Beneficial Effects of Tea and the Green Tea Catechin Epigallocatechin-3-gallate on Obesity
Abstract
:1. Introduction
2. Epidemiological Studies
3. Clinical Trials
4. Cell-Based and Animal Experiments
5. Molecular Mechanisms
5.1. Inhibition of Digestive Enzymes and Prevention of Absorption
5.2. Effects on Intestinal Microbiota
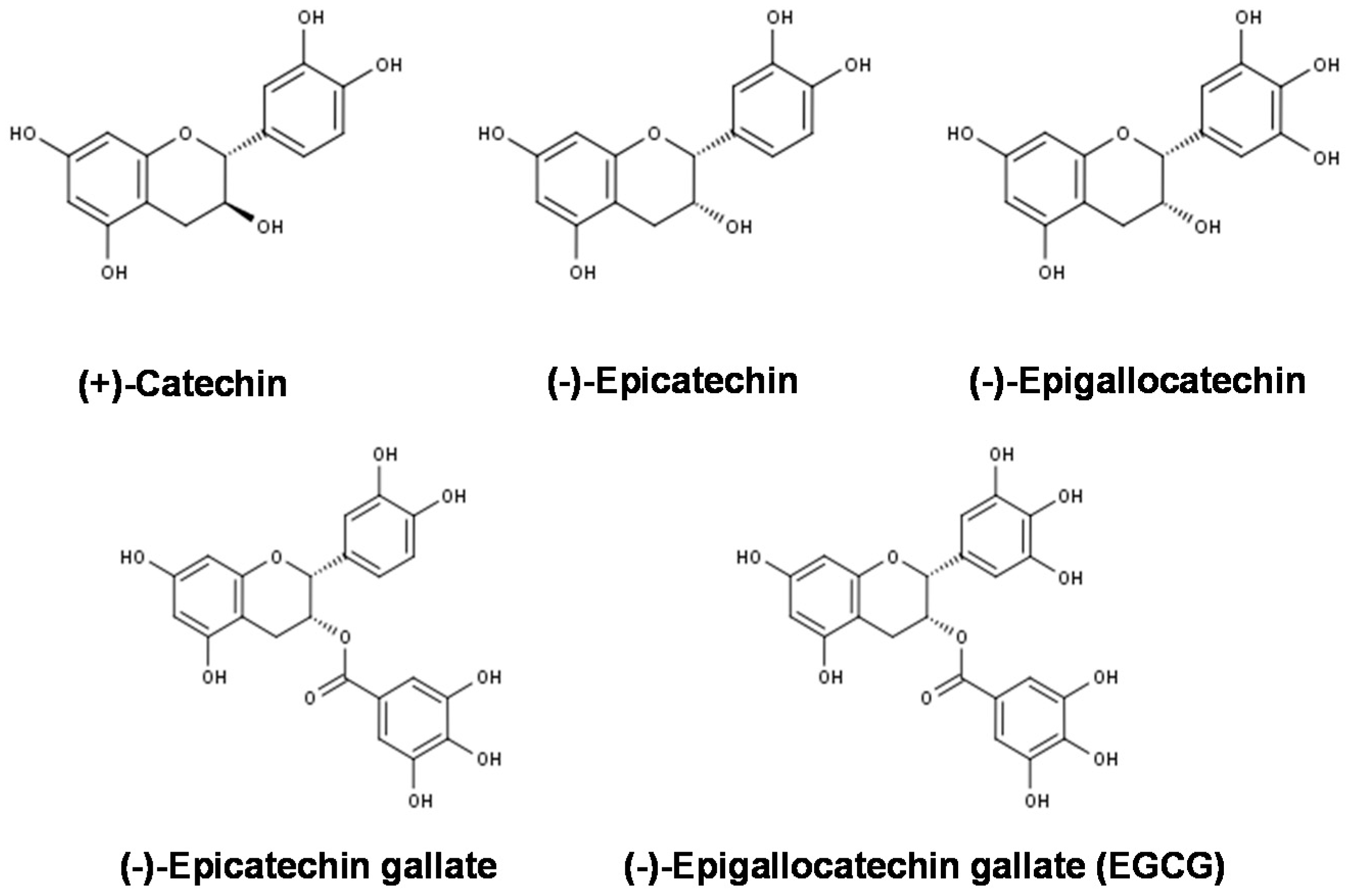
5.3. Effects on Gene and Protein Expression
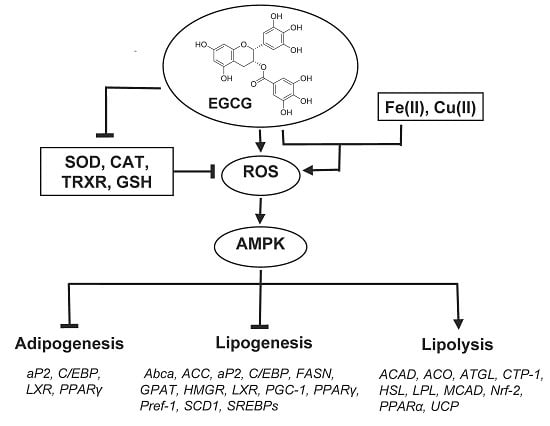
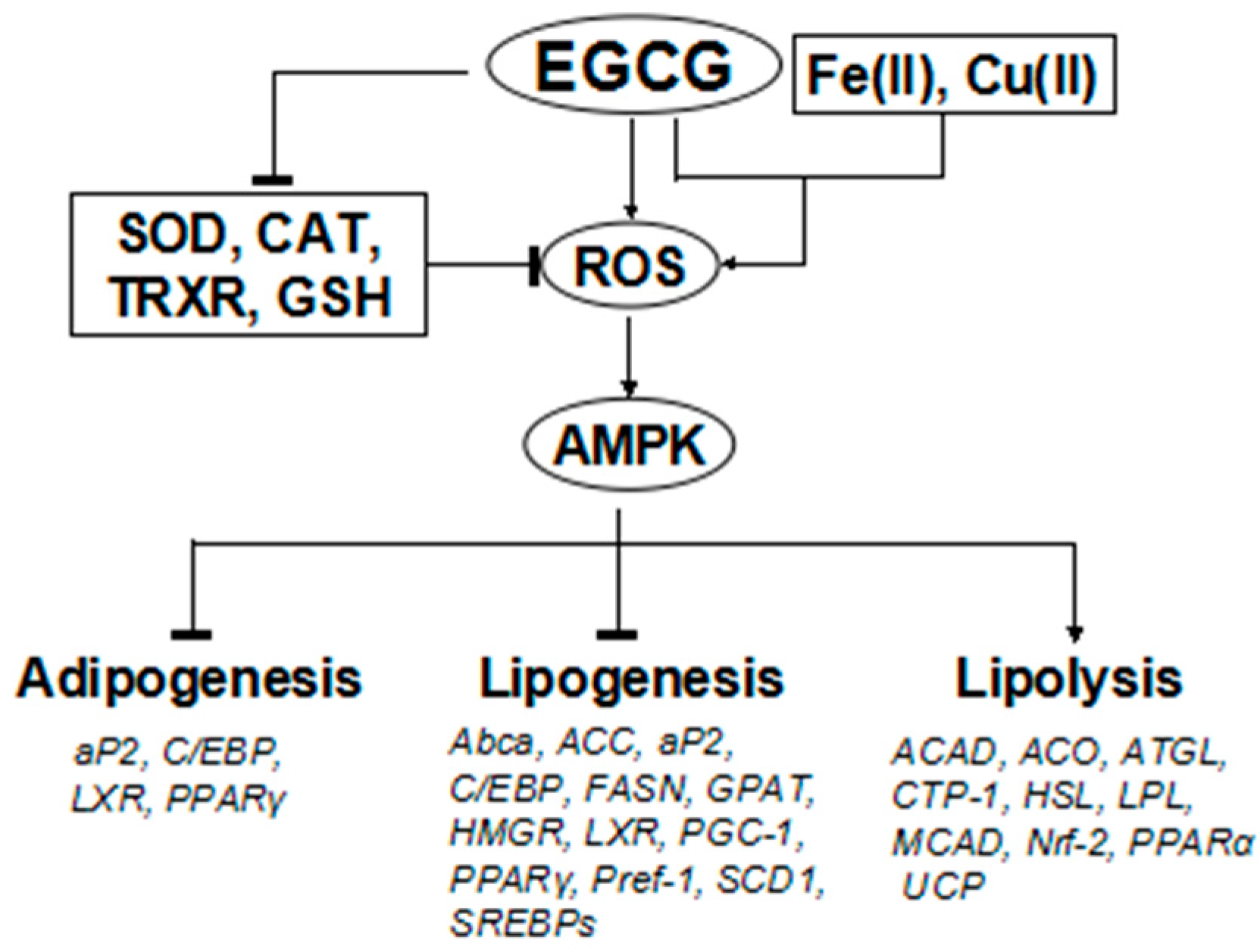
5.4. Effects on the Expression of Selected Genes and Action Mechanism of Green Tea Constituents
Acknowledgments
Conflicts of Interest
References
- Yang, C.S.; Chen, G.; Wu, Q. Recent scientific studies of a traditional Chinese medicine, tea, on prevention of chronic diseases. J. Tradit. Complement. Med. 2014, 4, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Miyoshi, N.; Hayakawa, S.; Imai, S.; Isemura, M.; Nakamura, Y. Health benefits of tea consumption. In Beverage Impacts on Health and Nutrition, 2nd ed.; Wilson, T., Temple, N.J., Eds.; Human Press: Cham, Switzerland, 2016; pp. 49–67. [Google Scholar]
- Hayat, K.; Iqbal, H.; Malik, U.; Bilal, U.; Mushtaq, S. Tea and its consumption: Benefits and risks. Crit. Rev. Food Sci. Nutr. 2015, 55, 939–954. [Google Scholar] [CrossRef] [PubMed]
- Kaibara, E. Yojokun: Life Lessons from A Samurai; Wilson, W.S., Translator; Kodansha International: Tokyo, Japan, 2008. [Google Scholar]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014, 25. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, Y.; Xie, Z.; Zhou, Y.; Zhang, Y.; Wan, X. The anti-obesity effects of green tea in human intervention and basic molecular studies. Eur. J. Clin. Nutr. 2014, 68, 1075–1087. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, N.; Pervin, M.; Suzuki, T.; Unno, K.; Isemura, M.; Nakamura, Y. Green tea catechins for well-being and therapy: Prospects and opportunities. Bot. Targets Ther. 2015, 5, 85–96. [Google Scholar]
- Yang, C.S.; Zhang, J.; Zhang, L.; Huang, J.; Wang, Y. Mechanisms of body weight reduction and metabolic syndrome alleviation by tea. Mol. Nutr. Food Res. 2016, 60, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Sorice, A.; Guerriero, E.; Capone, F.; Colonna, G.; Castello, G.; Costantini, S. Ascorbic acid: Its role in immune system and chronic inflammation diseases. Mini Rev. Med. Chem. 2014, 14, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.A.; Arts, I.C.; Ambergen, T.; Brants, H.A.; Dagnelie, P.C.; Goldbohm, R.A.; van den Brandt, P.A.; Weijenberg, M.P.; Netherlands Cohort Study. Higher dietary flavone, flavonol, and catechin intakes are associated with less of an increase in BMI over time in women: A longitudinal analysis from The Netherlands Cohort Study. Am. J. Clin. Nutr. 2008, 88, 1341–1352. [Google Scholar] [PubMed]
