Site-Selective Ribosylation of Fluorescent Nucleobase Analogs Using Purine-Nucleoside Phosphorylase as a Catalyst: Effects of Point Mutations
Abstract
:1. Introduction
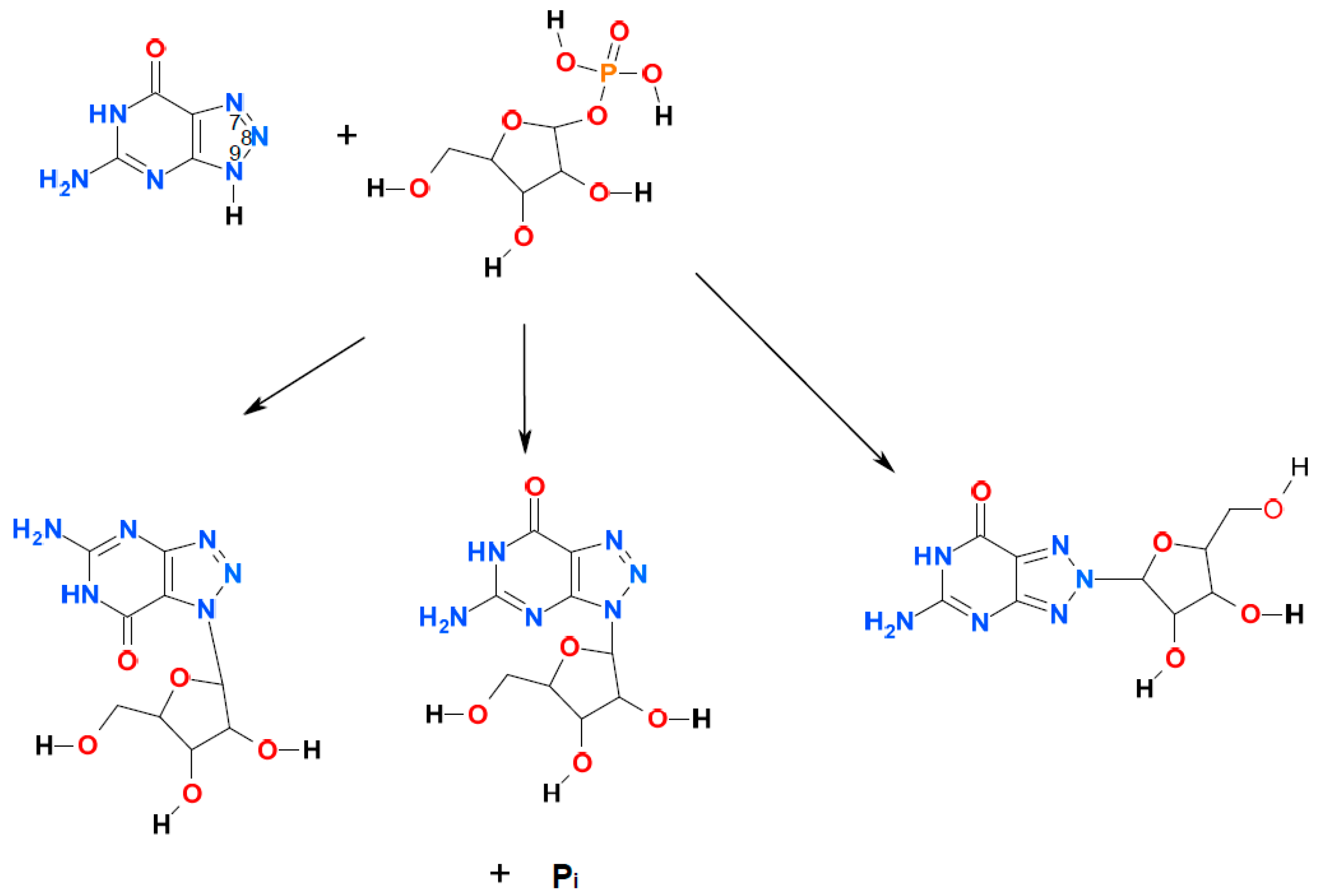
2. Results and Discussion
2.1. Ribosylation of 8-Azaguanine and 2,6-Diamino-8-azapurine

| Substrate/Enzyme | Km (µM) | Vmax (Relative) * | Approximate Product Ratio: N9-riboside: N8-riboside:N7-riboside |
|---|---|---|---|
| 8-azaGua/calf PNP-wt | ~90 | 21 | 1:0:0 |
| 8-azaGua/calf PNP-N243D | >100 | >0.2 *** | 1:0:2 |
| 8-azaGua/E. coli PNP-wt | >200 | ~1 *** | 20:1:0 |
| 8-azaGua/E. coli PNP-D204N | nd | traces | predominantly N9 |
| 8-azaDaPur/calf PNP-wt | 60 | ~1 | 0:1:1 ** |
| 8-azaDaPur/calf PNP-N243D | 35 | 0.6 | 1:0:3 |
| 8-azaDaPur/E. coli PNP-wt | >200 | ~3 *** | 1:2:0 |
| 8-azaDaPur/E. coli PNP-D204N | >200 | ~0.4 *** | 10:1:0 |
2.2. Phosphorolysis of 8-Azaguanine and 2,6-Diamino-8-azapurine Ribosides
| Substrate | Enzyme (wt = Wild Type) | Km (µM) | Vmax (Relative) * |
|---|---|---|---|
| N7-β-d-ribosyl-Gua ** | calf PNP-wt | 27 | 0.6 |
| N7-β-d-ribosyl-Gua ** | E. coli-wt | ~450 | 33 |
| N7-ribosyl-8-azaGua | calf PNP-wt | nd | >0.13 |
| N7-ribosyl-8-azaGua | calf PNP-N243D | nd | >1.5 |
| N7-ribosyl-8-azaDaPur | calf PNP-wt | 52 | ~20 |
| N7-ribosyl-8-azaDaPur | calf PNP-N243D | >50 | >60 |
| N7-ribosyl-8-azaDaPur | E. coli PNP-wt | ~80 | ~1.7 |
| N7-ribosyl-8-azaDaPur | E. coli PNP-D204N | nd | ~0.7 *** |
| N8-ribosyl-8-azaDaPur | E. coli PNP-wt | 7 | 1.1 |
| N8-ribosyl-8-azaDaPur | E. coli PNP-D204N | nd | ~1.6 *** |
| N9-ribosyl-8-azaDaPur | E. coli PNP-wt | ~20 | ~0.02 |
2.3. Fluorescence of 8-Azaguanine and 2,6-Diamino-8-azapurine Ribosides and Potential Applications
2.3.1. Fluorescence of 2,6-Diamino-8-azapurine Ribosides
| Compound | pKa | Form * (pH) | UV Absorption | Fluorescence | |||
|---|---|---|---|---|---|---|---|
| λmax (nm) | εmax (M−1·cm−1) | λmax (nm) | ϕ | τ (ns) | |||
| 9-β-d-ribofuranosyl-8-azaDaPur | 2.9 | n (7) | 285 | 10,800 | 368 | 0.9 | 6 |
| c (2) | 283 | 8100 | 360 | nd ** | nd ** | ||
| 8-β-d-ribofuranosyl-8-azaDaPur | 4.9 | n (7) | 313 | 8200 | 430 | 0.41 | 10.6 |
| c (2.7) | 264 | 13,200 | 430 | nd ** | nd ** | ||
| 7-β-d-ribofuranosyl-8-azaDaPur | 3.95 | n (7) | 314 | ~5500 | 420 | 0.063 | 1.5; 0.45 |
| c (2) | 258 | ~12,000 | 420 | nd ** | nd ** | ||
| 8-azaDaPur (free base) | 3.7; 7.7 | n (6) | 280 | 8500 | 365 | 0.40 | 7.5; 0.2 |
| 9-β-d-ribofuranosyl-8-azaGua | 8.05 | n (5) | 256 | 12,900 | 347 | ~0.01 | ~0.1 |
| ma (10) | 278 | 11,700 | 362 | 0.55 | 5.6 | ||
