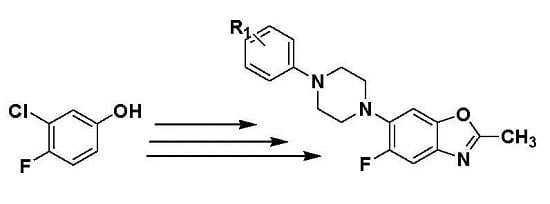
Design, Synthesis, and Cytotoxicity of 5-Fluoro-2-methyl-6-(4-aryl-piperazin-1-yl) Benzoxazoles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Cytotoxicity
3. Materials and Methods
3.1. Chemistry
3.1.1. General Procedure for Synthesis of 4-Fluoro-5-(substitutedphenyl-piperazin-1-yl)-2-nitro-phenol (6a, 6b, 6d, 6e, 6g, 6i, 6j)
3.1.2. General Procedure for Synthesis 5-[4-(o/m/p-Hydroxy-phenyl)-piperazin-1-yl]-4-fluoro-2-nitro-phenol (6c, 6f, 6h)
3.1.3. General Procedure for Synthesis of 5-Fluoro-6-(substitutedphenyl-piperazin-1-yl)-benzoxazoles (7a–j)
3.2. Cytotoxicity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chanda, K.; Maiti, B.; Yellol, G.S.; Chien, M.-H.; Kuoc, M.-L.; Sun, C.-M. Polymer supported synthesis of novel benzoxazole linked benzimidazoles under microwave conditions: In vitro evaluation of VEGFR-3 kinase inhibition activity. Org. Biomol. Chem. 2011, 9, 1917–1926. [Google Scholar] [CrossRef] [PubMed]
- Sheng, C.; Che, X.; Wang, W.; Wang, S.; Cao, Y.; Yao, J.; Miao, Z.; Zhang, W. Design and synthesis of antifungal benzoheterocyclic derivatives by scaffold hopping. Eur. J. Med. Chem. 2011, 46, 1706–1712. [Google Scholar] [CrossRef] [PubMed]
- Klimešováa, V.; Kočía, J.; Waissera, K.; Kaustováb, J.; Möllmannc, U. Preparation and in vitro evaluation of benzylsulfanyl benzoxazole derivatives as potential antituberculosis agents. Eur. J. Med. Chem. 2009, 44, 2286–2293. [Google Scholar] [CrossRef] [PubMed]
- Arhancet, G.B.; Walker, D.P.; Metz, S.; Fobian, Y.M.; Heasley, S.E.; Carter, J.S.; Springer, J.R.; Jones, D.E.; Hayes, M.J.; Shaffer, A.F.; et al. Discovery and SAR of PF-4693627, a potent, selective and orally bioavailable mPGES-1 inhibitor for the potential treatment of inflammation. Bioorg. Med. Chem. Lett. 2013, 23, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.G.; Lo, J.R.; Comery, T.A.; Zhang, G.M.; Zhang, J.Y.; Kowal, D.M.; Smith, D.L.; Di, L.; Kerns, E.H.; Schechter, L.E.; et al. Identification of a series of benzoxazoles as potent 5-HT6 ligands. Bioorg. Med. Chem. Lett. 2009, 19, 1115–1117. [Google Scholar] [CrossRef] [PubMed]
- Kallashi, F.; Kim, D.; Kowalchick, J.; Park, Y.J.; Hunt, J.A.; Ali, A.; Smith, C.J.; Hammond, M.L.; Pivnichny, J.V.; Tong, X.; et al. 2-Arylbenzoxazoles as CETP inhibitors: Raising HDL-C in cynoCETP transgenic mice. Bioorg. Med. Chem. Lett. 2011, 21, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Ongarora, D.S.B.; Gut, J.; Rosenthal, P.J.; Masimirembwa, C.M.; Chibale, K. Benzoheterocyclic amodiaquine analogues with potent antiplasmodial activity: Synthesis and pharmacological evaluation. Bioorg. Med. Chem. Lett. 2012, 22, 5046–5050. [Google Scholar] [CrossRef] [PubMed]