- Vernarelli, J.A.; Lambert, J.D. Tea consumption is inversely associated with weight status and other markers for metabolic syndrome in US adults. Eur. J. Nutr. 2013, 52, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Stepaniak, U.; Micek, A.; Topor-Mądry, R.; Pikhart, H.; Szafraniec, K.; Pająk, A. Association of daily coffee and tea consumption and metabolic syndrome: Results from the Polish arm of the HAPIEE study. Eur. J. Nutr. 2015, 54, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Gyntelberg, F.; Hein, H.O.; Suadicani, P. Sugar in coffee or tea and risk of obesity: A neglected issue. Int. J. Food Sci. Nutr. 2009, 60 (Suppl. 3), 56–64. [Google Scholar] [CrossRef] [PubMed]
- Takami, H.; Nakamoto, M.; Uemura, H.; Katsuura, S.; Yamaguchi, M.; Hiyoshi, M.; Sawachika, F.; Juta, T.; Arisawa, K. Inverse correlation between coffee consumption and prevalence of metabolic syndrome: Baseline survey of the Japan Multi-Institutional Collaborative Cohort (J-MICC) Study in Tokushima, Japan. J. Epidemiol. 2013, 23, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Miyoshi, N.; Isemura, M. Health-promoting effects of green tea. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2012, 88, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Rains, T.M.; Agarwal, S.; Maki, K.C. Antiobesity effects of green tea catechins: A mechanistic review. J. Nutr. Biochem. 2011, 22. [Google Scholar] [CrossRef] [PubMed]
- Lin, I.H.; Ho, M.L.; Chen, H.Y.; Lee, H.S.; Huang, C.C.; Chu, Y.H.; Lin, S.Y.; Deng, Y.R.; He, Y.H.; Lien, Y.H.; et al. Smoking, green tea consumption, genetic polymorphisms in the insulin-like growth factors and lung cancer risk. PLoS ONE 2012, 7, e30951. [Google Scholar] [CrossRef] [PubMed]
- Nagao, T.; Komine, Y.; Soga, S.; Meguro, S.; Hase, T.; Tanaka, Y.; Tokimitsu, I. Ingestion of a tea rich in catechins leads to a reduction in body fat and malondialdehyde-modified LDL in men. Am. J. Clin. Nutr. 2005, 81, 122–129. [Google Scholar] [PubMed]
- Matsuyama, T.; Tanaka, Y.; Kamimaki, I.; Nagao, T.; Tokimitsu, I. Catechin safely improved higher levels of fatness, blood pressure, and cholesterol in children. Obesity (Silver Spring) 2008, 16, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Maki, K.C.; Reeves, M.S.; Farmer, M.; Yasunaga, K.; Matsuo, N.; Katsuragi, Y.; Komikado, M.; Tokimitsu, I.; Wilder, D.; Jones, F.; et al. Green tea catechin consumption enhances exercise-induced abdominal fat loss in overweight and obese adults. J. Nutr. 2009, 139, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, G.A.; Salgado, J.M.; Cesar Mde, C.; Donado-Pestana, C.M. The effects of green tea consumption and resistance training on body composition and resting metabolic rate in overweight or