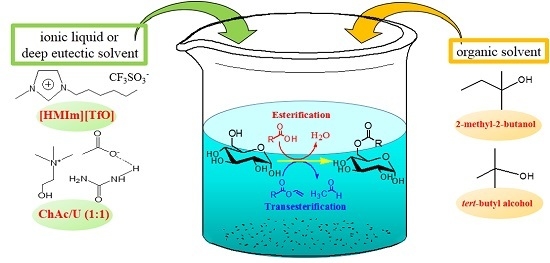
Enzymatic Synthesis of Glucose-Based Fatty Acid Esters in Bisolvent Systems Containing Ionic Liquids or Deep Eutectic Solvents
Abstract
:1. Introduction
2. Results and Discussion
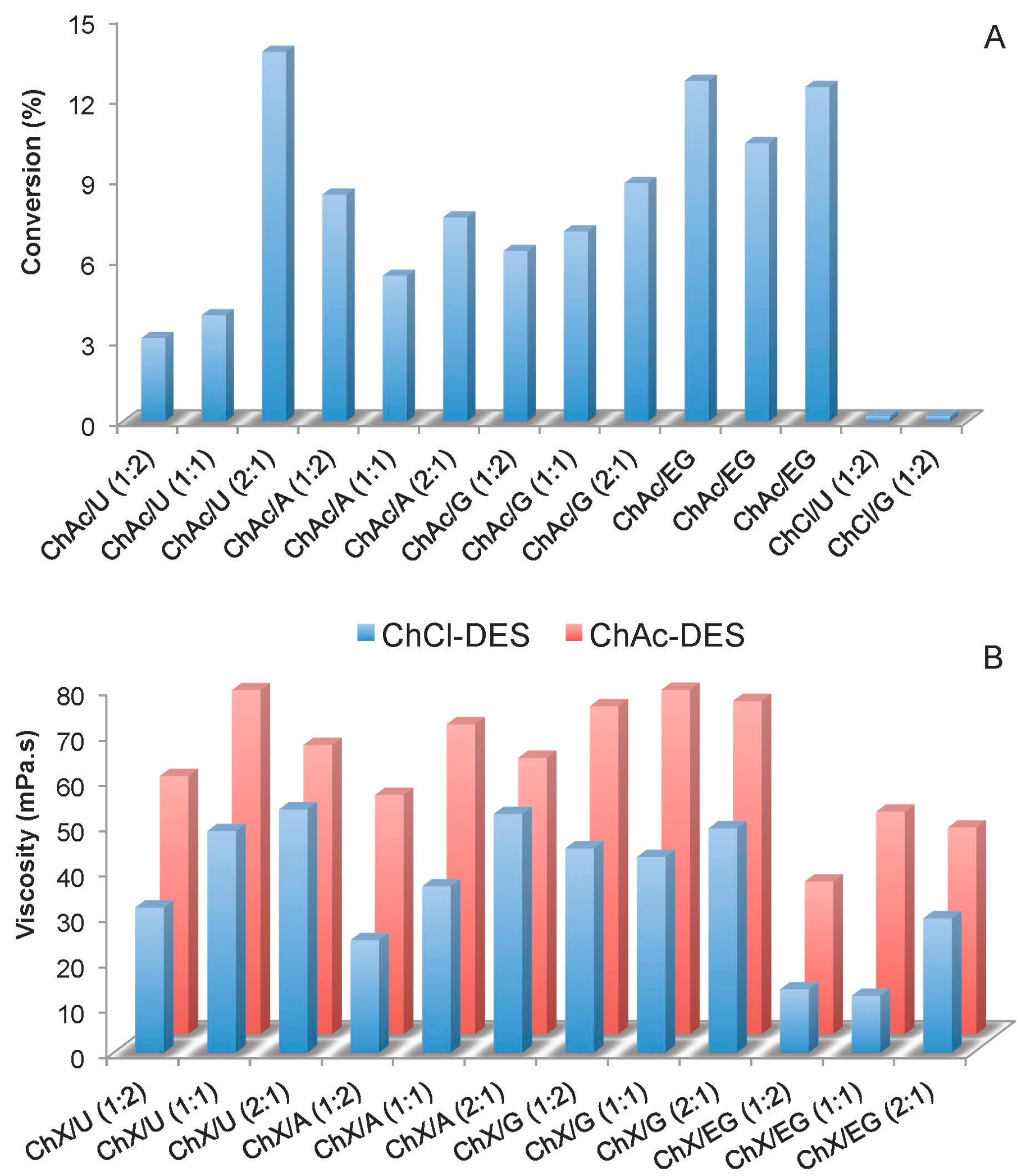
2.1. IL and DES Screening
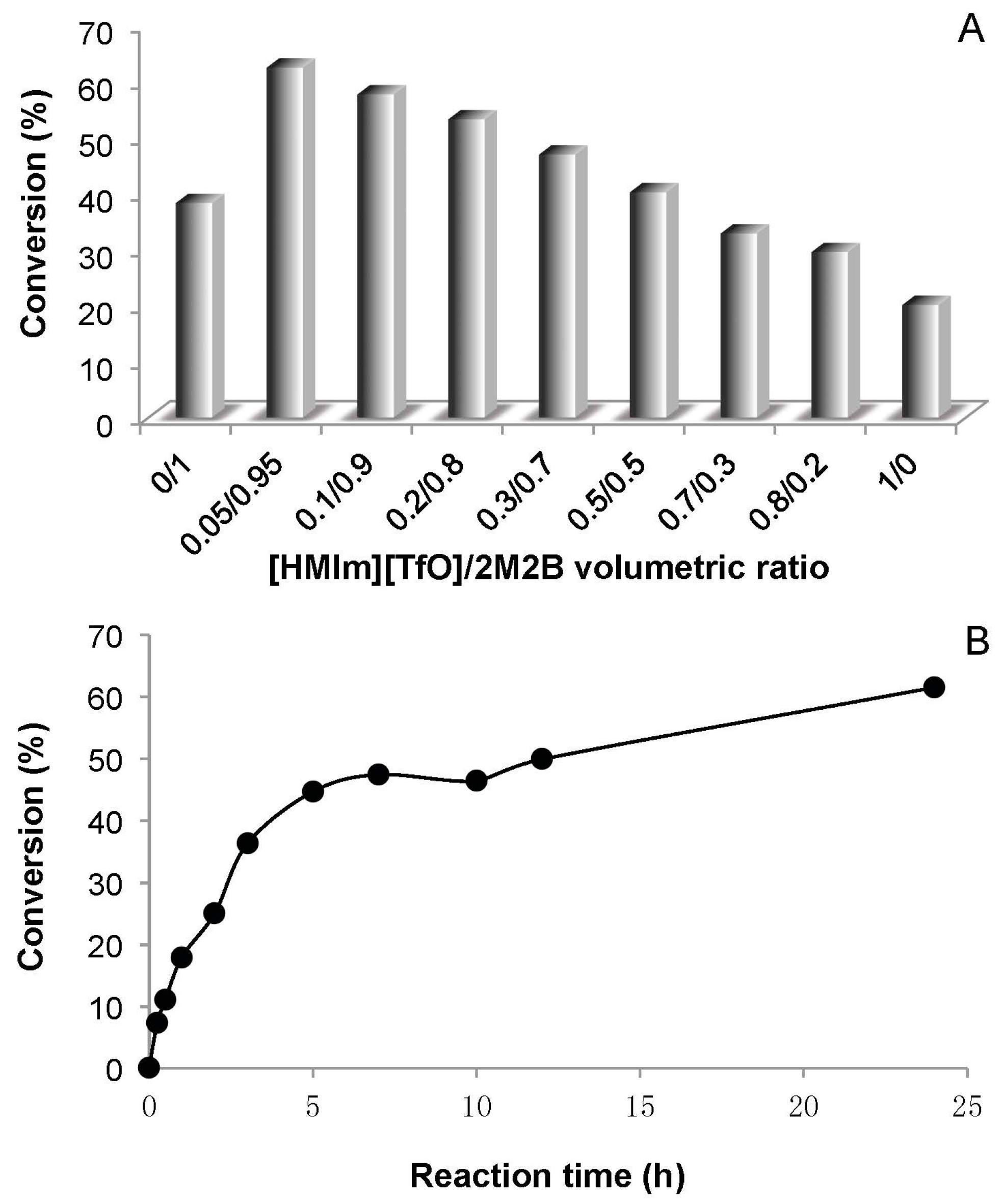
2.2. Methyl Glucoside vs. Glucose
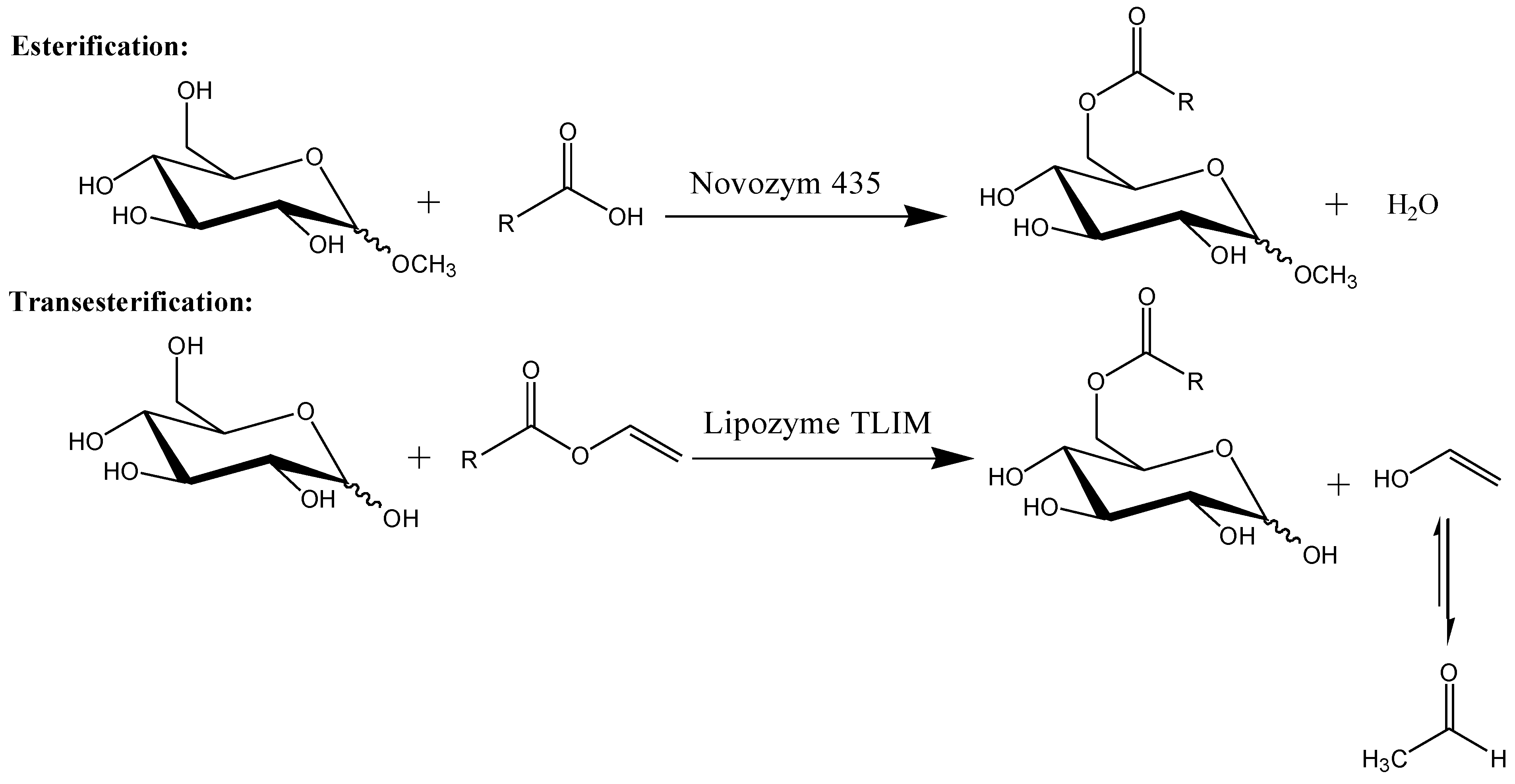
2.3. Esterification of Methyl Glucoside and Palmitic Acid, Catalyzed by Novozym 435
2.4. Transesterification of Glucose and Vinyl Laurate, Catalyzed by Lipozyme TLIM
2.5. Effect of Chain Length of the Acyl Donor
3. Materials and Methods
3.1. Materials
3.2. Preparation of DESs and Determination of Their Water Contents and Viscosities
3.3. Lipozyme TLIM-Catalyzed Transesterification of α-d-Glucose and Vinyl Esters
3.4. Novozym 435-Catalyzed Esterification of Methyl Glucoside and Fatty Acids
3.5. HPLC Analysis
3.6. RSM Experimental Design
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Garti, N.; Clement, V.; Fanun, M.; Leser, M.E. Some characteristics of sugar ester nonionic microemulsions in view of possible food applications. J. Agric. Food. Chem. 2000, 48, 3945–3956. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, M.; Costa, S.G.V.A.O. Biosurfactants in food industry. Trends Food. Sci. Technol. 2007, 18, 252–259. [Google Scholar] [CrossRef]
- Neta, N.S.; Teixeira, J.A.; Rodrigues, L.R. Sugar ester surfactants: Enzymatic synthesis and applications in food industry. Crit. Rev. Food. Sci. Nutr. 2015, 55, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.R.; Rathod, V.K. Enzyme catalyzed synthesis of cosmetic esters and its intensification: A review. Process Biochem. 2015, 50, 1793–1806. [Google Scholar] [CrossRef]
