Synthesis of a 1-Aryl-2,2-chlorosilyl(phospha)silene Coordinated by an N-Heterocyclic Carbene †
Abstract
:1. Introduction
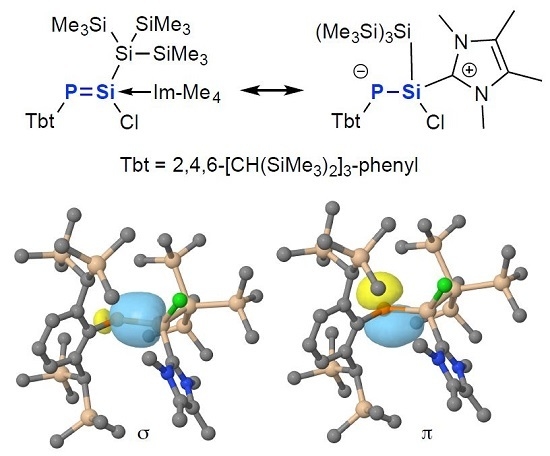
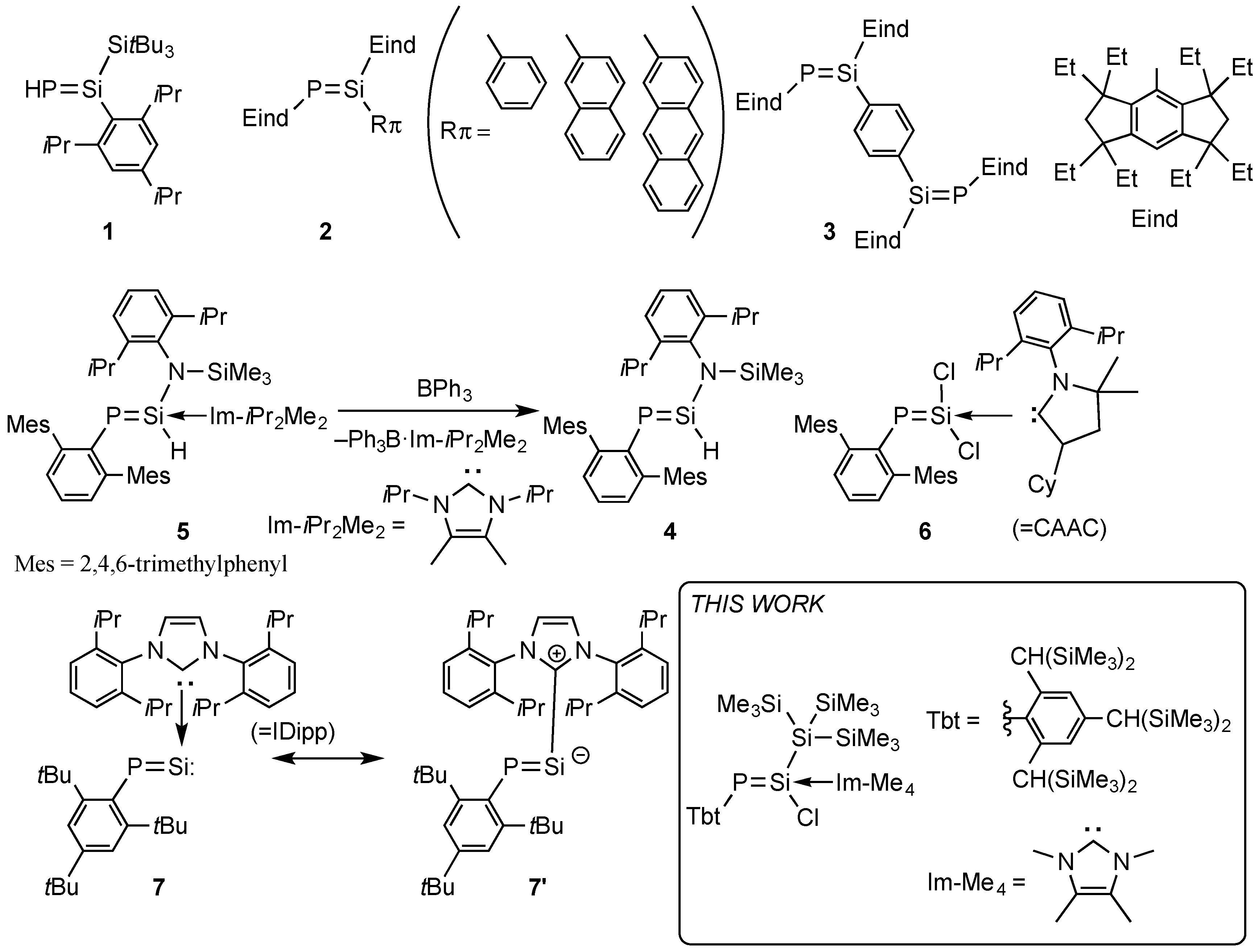
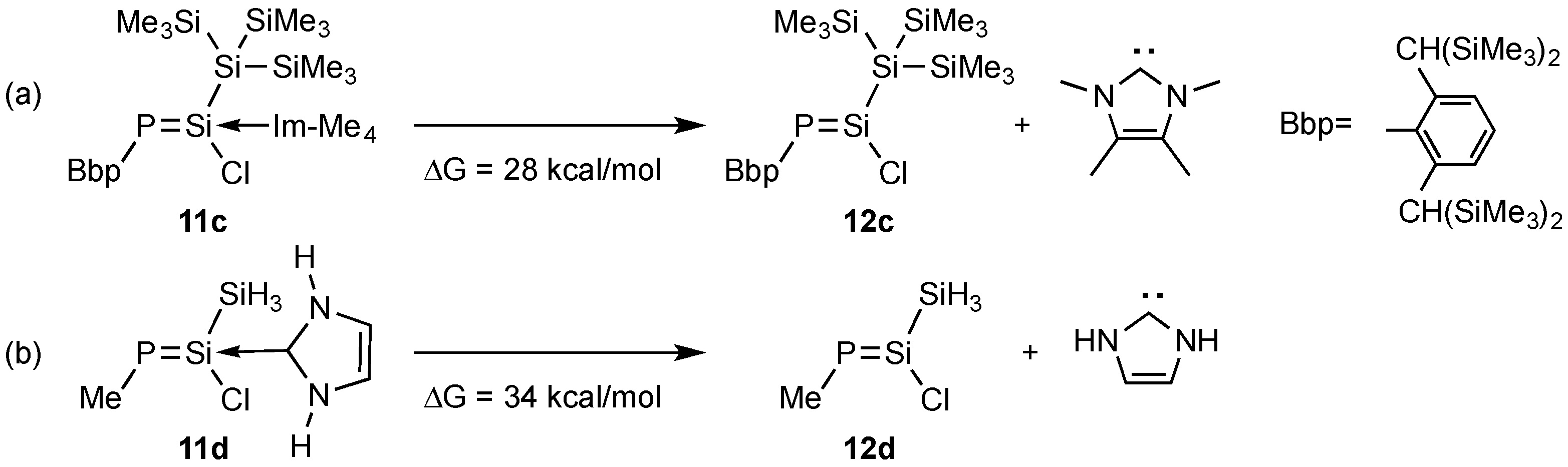
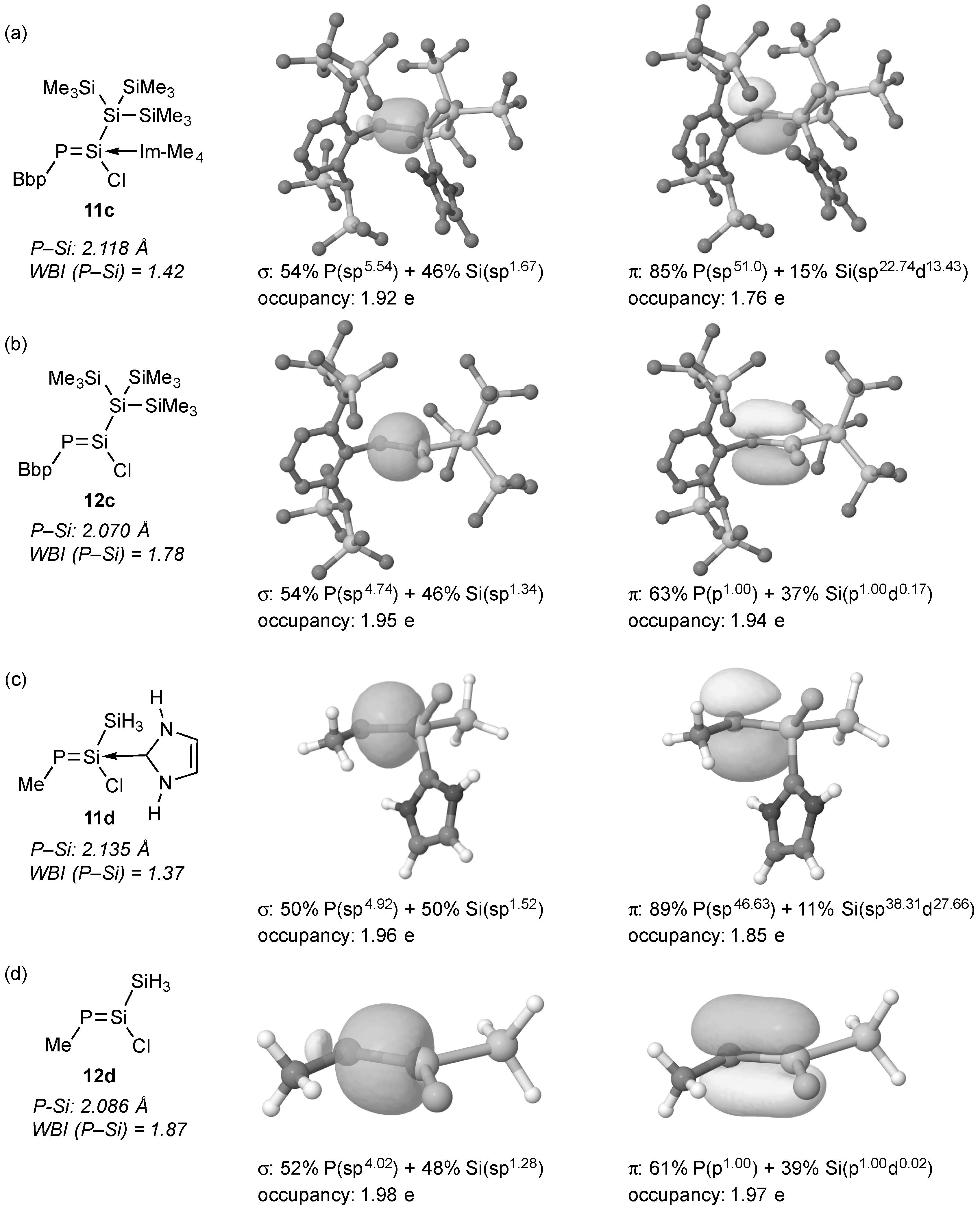
2. Results and Discussions
3. Materials and Methods
3.1. General Information
3.2. Synthesis
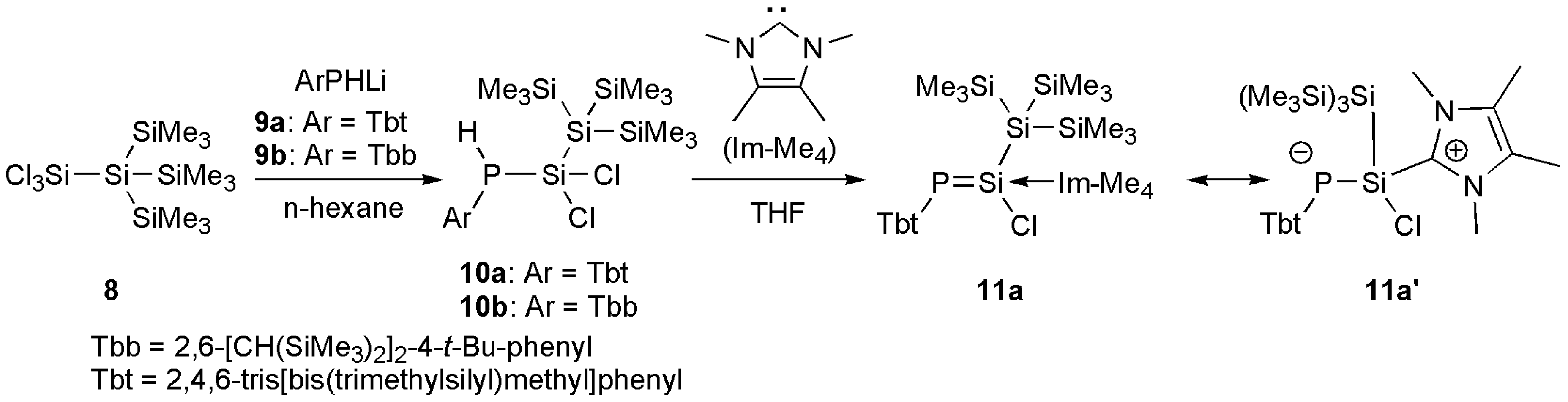
3.2.1. General Procedure for the Synthesis of Phosphinodichlorosilanes 10a and 10b
3.2.2. Synthesis of NHC-coordinated Phosphasilene 11a
3.3. Computational Methods
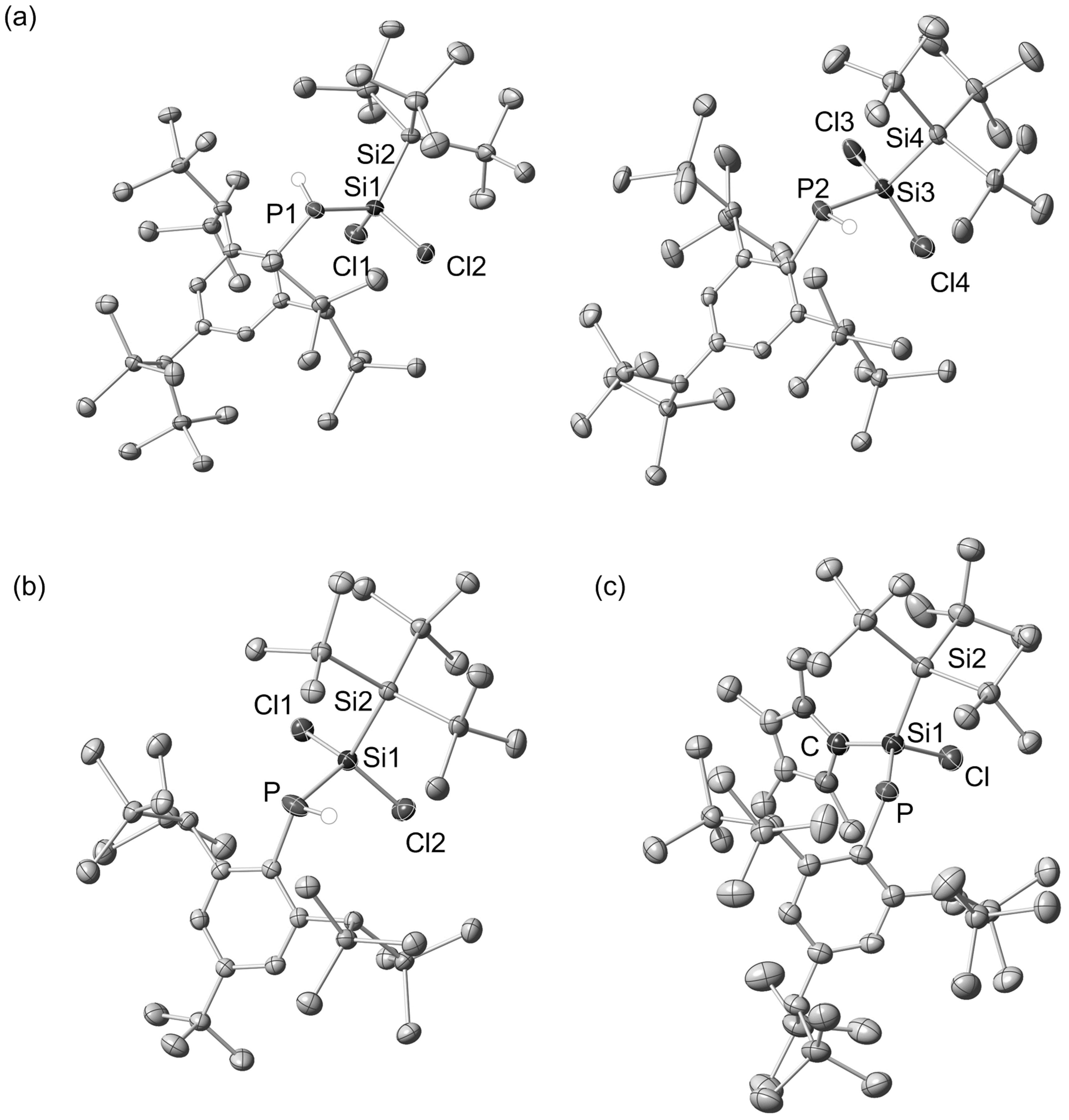
