier, J. Electric moments and structures of organosilicon compounds. II. The aromatic carbon-silicon bond. J. Am. Chem. Soc. 1953, 75, 2821–2824. [Google Scholar] [CrossRef]
- Yabe, M.; Shiotani, T.; Sato, H.; Akiyama, S. Tetraaminobiphenyls, Their Charge Transporting or Electroluminescent Materials, and Organic Electroluminescent Devices Using Them. JP 2005047811 A, 24 February 2005. [Google Scholar]
- Kipping, F.S.; Sands, J.E. Organic derivatives of silicon. Part XXV. Saturated and unsaturated silicohydrocarbons, Si4Ph8. J. Chem. Soc. Trans. 1921, 119, 830–847. [Google Scholar] [CrossRef]
- Miller, R.D.; Thompson, D.; Sooriyakumaran, R.; Fickers, G.N. The synthesis of soluble, substituted silane high polymer by Würtz coupling techniques. J. Polym. Sci. Part A Polym. Chem. 1991, 29, 813–824. [Google Scholar] [CrossRef]
- Goushi, K.; Kwong, R.; Brown, J.J.; Sasabe, H.; Adachi, C. Triplet exciton confinement and unconfinement by adjacent hole-transport layers. J. Appl. Phys. 2004, 95, 7798–7802. [Google Scholar] [CrossRef]
- Lee, J.; Chopra, N.; Eom, S.-H.; Zheng, Y.; Xue, J.; So, F.; Shi, J. Effects of triplet energies and transporting properties of carrier transporting materials on blue phosphorescent organic light emitting devices. Appl. Phys. Lett. 2008, 93, 123306. [Google Scholar] [CrossRef]
- Su, S.-J.; Gonmori, E.; Sasabe, H.; Kido, J. Highly efficient organic blue- and white-light-emitting devices having a carrier- and exciton-confining structure for reduced efficiency roll-off. Adv. Mater. 2008, 20, 4189–4194. [Google Scholar] [CrossRef]
- Fukagawa, H.; Shimizu, T.; Kawano, H.; Yui, S.; Shinnai, T.; Iwai, A.; Tsuchiya, K.; Yamamoto, T. Novel hole-transporting materials with high triplet energy for highly efficient and stable organic light-emitting diodes. J. Phys. Chem. C 2016, 120, 18748–18755. [Google Scholar] [CrossRef]