- Therisod, M.; Klibanov, A.M. Facile Enzymatic preparation of monoacylated sugars in pyridine. J. Am. Chem. Soc. 1986, 108, 5638–5640. [Google Scholar] [CrossRef]
- Kobayashi, T. Lipase-catalyzed syntheses of sugar esters in non-aqueous media. Biotechnol. Lett. 2011, 33, 1911–1919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Huang, Z.-L. Enzymatic synthesis of sugar fatty acid esters in ionic liquids. Catal. Sci. Technol. 2012, 2, 1767–1775. [Google Scholar] [CrossRef]
- Cao, L.; Bornscheuer, U.T.; Schimid, R.D. Lipase-catalyzed solid phase synthesis of sugar esters. Fett/Lipid 1996, 98, 332–335. [Google Scholar] [CrossRef]
- Otto, R.T.; Bornscheuer, U.T.; Syldatk, C.; Schmid, R.D. Lipase-catalyzed synthesis of arylaliphatic esters of β-d(+)-glucose, n-alkyl- and arylglucosides and characterization of their surfactant properties. J. Biotechnol. 1998, 64, 231–237. [Google Scholar] [CrossRef]
- Laane, C.; Boeren, S.; Vos, K.; Veeger, C. Rules for optimization of biocatalysis in organic solvents. Biotechnol. Bioeng. 1987, 30, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Adlehorst, K.; Björkling, F.; Godtfredsen, S.E.; Kirk, O. Enzyme catalyzed preparation of 6-O-acylglucopyranosides. Synthesis 1990, 1990, 112–115. [Google Scholar] [CrossRef]
- Ikeda, I.; Klibanov, A.M. Lipase-catalyzed acylation of sugars solubilized in hydrophobic solvents by complexation. Biotechnol. Bioeng. 1993, 42, 788–791. [Google Scholar] [CrossRef] [PubMed]
- Sarney, D.B.; Vulfson, E.N. Application of enzymes to the synthesis of surfactants. Trends Biotechnol. 1995, 13, 164–172. [Google Scholar] [CrossRef]
- Ruela, H.S.; Sutili, F.K.; Leal, I.C.R.; Carvalho, N.M.F.; Miranda, L.S.M.; de Souza, R.O.M.A. Lipase-catalyzed synthesis of secondary glucose esters under continuous flow conditions. Eur. J. Lipid Sci. Technol. 2013, 115, 464–467. [Google Scholar] [CrossRef]