3.4. X-ray Crystallographic Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Fischer, R.C.; Power, P.P. π-Bonding and the Lone Pair Effect in Multiple Bonds Involving Heavier Main Group Elements: Developments in the New Millennium. Chem. Rev. 2010, 110, 3877–3923. [Google Scholar] [CrossRef] [PubMed]
- Smit, C.N.; Lock, F.M.; Bickelhaupt, F. 2,4,6-Tri-tert-Butylphenylphosphene-Dimesitylsilene—The 1st Phosphasilaalkene. Tetrahedron Lett. 1984, 25, 3011–3014. [Google Scholar] [CrossRef]
- Driess, M. Some aspects of the chemistry of silylidene-phosphanes and -arsanes. Coord. Chem. Rev. 1995, 145, 1–25. [Google Scholar] [CrossRef]
- Lee, V.Y.; Sekiguchi, A.; Escudie, J.; Ranaivonjatovo, H. Heteronuclear Double Bonds E=E′ (E = Heavy Group 14 Element, E′ = Group 13–16 Element). Chem. Lett. 2010, 39, 312–318. [Google Scholar] [CrossRef]
- Phosphaalkenes, P=C compounds, are known to be an effective π-accepting ligands towards transition metals. For examples, see refs. 6–11.
- Floch, P.L. Phosphaalkene, phospholyl and phosphinine ligands: New tools in coordination chemistry and catalysis. Coord. Chem. Rev. 2006, 250, 627–681. [Google Scholar] [CrossRef]
- Mathey, F. Phospha-organic chemistry: Panorama and perspectives. Angew. Chem. Int. Ed. 2003, 42, 1578–1604. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, F.; Kawagishi, S.; Ishiyama, T.; Yoshifuji, M. Synthesis, structures, and reactions of methylplatinum(II) and -palladium(II) complexes bearing diphosphinidenecyclobutene ligands (DPCB-Y). Organometallics 2004, 23, 1325–1332. [Google Scholar] [CrossRef]
- Nakajima, Y.; Nakao, Y.; Sakaki, S.; Tamada, Y.; Ono, T.; Ozawa, F. Electronic Structure of Four-Coordinate Iron(I) Complex Supported by a Bis(phosphaethenyl)pyridine Ligand. J. Am. Chem. Soc. 2010, 132, 9934–9936. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, M.; Hayashi, A.; Fu, C.F.; Liu, S.T.; Ozawa, F. Synthesis, Structures, and Reactions of Dirhodium Complexes Bearing a 1,2-Diphenyl-3,4-diphosphinidenecyclobutene Ligand (DPCB). Organometallics 2009, 28, 902–908. [Google Scholar] [CrossRef]
- Sasamori, T.; Hirano, K.; Miyake, H.; Tokitoh, N. Photochemical (E)-(Z) Isomerization of the P=C Double Bond in Triphospha[3]radialene-[M(CO)5] (M= W, Cr) Complexes. Chem. Lett. 2015, 44, 1240–1242. [Google Scholar] [CrossRef]
- Driess, M.; Block, S.; Brym, M.; Gamer, M.T. Synthesis of a “half”-parent phosphasilene R2Si=PH and its metalation to the corresponding P-zinciophosphasilene [R2Si=PM]. Angew. Chem. Int. Ed. 2006, 45, 2293–2296. [Google Scholar] [CrossRef] [PubMed]
- Recently, several numbers of stable phosphasilenes bearing a fundtional group have been reported. For examples, see refs 14–20.