obese women. J. Med. Food 2013, 16, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Mähler, A.; Steiniger, J.; Bock, M.; Klug, L.; Parreidt, N.; Lorenz, M.; Zimmermann, B.F.; Krannich, A.; Paul, F.; Boschmann, M. Metabolic response to epigallocatechin-3-gallate in relapsing-remitting multiple sclerosis: A randomized clinical trial. Am. J. Clin. Nutr. 2015, 101, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Boschmann, M.; Thielecke, F. The effects of epigallocatechin-3-gallate on thermogenesis and fat oxidation in obese men: A pilot study. J. Am. Coll. Nutr. 2007, 26, 389S–395S. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wen, Y.; Du, Y.; Yan, X.; Guo, H.; Rycroft, J.A.; Boon, N.; Kovacs, E.M.; Mela, D.J. Effects of catechin enriched green tea on body composition. Obesity (Silver Spring) 2010, 18, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Sanchez, K.; Leyva, M.J.; Wu, M.; Betts, N.M.; Aston, C.E.; Lyons, T.J. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J. Am. Coll. Nutr. 2010, 29, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, H.; Hitoe, S.; Nakamura, S.; Matsuda, H. Purple tea and its extract suppress diet-induced fat accumulation in mice and human subjects by inhibiting fat absorption and enhancing hepatic carnitine palmitoyltransferase expression. Int. J. Biomed. Sci. 2015, 11, 67–75. [Google Scholar] [PubMed]
- Ashigai, H.; Taniguchi, Y.; Suzuki, M.; Ikeshima, E.; Kanaya, T.; Zembutsu, K.; Tomita, S.; Miyake, M.; Fukuhara, I. Fecal lipid excretion after consumption of a black tea polyphenol containing beverage-randomized, placebo-controlled, double-blind, crossover study. Biol. Pharm. Bull. 2016, 39, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.J.; Liu, C.Y.; Chiu, J.P.; Hsu, C.H. Therapeutic effect of high-dose green tea extract on weight reduction: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. 2016, 35, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Nagao, T.; Meguro, S.; Hase, T.; Otsuka, K.; Komikado, M.; Tokimitsu, I.; Yamamoto, T.; Yamamoto, K. A catechin-rich beverage improves obesity and blood glucose control in patients with type 2 diabetes. Obesity (Silver Spring) 2009, 17, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, Y.; Thabane, L.; Mbuagbaw, L.; Liu, A.; Levine, M.A.; Holbrook, A. Effect of green tea supplementation on blood pressure among overweight and obese adults: A systematic review and meta-analysis. J. Hypertens. 2015, 33, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, Y.; Mbuagbaw, L.; Holbrook, A.; Levine, M.A.; Thabane, L. Effect of green tea supplementation on blood pressure among overweight and obese adults: A protocol for a systematic review. BMJ Open 2014, 4, e004971. [Google Scholar] [CrossRef] [PubMed]