- Sutili, F.K.; Ruela, H.S.; Leite, S.G.F.; de M Miranda, L.S.; Leal, I.C.R.; de Souza, R.O.M.A. Lipase-catalyzed esterification of steric hindered fructose derivative by continuous flow and batch conditions. J. Mol. Catal. B Enzym. 2013, 85–86, 37–42. [Google Scholar] [CrossRef]
- Katsoura, M.H.; Katapodis, P.; Kolisis, F.N.; Stamatis, H. Effect of different reaction parameters on the lipase-catalyzed selective acylation of polyhydroxylated natural compounds in ionic liquids. Process Biochem. 2007, 42, 1326–1334. [Google Scholar] [CrossRef]
- Rahman, M.B.A.; Arumugam, M.; Khairuddin, N.S.K.; Abdulmalek, E.; Basri, M.; Salleh, A. Microwave assisted enzymatic synthesis of fatty acid sugar ester in ionic liquid-tert-butanol biphasic solvent system. Asian J. Chem. 2012, 24, 5058–5062. [Google Scholar]
- Galonde, N.; Brostaux, Y.; Richard, G.; Nott, K.; Jerôme, C.; Fauconnier, C. Use of response surface methodology for the optimization of the lipase-catalyzed synthesis of mannosyl myristate in pure ionic liquid. Process Biochem. 2013, 48, 1914–1920. [Google Scholar] [CrossRef]
- Fischer, F.; Happe, M.; Emery, J.; Fornage, A.; Schütz, R. Enzymatic synthesis of 6- and 6’-O-linoleyl-α-d-maltose: From solvent-free to binary ionic liquid reaction media. J. Mol. Catal. B Enzym. 2013, 90, 98–106. [Google Scholar] [CrossRef]
- Mai, N.L.; Ahn, K.; Bae, S.W.; Shin, D.W.; Morya, K.; Koo, Y.-M. Ionic liquids as novel solvents for the synthesis of sugar fatty acid ester. Biotechnol. J. 2014, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ji, F.; Wang, J.; Li, Y.; Bao, Y. Esterification degree of fructose laurate exerted by Candida antarctica lipase B in organic solvents. Enzyme Microb. Technol. 2015, 69, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Findrik, Z.; Megyeri, G.; Gubicza, L.; Bélafi-Bakó, K.; Nemestóthy, N.; Sudar, M. Lipase catalyzed synthesis of glucose palmitate in ionic liquid. J. Clean. Prod. 2016, 112, 1106–1111. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef]