- Szilvási, T.; Veszprémi, T. On the mechanism of the reaction of white phosphorus with silylenes. Dalton Trans. 2011, 40, 7193–7200. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.; Szilvási, T.; Blom, B.; Inoue, S.; Epping, J.; Driess, M. A Fragile Zwitterionic Phosphasilene as a Transfer Agent of the Elusive Parent Phosphinidene (:PH). J. Am. Chem. Soc. 2013, 135, 11795–11798. [Google Scholar] [CrossRef] [PubMed]
- Willmes, P.; Cowley, M.J.; Hartmann, M.; Zimmer, M.; Huch, V.; Scheschkewitz, D. From Disilene (Si=Si) to Phosphasilene (Si=P) and Phosphacumulene (P=C=N). Angew. Chem. Int. Ed. 2014, 53, 2216–2220. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.; Szilvási, T.; Blom, B.; Irran, E.; Driess, M. A Donor-Stabilized Zwitterionic “Half-Parent” Phosphasilene and Its Unusual Reactivity towards Small Molecules. Chem. Eur. J. 2014, 20, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.; Szilvási, T.; Blom, B.; Driese, M. Transition Metal Complexes of a “Half-Parent” Phosphasilene Adduct Representing Silylene -> Phosphinidene -> Metal Complexes. Organometallics 2015, 34, 5703–5708. [Google Scholar] [CrossRef]
- Breit, N.C.; Szilvási, T.; Inoue, S. Facile rotation around a silicon-phosphorus double bond enabled through coordination to tungsten. Chem. Commun. 2015, 51, 11272–11275. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.; Szilvasi, T.; Blom, B.; Driess, M. A Persistent 1,2-Dihydrophosphasilene Adduct. Angew. Chem. Int. Ed. 2015, 54, 15060–15063. [Google Scholar] [CrossRef] [PubMed]
- Li, B.L.; Matsuo, T.; Hashizume, D.; Fueno, H.; Tanaka, K.; Tamao, K. π-Conjugated Phosphasilenes Stabilized by Fused-Ring Bulky Groups. J. Am. Chem. Soc. 2009, 131, 13222–13223. [Google Scholar] [CrossRef] [PubMed]
- Li, B.L.; Matsuo, T.; Fukunaga, T.; Hashizume, D.; Fueno, H.; Tanaka, K.; Tamao, K. Neutral and Cationic Gold(I) Complexes with π-Conjugated Phosphasilene Ligands. Organometallics 2011, 30, 3453–3456. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Köcher, C. N-heterocyclic carbenes. Angew. Chem. Int. Ed. 1997, 36, 2162–2187. [Google Scholar] [CrossRef]
- Cui, H.Y.; Zhang, J.Y.; Cui, C.M. 2-Hydro-2-aminophosphasilene with N–Si–P π Conjugation. Organometallics 2013, 32, 1–4. [Google Scholar] [CrossRef]
- Roy, S.; Stollberg, P.; Herbst-Irmer, R.; Stalke, D.; Andrada, D.M.; Frenking, G.; Roesky, H.W. Carbene-Dichlorosilylene Stabilized Phosphinidenes Exhibiting Strong Intramolecular Charge Transfer Transition. J. Am. Chem. Soc. 2015, 137, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Lavallo, V.; Canac, Y.; Präsang, C.; Donnadieu, B.; Bertrand, G. Stable cyclic (alkyl) (amino) carbenes as rigid or flexible, bulky, electron-rich ligands for transition-metal catalysts: A quaternary carbon atom makes the difference. Angew. Chem. Int. Ed. 2005, 44, 5705–5709. [Google Scholar] [CrossRef] [PubMed]
- Geiß, D.; Arz, M.I.; Straßmann, M.; Schnakenburg, G.; Filippou, A.C. Si=P Double Bonds: Experimental and Theoretical Study of an NHC-Stabilized Phosphasilenylidene. Angew. Chem. Int. Ed. 2015, 54, 2739–2744. [Google Scholar] [CrossRef] [PubMed]