- Bajerska, J.; Mildner-Szkudlarz, S.; Walkowiak, J. Effects of rye bread enriched with green tea extract on weight maintenance and the characteristics of metabolic syndrome following weight loss: A pilot study. J. Med. Food 2015, 18, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.L.; Lane, J.; Holyoak, C.; Nicol, B.; Mayes, A.E.; Dadd, T. Health effects of green tea catechins in overweight and obese men: A randomised controlled cross-over trial. Br. J. Nutr. 2011, 106, 1880–1889. [Google Scholar] [CrossRef] [PubMed]
- Dulloo, A.G.; Duret, C.; Rohrer, D.; Girardier, L.; Mensi, N.; Fathi, M.; Chantre, P.; Vandermander, J. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am. J. Clin. Nutr. 1999, 70, 1040–1045. [Google Scholar] [PubMed]
- Hursel, R.; Viechtbauer, W.; Dulloo, A.G.; Tremblay, A.; Tappy, L.; Rumpler, W.; Westerterp-Plantenga, M.S. The effects of catechin rich teas and caffeine on energy expenditure and fat oxidation: A meta-analysis. Obes. Rev. 2011, 12, e573–e581. [Google Scholar] [CrossRef] [PubMed]
- Mielgo-Ayuso, J.; Barrenechea, L.; Alcorta, P.; Larrarte, E.; Margareto, J.; Labayen, I. Effects of dietary supplementation with epigallocatechin-3-gallate on weight loss, energy homeostasis, cardiometabolic risk factors and liver function in obese women: Randomised, double-blind, placebo-controlled clinical trial. Br. J. Nutr. 2014, 111, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Dostal, A.M.; Samavat, H.; Espejo, L.; Arikawa, A.Y.; Stendell-Hollis, N.R.; Kurzer, M.S. Green tea extract and catechol-O-methyltransferase genotype modify fasting serum insulin and plasma adiponectin concentrations in a randomized controlled trial of overweight and obese postmenopausal women. J. Nutr. 2016, 146, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Dostal, A.M.; Arikawa, A.; Espejo, L.; Kurzer, M.S. Long-term supplementation of green tea extract does not modify adiposity or bone mineral density in a randomized trial of overweight and obese postmenopausal women. J. Nutr. 2016, 146, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.S.; Chung, C.S.; Lee, H.G.; Kim, T.G.; Choi, Y.J.; Cho, C.S. Inhibitory effect of (−)-epigallocatechin-3-gallate on lipid accumulation of 3T3-L1 cells. Obesity (Silver Spring) 2007, 15, 2571–2582. [Google Scholar] [CrossRef] [PubMed]
- Klaus, S.; Pültz, S.; Thöne-Reineke, C.; Wolfram, S. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int. J. Obes. (Lond.) 2005, 29, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Bose, M.; Lambert, J.D.; Ju, J.; Reuhl, K.R.; Shapses, S.A.; Yang, C.S. The major green tea polyphenol, (−)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J. Nutr. 2008, 138, 1677–1683. [Google Scholar] [PubMed]