- Sample Availability: Samples of SimCP3 are available from the authors.




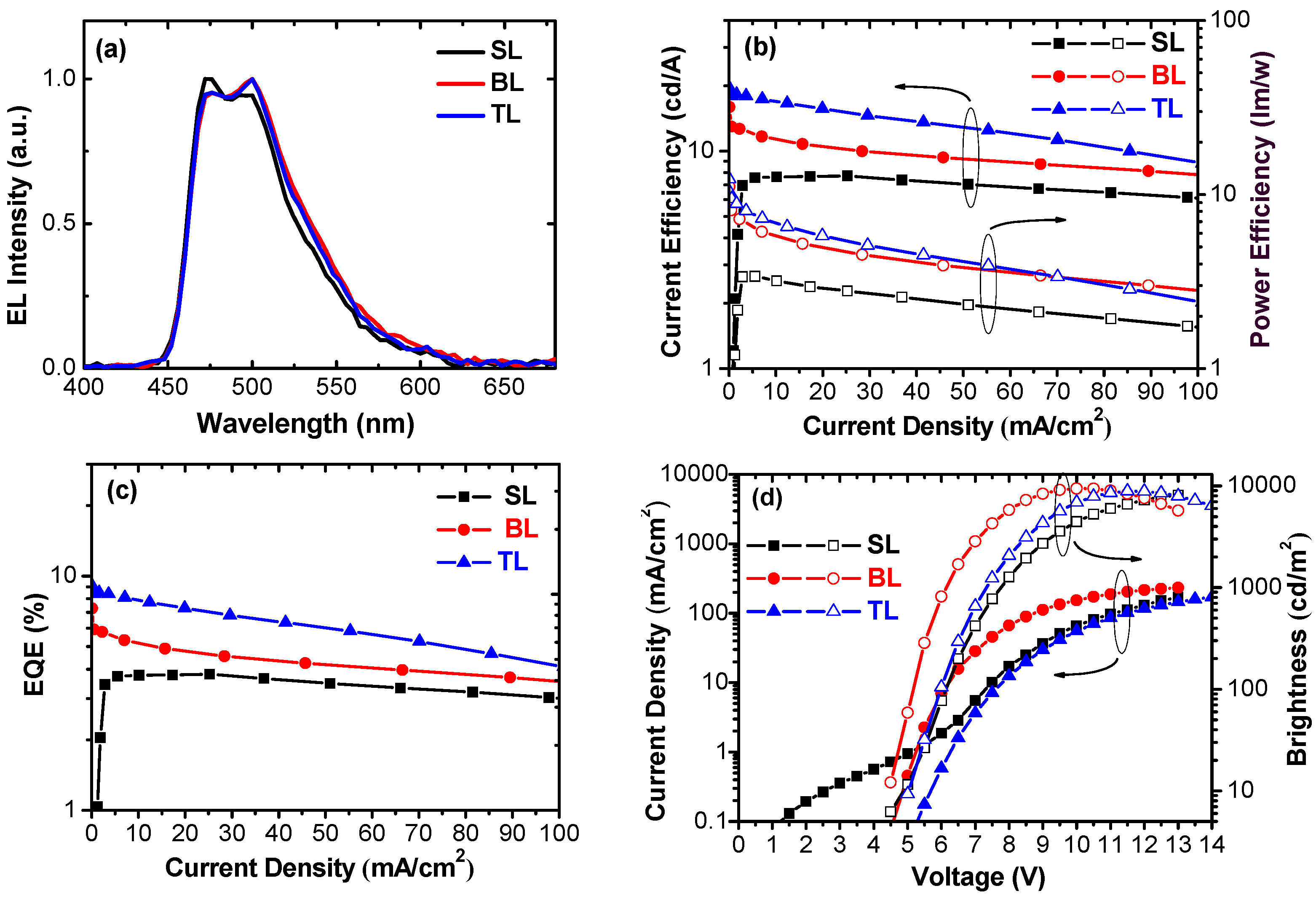
| Device | λmax (nm) | Von a (V) | Lmax b (cd/m2) | CE c (cd/A) | PE d (lm/W) | EQE e (%) | CIEx,y f |
|---|---|---|---|---|---|---|---|
| SL | 472, 492 | 5.0 | 8078 | 7.7, 7.0, 7.7 | 3.4, 3.4, 3.0 | 3.8, 3.4, 3.8 | 0.15, 0.32 |
| BL | 476, 500 | 4.5 | 9440 | 16.0, 12.7, 10.8 | 11.1, 7.2, 5.2 | 7.3, 5.8, 4.9 | 0.16, 0.34 |
| TL | 476, 500 | 5.0 | 8880 | 19.6, 18.0, 17.4 | 12.3, 9.5, 7.3 | 9.1, 8.4, 8.1 | 0.16, 0.34 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-T.; Chang, Y.-T.; Wu, C.-L.; Golder, J.; Chen, C.-T.; Chen, C.-T. SimCP3—An Advanced Homologue of SimCP2 as a Solution-Processed Small Molecular Host Material for Blue Phosphorescence Organic Light-Emitting Diodes. Molecules 2016, 21, 1315. https://doi.org/10.3390/molecules21101315
Lee Y-T, Chang Y-T, Wu C-L, Golder J, Chen C-T, Chen C-T. SimCP3—An Advanced Homologue of SimCP2 as a Solution-Processed Small Molecular Host Material for Blue Phosphorescence Organic Light-Emitting Diodes. Molecules. 2016; 21(10):1315. https://doi.org/10.3390/molecules21101315
Chicago/Turabian StyleLee, Yi-Ting, Yung-Ting Chang, Cheng-Lung Wu, Jan Golder, Chin-Ti Chen, and Chao-Tsen Chen. 2016. "SimCP3—An Advanced Homologue of SimCP2 as a Solution-Processed Small Molecular Host Material for Blue Phosphorescence Organic Light-Emitting Diodes" Molecules 21, no. 10: 1315. https://doi.org/10.3390/molecules21101315
APA StyleLee, Y.-T., Chang, Y.-T., Wu, C.-L., Golder, J., Chen, C.-T., & Chen, C.-T. (2016). SimCP3—An Advanced Homologue of SimCP2 as a Solution-Processed Small Molecular Host Material for Blue Phosphorescence Organic Light-Emitting Diodes. Molecules, 21(10), 1315. https://doi.org/10.3390/molecules21101315