- Ghadwal, R.S.; Roesky, H.W.; Merkel, S.; Henn, J.; Stalke, D. Lewis Base Stabilized Dichlorosilylene. Angew. Chem. Int. Ed. 2009, 48, 5683–5686. [Google Scholar] [CrossRef] [PubMed]
- Märkl, G.; Raab, K.M. Phosphapentafulvene. Tetrahedron Lett. 1989, 30, 1077–1080. [Google Scholar] [CrossRef]
- Generation of P=C double bond compounds via 1,2-Me3SiCl elimination has been reported, see refs 31 and 32.
- Sugamata, K.; Sasamori, T.; Tokitoh, N. Unique Synthetic Approach toward a Phosphaalkene: Synthesis of a Selenium-substituted Phosphaalkene with Bulky Substituents. Chem. Lett. 2014, 43, 95–96. [Google Scholar] [CrossRef]
- Sasamori, T.; Villalba Franco, J.M.; Guo, J.-D.; Sugamata, K.; Nagase, S.; Streubel, R.; Tokitoh, N. Selenium-Substituted Phosphaalkenes Obtained through 1,2-Elimination of Chlorosilanes from Selenenylchlorophosphines. Eur. J. Inorg. Chem. 2016, 678–684. [Google Scholar] [CrossRef]
- Haase, M.; Klingebiel, U. Halogenofunctional and Aminofunctional Tris(Trimethylsilyl)Silyl-Silanes. Z. Anorg. Allg. Chem. 1985, 524, 106–110. [Google Scholar] [CrossRef]
- Nagahora, N.; Sasamori, T.; Takeda, N.; Tokitoh, N. A Kinetically Stabilized Ferrocenyl Diphosphene: Synthesis, Structure, Properties, and Redox Behavior. Chem. Eur. J. 2004, 10, 6146–6151. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, N.; Kratz, T. Synthesis of Imidazol-2-Ylidenes by Reduction of Imidazole-2(3H)-Thiones. Synthesis 1993, 561–562. [Google Scholar] [CrossRef]
- X-ray crystallographic data for 10a, 10b and 11a. Data for 10a (C36H87Cl2PSi11): M = 930.91, triclinic, P–1 (no. 2), a = 18.2047(2) Å, b = 18.3912(2) Å, c = 19.4617(3) Å, α = 66.2398(6)°, β = 84.2173(8)°, γ = 75.3258(9)°, V = 5769.08(13) Å3, Z = 4, Dcalc = 1.072 g cm−3, µ = 0.391 mm−1, 2θmax = 51.0°, measd./unique refls. = 60710/21192 (Rint = 0.0449), param = 963, GOF = 1000, R1 = 0.0386/0.0509 [I > 2σ(I)/for all data], wR2 = 0.1008/0.1106 [I > 2σ(I)/for all data], largest diff. peak and hole 0.897 and −0.614 e·Å−3 (CCDC-1501067). Data for [10b·C6H14] (C33H77Cl2PSi9·C6H14): M = 914.80, triclinic, Pna21 (no.33), a = 17.3272(4) Å, b = 23.7816(4) Å, c = 13.7943(3) Å, V = 5684.2(2) Å3, Z = 4, Dcalc = 1.069 g cm−3, µ = 0.356 mm−1, 2θmax = 53.0°, measd./unique refls. = 78838/11766 (Rint = 0.0563), param = 516, GOF = 1017, R1 = 0.0293/0.0363 [I > 2σ(I)/for all data], wR2 = 0.0656/0.0685 [I > 2σ(I)/for all data], largest diff. peak and hole 0.268 and −0.237 e·Å−3 (CCDC-1501065). Data for 11a (C46H105ClN2PSi11): M = 1061.72, triclinic, P–1 (no. 2), a = 12.0497(3) Å, b = 15.4180(4) Å, c = 18.9168(4) Å, α = 82.5932(14)°, β = 72.2764(10)°, γ = 79.7072(17)°, V = 3283.30(14) Å3, Z = 2, Dcalc = 1.074 g cm−3, µ = 0.313 mm−1, 2θmax = 51.0°, measd./unique refls. = 34462/12078 (Rint = 0.0512), param = 582, GOF = 1091, R1 = 0.0583/0.0695 [I > 2σ(I)/for all data], wR2 = 0.1450/0.1553 [I > 2σ(I)/for all data], largest diff. peak and hole 0.903 and −0.407 e·Å−3 (CCDC-1501066).