- Heber, D.; Zhang, Y.; Yang, J.; Ma, J.E.; Henning, S.M.; Li, Z. Green tea, black tea, and oolong tea polyphenols reduce visceral fat and inflammation in mice fed high-fat, high-sucrose obesogenic diets. J. Nutr. 2014, 144, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Sayama, K.; Okubo, T.; Juneja, L.R.; Oguni, I. Anti-obesity effects of three major components of green tea, catechins, caffeine and theanine, in mice. In Vivo 2004, 18, 55–62. [Google Scholar] [PubMed]
- Unno, T.; Osada, C.; Motoo, Y.; Suzuki, Y.; Kobayashi, M.; Nozawa, A. Dietary tea catechins increase fecal energy in rats. J. Nutr. Sci. Vitaminol. 2009, 55, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Grove, K.A.; Sae-tan, S.; Kennett, M.J.; Lambert, J.D. (−)-Epigallocatechin-3-gallate inhibits pancreatic lipase and reduces body weight gain in high fat-fed obese mice. Obesity (Silver Spring) 2012, 20, 2311–2313. [Google Scholar] [CrossRef] [PubMed]
- Yuda, N.; Tanaka, M.; Suzuki, M.; Asano, Y.; Ochi, H.; Iwatsuki, K. Polyphenols extracted from black tea (Camellia sinensis) residue by hot-compressed water and their inhibitory effect on pancreatic lipase in vitro. J. Food Sci. 2012, 77, H254–H261. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, I.; Tsuda, K.; Suzuki, Y.; Kobayashi, M.; Unno, T.; Tomoyori, H.; Goto, H.; Kawata, Y.; Imaizumi, K.; Nozawa, A.; et al. Tea catechins with a galloyl moiety suppress postprandial hypertriacylglycerolemia by delaying lymphatic transport of dietary fat in rats. J. Nutr. 2005, 135, 155–159. [Google Scholar] [PubMed]
- Fei, Q.; Gao, Y.; Zhang, X.; Sun, Y.; Hu, B.; Zhou, L.; Jabbar, S.; Zeng, X. Effects of Oolong tea polyphenols, EGCG, and EGCG3″Me on pancreatic α-amylase activity in vitro. J. Agric. Food Chem. 2014, 62, 9507–9514. [Google Scholar] [CrossRef] [PubMed]
- Walkowiak, J.; Bajerska, J.; Kargulewicz, A.; Lisowska, A.; Siedlerski, G.; Szczapa, T.; Kobelska-Dubiel, N.; Grzymisławski, M. Single dose of green tea extract decreases lipid digestion and absorption from a test meal in humans. Acta Biochim. Pol. 2013, 60, 481–483. [Google Scholar] [PubMed]
- Nishiumi, S.; Bessyo, H.; Kubo, M.; Aoki, Y.; Tanaka, A.; Yoshida, K.; Ashida, H. Green and black tea suppress hyperglycemia and insulin resistance by retaining the expression of glucose transporter 4 in muscle of high-fat diet-fed C57BL/6J mice. J. Agric. Food Chem. 2010, 58, 12916–12923. [Google Scholar] [CrossRef] [PubMed]
- Unno, T.; Sakuma, M.; Mitsuhashi, S. Effect of dietary supplementation of (−)-epigallocatechin gallate on gut microbiota and biomarkers of colonic fermentation in rats. J. Nutr. Sci. Vitaminol. 2014, 60, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Murase, T.; Nagasawa, A.; Suzuki, J.; Hase, T.; Tokimitsu, I. Beneficial effects of tea catechins on diet-induced obesity: Stimulation of lipid catabolism in the liver. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 1459–1464. [Google Scholar] [CrossRef] [PubMed]