- Yang, Z.; Wen, Q. Deep eutectic solvents as a new reaction medium for biotransformations. In Ionic Liquid Based Surfactant Science: Formulation, Characterization and Applications; Paul, B.K., Moulik, S.P., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 517–531. [Google Scholar]
- Pöhnlein, M.; Ulrich, J.; Kirschhöfer, F.; Nusser, M.; Muhle-Goll, C.; Kannengiesser, B.; Brenner-Weiß, G.; Luy, B.; Liese, A.; Syldatk, C.; et al. Lipase-catalyzed synthesis of glucose-6-O-hexanoate in deep eutectic solvents. Eur. J. Lipid Sci. Technol. 2015, 117, 161–166. [Google Scholar] [CrossRef]
- Lin, X.-S.; Wen, Q.; Huang, Z.-L.; Cai, Y.-Z.; Halling, P.J.; Yang, Z. Impacts of ionic liquids on enzymatic synthesis of glucose laurate and optimization with superior productivity by response surface methodology. Process Biochem. 2015, 50, 1852–1858. [Google Scholar] [CrossRef]
- Lin, X.-S.; Zou, Y.; Zhao, K.-H.; Yang, T.-X.; Halling, P.J.; Yang, Z. Tetraalkylammonium ionic liquids as dual solvents–catalysts for direct synthesis of sugar fatty acid esters. J. Surfactants Deterg. 2016, 19, 511–517. [Google Scholar] [CrossRef]
- Huang, Z.-L.; Wu, B.-P.; Wen, Q.; Yang, T.-X.; Yang, Z. Deep eutectic solvents can be viable enzyme activators and stabilizers. J. Chem. Technol. Biotechnol. 2014, 89, 1975–1981. [Google Scholar] [CrossRef]
- Durand, E.; Lecomte, J.; Baréa, B.; Villeneuve, P. Towards a better understanding of how to improve lipase-catalyzed reactions using deep eutectic solvents based on choline chloride. Eur. J. Lipid Sci. Technol. 2014, 116, 16–23. [Google Scholar] [CrossRef]