- Murty, M.S.R.; Rao, B.R.; Katiki, M.R.; Nath, L.R.; Anto, R.J. Synthesis of piperazinyl benzothiazole/benzoxazole derivatives coupled with 1,3,4-oxadiazole-2-thiol: Novel hybrid heterocycles as anticancer agents. Med. Chem. Res. 2013, 22, 4980–4991. [Google Scholar] [CrossRef]
- Shao, K.-P.; Zhang, X.-Y.; Chen, P.-J.; Xue, D.-Q.; He, P.; Ma, L.-Y.; Zheng, J.-X.; Zhang, Q.-R.; Liu, H.-M. Synthesis and biological evaluation of novel pyrimidine-benzimidazol hybrids as potential anticancer agents. Bioorg. Med. Chem. Lett. 2014, 24, 3877–3881. [Google Scholar] [CrossRef] [PubMed]
- Shaquiquzzaman, M.; Verma, G.; Marella, A.; Akhter, M.; Akhtar, W.; Khan, M.F.; Tasneem, S.; Alam, M.M. Piperazine scaffold: A remarkable tool in generation of diverse pharmacological agents. Eur. J. Org. Chem. 2015, 102, 487–529. [Google Scholar] [CrossRef] [PubMed]
- Filler, R.; Saha, R. Fluorine in medicinal chemistry: a century of progress and a 60-year retrospective of selected highlights. Future Med. Chem. 2009, 1, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.P.; Domagala, J.M.; Hagen, S.E.; Heifetz, C.L.; Hutt, M.P.; Nichols, J.B.; Trehan, A.K. Quinolone Antibacterial Agents. Synthesis and Structure-Activity Relationships of 8-Substituted Quinoline-3-carboxylic Acids and 1,8-Naphthyridine-3-carboxylic Acids. J. Med. Chem. 1988, 31, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Abdel-jalil, R.J.; Al-Qawasemeh, R.A.; Voleter, W.; Heeg, P.; El-Abadelah, M.M.; Sabri, S.S. Synthesis and properties of some 2,3-disubstituted 6-fluoro-7-(4-methyl-1-piperazinyl)quinoxalines. J. Heterocycl. Chem. 2009, 37, 1273–1275. [Google Scholar] [CrossRef]
- Abu-Elteen, K.H.; Abdel-Jalil, R.J.; Hamad, M.A.; Ghaleb, M.; Khan, K.M.; Voelter, W. fungicidal effects of some derivatives of 2-ferrocenyl-benzimidazoles: A possible template for antifungal drug design. J. Med. Sci. 2008, 8, 673–681. [Google Scholar] [CrossRef]
- Abdel-Jalil, R.J.; Aldoqum, H.M.; Ayoub, M.T.; Voelter, W. Synthesis and antitumor activity of 2-aryl-6-fluoro-6-(4-methyl-1-piperazinyl)-4-(3H)-Quinazolinones. Heterocycles 2005, 65, 2061–2070. [Google Scholar] [CrossRef]
- Al-Harthy, T.; Abdel-Jalil, R.; Zoghaib, W.; Pflüger, M.; Hofmann, E.; Hundsberger, H. Design and Synthesis of Benzothiazole Schiff Bases of Potential Antitumor Activitiy. Heterocycles 2016, 92, 1282–1292. [Google Scholar]
- Crouch, S.; Kozlowski, R.; Slater, K.; Fletcher, J. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J. Immunol. Methods. 1993, 160, 81–88. [Google Scholar] [CrossRef]
- Guidance Document on Using in Vitro Data to Estimate in Vivo Starting Doses for Acute Toxicity. Available online: http://www.epa.gov/hpv/pubs/general/nih2001b.pdf (accessed on 5 November 2014).
- Sample Availability: Samples of the compounds are available from the authors.