- Ferré, P.; Foufelle, F. SREBP-1c transcription factor and lipid homeostasis: Clinical perspective. Horm. Res. 2007, 68, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Murase, T.; Misawa, K.; Haramizu, S.; Hase, T. Catechin-induced activation of the LKB1/AMP-activated protein kinase pathway. Biochem. Pharmacol. 2009, 78, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Santamarina, A.B.; Oliveira, J.L.; Silva, F.P.; Carnier, J.; Mennitti, L.V.; Santana, A.A.; de Souza, G.H.; Ribeiro, E.B.; Oller do Nascimento, C.M.; Lira, F.S.; et al. Green tea extract rich in epigallocatechin-3-gallate prevents fatty liver by AMPK activation via LKB1 in mice fed a high-fat diet. PLoS ONE 2015, 10, e0141227. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Okada, N.; Tanabe, H.; Fukutomi, R.; Yasui, K.; Isemura, M.; Kinae, N. Effects of chronic ingestion of catechin-rich green tea on hepatic gene expression of gluconeogenic enzymes in rats. Biomed. Res. 2009, 30, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Yasui, K.; Paeng, N.; Miyoshi, N.; Suzuki, T.; Taguchi, K.; Ishigami, Y.; Fukutomi, R.; Imai, S.; Isemura, M.; Nakayama, T. Effects of a catechin-free fraction derived from green tea on gene expression of enzymes related to lipid metabolism in the mouse liver. Biomed. Res. 2012, 33, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Suh, K.S.; Chon, S.; Oh, S.; Kim, S.W.; Kim, J.W.; Kim, Y.S.; Woo, J.T. Prooxidative effects of green tea polyphenol (−)-epigallocatechin-3-gallate on the HIT-T15 pancreatic beta cell line. Cell. Biol. Toxicol. 2010, 26, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Li, G.X.; Chen, Y.K.; Hou, Z.; Xiao, H.; Jin, H.; Lu, G.; Lee, M.J.; Liu, B.; Guan, F.; Yang, Z.; et al. Pro-oxidative activities and dose-response relationship of (−)-epigallocatechin-3-gallate in the inhibition of lung cancer cell growth: a comparative study in vivo and in vitro. Carcinogenesis 2010, 31, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, S.; Saito, K.; Miyoshi, N.; Ohishi, T.; Oishi, Y.; Miyoshi, M.; Nakamura, Y. Anti-cancer effects of green tea by either anti- or pro-oxidative mechanisms. Asian Pac. J. Cancer Prev. 2016, 17, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Sun, Z.; Xu, Z.; Hua, Y. Chemiluminescence analysis of the prooxidant and antioxidant effects of epigallocatechin-3-gallate. Asia Pac. J. Clin. Nutr. 2007, 16, 153–157. [Google Scholar] [PubMed]