- Yang, Z.; Pan, W. Ionic liquids: green solvents for nonaqueous biocatalysis. Enzyme Microb. Technol. 2005, 37, 19–28. [Google Scholar] [CrossRef]
- Pöhnlein, M.; Slomka, C.; Kukharenko, O.; Gärtner, T.; Wiemann, L.O.; Sieber, V.; Syldatk, C.; Hausmann, R. Enzymatic synthesis of amino sugar fatty acid esters. Eur. J. Lipid Sci. Technol. 2014, 116, 423–428. [Google Scholar] [CrossRef]
- Yan, Y.; Bornscheuer, U.T.; Cao, L.; Schmid, R.D. Lipase-catalyzed solid-phase synthesis of sugar fatty acid esters. Removal of byproducts by azeotropic distillation. Enzyme Microb. Technol. 1999, 25, 725–728. [Google Scholar] [CrossRef]
- Ferrer, M.; Cruces, M.A.; Plou, F.J.; Bernabé, M.; Ballesteros, A. A simple procedure for the regioselective synthesis of fatty acid esters of maltose, leucrose, maltotriose and n-dodecyl maltosides. Tetrahedron 2000, 56, 4053–4061. [Google Scholar] [CrossRef]
- Ferrer, M.; Soliveri, J.; Plou, F.J.; López-Cortés, N.; Reyes-Duarte, D.; Christensen, M.; Copa-Patiño, J.L.; Ballesteros, A. Synthesis of sugar esters in solvent mixtures by lipases from Thermomyces lanuginosus and Candida antarctica B, and their antimicrobial properties. Enzyme Microb. Technol. 2005, 36, 391–398. [Google Scholar] [CrossRef]
- Zhang, X.; Nie, K.; Zheng, Y.; Wang, F.; Deng, L.; Tan, T. Enzymatic production and functional characterization of d-sorbitol monoesters with various fatty acids. Catal. Comm. 2015, 72, 138–141. [Google Scholar] [CrossRef]
- Yang, R.; Zhao, X.; Liu, X. Novel and highly efficient regioselective route to helicid esters by Lipozyme TLL. PLoS ONE 2013, 8, e80715. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.; Holmberg, K.; Watzke, H.; Leser, M.E.; Miller, R. Lipases at interfaces: A review. Adv. Colloid Interface Sci. 2009, 147–148, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.