- Glendening, E.D.; Landis, C.R.; Weinhold, F. NBO 6.0: Natural bond orbital analysis program. J. Comp. Chem. 2013, 34, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- The dissociation energy was calculated as ΔG = −0.5 at B3PW91/6–31+G(d,p)//B3PW91/3–21G(d) level, suggesting the importance of dispersion effects.
- Majhi, P.K.; Chow, K.C.F.; Hsieh, T.H.H.; Bowes, E.G.; Schnakenburg, G.; Kennepohl, P.; Streubel, R.; Gates, D.P. Even the normal is abnormal: N-heterocyclic carbene C2 binding to a phosphaalkene without breaking the P=C π-bond. Chem. Commun. 2016, 52, 998–1001. [Google Scholar] [CrossRef] [PubMed]
- Pangborn, A.B.; Giardello, M.A.; Grubbs, R.H.; Rosen, R.K.; Timmers, F.J. Safe and Convenient Procedure for Solvent Purification. Organometallics 2004, 15, 1518–1520. [Google Scholar] [CrossRef]
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; de Caro, L.; Giacovazzo, C.; Polidori, G.; Spagna, R. SIR2004: An improved tool for crystal structure determination and refinement. J. Appl. Cryst. 2005, 38, 381–388. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyri, A.W.; Majhi, P.K.; Sasamori, T.; Agou, T.; Nesterov, V.; Guo, J.-D.; Nagase, S.; Tokitoh, N.; Streubel, R. Synthesis of a 1-Aryl-2,2-chlorosilyl(phospha)silene Coordinated by an N-Heterocyclic Carbene. Molecules 2016, 21, 1309. https://doi.org/10.3390/molecules21101309
Kyri AW, Majhi PK, Sasamori T, Agou T, Nesterov V, Guo J-D, Nagase S, Tokitoh N, Streubel R. Synthesis of a 1-Aryl-2,2-chlorosilyl(phospha)silene Coordinated by an N-Heterocyclic Carbene. Molecules. 2016; 21(10):1309. https://doi.org/10.3390/molecules21101309
Chicago/Turabian StyleKyri, Andreas Wolfgang, Paresh Kumar Majhi, Takahiro Sasamori, Tomohiro Agou, Vitaly Nesterov, Jing-Dong Guo, Shigeru Nagase, Norihiro Tokitoh, and Rainer Streubel. 2016. "Synthesis of a 1-Aryl-2,2-chlorosilyl(phospha)silene Coordinated by an N-Heterocyclic Carbene" Molecules 21, no. 10: 1309. https://doi.org/10.3390/molecules21101309
APA StyleKyri, A. W., Majhi, P. K., Sasamori, T., Agou, T., Nesterov, V., Guo, J.-D., Nagase, S., Tokitoh, N., & Streubel, R. (2016). Synthesis of a 1-Aryl-2,2-chlorosilyl(phospha)silene Coordinated by an N-Heterocyclic Carbene. Molecules, 21(10), 1309. https://doi.org/10.3390/molecules21101309