- Farhan, M.; Khan, H.Y.; Oves, M.; Al-Harrasi, A.; Rehmani, N.; Arif, H.; Hadi, S.M.; Ahmad, A. Cancer therapy by catechins involves redox cycling of copper ions and generation of reactive oxygen species. Toxins (Basel) 2016, 8, 37. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, D.; Cui, W.; Ji, M.; Qian, X.; Zhong, L. Molecular bases of thioredoxin and thioredoxin reductase-mediated prooxidant actions of (−)-epigallocatechin-3-gallate. Free Radic. Biol. Med. 2010, 49, 2010–2018. [Google Scholar] [CrossRef] [PubMed]
- Manohar, M.; Fatima, I.; Saxena, R.; Chandra, V.; Sankhwar, P.L.; Dwivedi, A. (−)-Epigallocatechin-3-gallate induces apoptosis in human endometrial adenocarcinoma cells via ROS generation and p38 MAP kinase activation. J. Nutr. Biochem. 2013, 24, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Forester, S.C.; Lambert, J.D. The role of the mitochondrial oxidative stress in the cytotoxic effects of the green tea catechin, (−)-epigallocatechin-3-gallate, in oral cells. Mol. Nutr. Food Res. 2014, 58, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Ijiri, M.; Suzuki, T.; Taguchi, K.; Koyama, Y.; Isemura, M. Green tea with a high catechin content suppresses inflammatory cytokine expression in the galactosamine-injured rat liver. Biomed. Res. 2005, 26, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhu, W.; Shen, C.L.; Gao, W. Green tea polyphenols reduce body weight in rats by modulating obesity-related genes. PLoS ONE 2012, 7, e38332. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Cao, J.J.; Dagda, R.Y.; Chanjaplammootil, S.; Lu, C.; Chyu, M.C.; Gao, W.; Wang, J.S.; Yeh, J.K. Green tea polyphenols benefits body composition and improves bone quality in long-term high-fat diet-induced obese rats. Nutr. Res. 2012, 32, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Bao, S.; Yang, W.; Zhang, J.; Li, L.; Shan, Z.; Teng, W. Epigallocatechin gallate prevents inflammation by reducing macrophage infiltration and inhibiting tumor necrosis factor-α signaling in the pancreas of rats on a high-fat diet. Nutr. Res. 2014, 34, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Bogdanski, P.; Suliburska, J.; Szulinska, M.; Stepien, M.; Pupek-Musialik, D.; Jablecka, A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr. Res. 2012, 32, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Ethanol and liver: Recent insights into the mechanisms of ethanol-induced fatty liver. World J. Gastroenterol. 2014, 20, 14672–14685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, Q.; Wang, L.; Chen, Y.; Wang, L.; Zhang, W. Interleukin-1β enhances the intracellular accumulation of cholesterol by up-regulating the expression of low-density lipoprotein receptor and 3-hydroxy-3-methylglutaryl coenzyme A reductase in podocytes. Mol. Cell. Biochem. 