| No. | Ionic Liquid a | Transesterification b | Esterification c |
|---|---|---|---|
| 1 | [BMIm][PF6] | 0.20 | 6.21 |
| 2 | [BMIm][BF4] | 2.32 | 22.46 |
| 3 | [EMIm][MeSO4] | 0.20 | 0.21 |
| 4 | [EMIm][MeSO4] | 0.20 | 0.21 |
| 5 | [BMIm][MeSO4] | 0.60 | 0.21 |
| 6 | [EMIm][Tf2N] | 2.11 | |
| 7 | [BMIm][Tf2N] | 2.08 | 5.71 |
| 8 | [C7-MIm][Tf2N] | 0.20 | 7.29 |
| 9 | [C12-MIm][Tf2N] | 0.20 | 6.59 |
| 10 | [BTMA][Tf2N] | 0.20 | 3.74 |
| 11 | [HTMA][Tf2N] | 0.59 | 3.47 |
| 12 | [EMIm][TfO] | 2.38 | |
| 13 | [BMIm][TfO] | 26.50 | 30.89 |
| 14 | [HMIm][TfO] | 26.84 | 30.67 |
| Reagent | IL/2M2B (v/v) | ||
|---|---|---|---|
| 0/10 | 1/9 | 3/7 | |
| Glucose | 2.5 | 3.5 | 7.0 |
| Methyl glucoside | 10 | 12 | 20 |
| Variable | Symbol | Level | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Enzyme dosage (mg) | A | 5 | 20 | 35 |
| VL/Glc molar ratio | B | 0.5 | 1 | 1.5 |
| Reaction time (h) | C | 18 | 22 | 26 |
| 2M2B/IL (v/v) | D | 1.0 | 2.5 | 4.0 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, K.-H.; Cai, Y.-Z.; Lin, X.-S.; Xiong, J.; Halling, P.J.; Yang, Z. Enzymatic Synthesis of Glucose-Based Fatty Acid Esters in Bisolvent Systems Containing Ionic Liquids or Deep Eutectic Solvents. Molecules 2016, 21, 1294. https://doi.org/10.3390/molecules21101294
Zhao K-H, Cai Y-Z, Lin X-S, Xiong J, Halling PJ, Yang Z. Enzymatic Synthesis of Glucose-Based Fatty Acid Esters in Bisolvent Systems Containing Ionic Liquids or Deep Eutectic Solvents. Molecules. 2016; 21(10):1294. https://doi.org/10.3390/molecules21101294
Chicago/Turabian StyleZhao, Kai-Hua, Yu-Zheng Cai, Xiao-Sheng Lin, Jun Xiong, Peter J. Halling, and Zhen Yang. 2016. "Enzymatic Synthesis of Glucose-Based Fatty Acid Esters in Bisolvent Systems Containing Ionic Liquids or Deep Eutectic Solvents" Molecules 21, no. 10: 1294. https://doi.org/10.3390/molecules21101294