| Compound | R | Time (h) | Yield * % | Solvent ** | Compound | Time (h) | Yield * % | Solvent ** |
|---|---|---|---|---|---|---|---|---|
| 6a | H | 5.5 | 72 | Toluene | 7a | 4 | 71 | Benzene |
| 6b | p-CH3 | 2 | 73 | Toluene | 7b | 3 | 53 | Benzene |
| 6c | p-OH | 24 | 37 | Chlorobenzene | 7c | 4 | 63 | Benzene |
| 6d | p-F | 2 | 73 | Toluene | 7d | 2.5 | 51 | Benzene |
| 6e | p-OCH3 | 1.5 | 27 | Toluene | 7e | 1 | 70 | Benzene |
| 6f | o-OH | 3 | 71 | Chlorobenzene | 7f | 1.5 | 55 | Benzene |
| 6g | o-F | 2.5 | 83 | Toluene | 7g | 2 | 74 | Benzene |
| 6h | m-OH | 24 | 28 | Chlorobenzene | 7h | 2 | 74 | Benzene |
| 6i | m-OCH3 | 2 | 73 | Toluene | 7i | 1 | 77 | Benzene |
| 6j | 3,4-Cl | 5 | 33 | Toluene | 7j | 1 | 75 | Benzene |
| Concentration (C) µM | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liver Cells, Hepatocytes | Lung Cancer Cells | |||||||||||||||
| Compound | 0 | 0.1 | 1 | 10 | 50 | 100 | 250 | 500 | 0 | 0.1 | 1 | 10 | 50 | 100 | 250 | 500 |
| 6a | 100 | 86 | 97 | 112 | 90 | 76 | 100 | 97 | 104 | 64 | 61 | 58 | ||||
| 6b | 100 | 107 | 123 | 120 | 97 | 96 | 100 | 99 | 100 | 68 | 65 | |||||
| 6c | 100 | 95 | 101 | 110 | 101 | 89 | 100 | 104 | 103 | 102 | 94 | |||||
| 6d | 100 | 94 | 87 | 101 | 57 | 62 | 44 | 13 | 100 | 103 | 111 | 78 | 58 | 54 | 46 | 34 |
| 6e | 100 | 107 | 110 | 104 | 74 | 79 | 100 | 101 | 104 | 96 | 71 | |||||
| 6f | 100 | 105 | 104 | 110 | 76 | 100 | 97 | 100 | 93 | 60 | 46 | |||||
| 6g | 100 | 110 | 109 | 112 | 87 | 100 | 96 | 96 | 62 | 51 | 42 | |||||
| 6h | 100 | 117 | 109 | 108 | 83 | 58 | 100 | 106 | 106 | 105 | 87 | |||||
| 6i | 100 | 79 | 95 | 99 | 100 | 95 | 93 | 96 | 69 | |||||||
| 6j | 100 | 119 | 115 | 81 | 102 | 108 | 100 | 98 | 94 | 65 | 62 | |||||
| 7a | 100 | 101 | 97 | 94 | 100 | 101 | 96 | 90 | 60 | |||||||
| 7b | 100 | 105 | 99 | 91 | 63 | 60 | 100 | 92 | 94 | 92 | 87 | 75 | ||||
| 7c | 100 | 98 | 107 | 101 | 93 | 100 | 92 | 97 | 101 | 90 | 83 | |||||
| 7d | 100 | 91 | 94 | 85 | 100 | 98 | 98 | 87 | 87 | 82 | ||||||
| 7e | 100 | 107 | 108 | 91 | 71 | 100 | 93 | 92 | 75 | 74 | 68 | |||||
| 7f | 100 | 106 | 97 | 86 | 59 | 60 | 53 | 54 | 100 | 103 | 99 | 91 | 91 | 95 | 83 | 63 |
| 7g | 100 | 96 | 106 | 87 | 100 | 96 | 92 | 92 | 73 | 63 | ||||||
| 7h | 100 | 100 | 102 | 99 | 100 | 100 | 93 | 97 | 95 | 90 | ||||||
| 7i | 100 | 100 | 102 | 91 | 100 | 105 | 102 | 88 | 76 | 75 | ||||||
| 7j | 100 | 100 | 90 | 85 | 65 | 27 | 7 | 7 | 100 | 98 | 94 | 81 | 70 | 47 | 28 | |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Harthy, T.; Zoghaib, W.M.; Pflüger, M.; Schöpel, M.; Önder, K.; Reitsammer, M.; Hundsberger, H.; Stoll, R.; Abdel-Jalil, R. Design, Synthesis, and Cytotoxicity of 5-Fluoro-2-methyl-6-(4-aryl-piperazin-1-yl) Benzoxazoles. Molecules 2016, 21, 1290. https://doi.org/10.3390/molecules21101290
Al-Harthy T, Zoghaib WM, Pflüger M, Schöpel M, Önder K, Reitsammer M, Hundsberger H, Stoll R, Abdel-Jalil R. Design, Synthesis, and Cytotoxicity of 5-Fluoro-2-methyl-6-(4-aryl-piperazin-1-yl) Benzoxazoles. Molecules. 2016; 21(10):1290. https://doi.org/10.3390/molecules21101290
Chicago/Turabian StyleAl-Harthy, Thuraya, Wajdi Michel Zoghaib, Maren Pflüger, Miriam Schöpel, Kamil Önder, Maria Reitsammer, Harald Hundsberger, Raphael Stoll, and Raid Abdel-Jalil. 2016. "Design, Synthesis, and Cytotoxicity of 5-Fluoro-2-methyl-6-(4-aryl-piperazin-1-yl) Benzoxazoles" Molecules 21, no. 10: 1290. https://doi.org/10.3390/molecules21101290