2011, 346, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Bone, R.N.; Ali, T.; Zhang, S.; Bohrer, A.; Tse, H.M.; Bidasee, K.R.; Ramanadham, S. Evidence of contribution of iPLA2β-mediated events during islet β-cell apoptosis due to proinflammatory cytokine suggests a role for iPLA2β in T1D development. Endocrinology 2014, 155, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.T.; Ha, J.; Park, I.J.; Lee, S.K.; Baik, H.W.; Kim, Y.M.; Park, O.J. Apoptotic effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway. Cancer Lett. 2007, 247, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Collins, Q.F.; Liu, H.Y.; Pi, J.; Liu, Z.; Quon, M.J.; Cao, W. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5′-AMP-activated protein kinase. J. Biol. Chem. 2007, 282, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Chan, Y.C.; Wang, M.F.; Wei, C.C.; Chang, S.J. Dietary (−)-Epigallocatechin-3-gallate supplementation counteracts aging-associated skeletal muscle insulin resistance and fatty liver in senescence-accelerated mouse. J. Agric. Food Chem. 2015, 63, 8407–8417. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Kwon, S.J.; Hong, J.; Yang, C.S. Salivary hydrogen peroxide produced by holding or chewing green tea in the oral cavity. Free Radic. Res. 2007, 41, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Wang, X.; Lu, G.; Picinich, S.C. Cancer prevention by tea: Animal studies, molecular mechanisms and human relevance. Nat. Rev. Cancer 2009, 9, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Kanadzu, M.; Lu, Y.; Morimoto, K. Dual function of (−)-epigallocatechin gallate (EGCG) in healthy human lymphocytes. Cancer Lett. 2006, 241, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, Y.; Suzuki, T.; Mochizuki, K.; Goda, T. Dietary supplementation with (−)-epigallocatechin-3-gallate reduces inflammatory response in adipose tissue of non-obese type 2 diabetic Goto-Kakizaki (GK) rats. J. Agric. Food Chem. 2013, 61, 11410–11417. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, T.; Pervin, M.; Goto, S.; Isemura, M.; Nakamura, Y. Beneficial Effects of Tea and the Green Tea Catechin Epigallocatechin-3-gallate on Obesity. Molecules 2016, 21, 1305. https://doi.org/10.3390/molecules21101305
Suzuki T, Pervin M, Goto S, Isemura M, Nakamura Y. Beneficial Effects of Tea and the Green Tea Catechin Epigallocatechin-3-gallate on Obesity. Molecules. 2016; 21(10):1305. https://doi.org/10.3390/molecules21101305
Chicago/Turabian StyleSuzuki, Takuji, Monira Pervin, Shingo Goto, Mamoru Isemura, and Yoriyuki Nakamura. 2016. "Beneficial Effects of Tea and the Green Tea Catechin Epigallocatechin-3-gallate on Obesity" Molecules 21, no. 10: 1305. https://doi.org/10.3390/molecules21101305
APA StyleSuzuki, T., Pervin, M., Goto, S., Isemura, M., & Nakamura, Y. (2016). Beneficial Effects of Tea and the Green Tea Catechin Epigallocatechin-3-gallate on Obesity. Molecules, 21(10), 1305. https://doi.org/10.3390/molecules21101305