| 7-β-d-ribofuranosyl-8-azaGua | 7.4 | n (5) | 302 | 4900 | 410 | ~0.04 | nd ** |
| ma (10) | 304 | 5100 | 420 | ~0.03 | nd ** | ||
| 8-azaGua (free base) | 6.5 | n (4.5) | 249 | 11,200 | 395 | 0.05–0.33 | 6.2 |
2.3.2. Blood PNP Determination Using Fluorogenic Ribosides

3. Experimental Section
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Bzowska, A.; Kulikowska, E.; Shugar, D. Purine nucleoside phosphorylases: Properties, functions, and clinical aspects. Pharmacol. Ther. 2000, 88, 349–425. [Google Scholar] [CrossRef]
- Grunebaum, E.; Cohen, A.; Roifman, C.M. Recent advances in understanding and managing adenosine deaminase and purine nucleoside phosphorylase deficiencies. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Edwards, P.N. A kinetic, modeling and mechanistic re-analysis of thymidine phosphorylase and some related enzymes. J. Enzym. Inhib. Med. Chem. 2006, 21, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Taylor Ringia, E.A.; Schramm, V.L. Transition states and inhibitors of the purine nucleoside phosphorylase family. Curr. Top. Med. Chem. 2005, 5, 1237–1258. [Google Scholar] [CrossRef] [PubMed]
- Robak, P.; Robak, T. Older and new purine nucleoside analogs for patients with acute leukemias. Cancer Treat. Rev. 2013, 39, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Al-Kali, A.; Gandhi, V.; Ayoubi, M.; Keating, M.; Ravandi, F. Forodesine: Review of preclinical and clinical data. Future Oncol. 2010, 6, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- De Jersey, J.; Holý, A.; Hocková, D.; Naesens, L.; Keough, D.T.; Guddat, L.W. 6-oxopurine phosphoribosyltransferase: A target for the development of antimalarial drugs. Curr. Top. Med. Chem. 2011, 11, 2085–2102. [Google Scholar] [CrossRef] [PubMed]
- Portsmouth, D.; Hlavaty, J.; Renner, M. Suicide genes for cancer therapy. Mol. Asp. Med. 2007, 28, 4–41. [Google Scholar] [CrossRef] [PubMed]
- Mikhailopulo, I.A.; Miroshnikov, A.I. Biologically important nucleosides: Modern trends in biotechnology and application. Mendeleev Commun. 2011, 21, 57–68. [Google Scholar] [CrossRef]
- Zhou, X.; Mikhailopulo, I.A.; Cruz Bournazou, M.N.; Neubauer, P. Immobilization of thermostable nucleoside phosphorylases on MagReSyn® epoxide microspheres and their application for the synthesis of 2,6-dihalogenated purine nucleosides. J. Mol. Catal. B Enzym. 2015, 115, 119–127. [Google Scholar] [CrossRef]
- Wierzchowski, J.; Antosiewicz, J.M.; Shugar, D. 8-Azapurines as isosteric purine fluorescent probes for nucleic acid and enzymatic research. Mol. BioSyst. 2014, 10, 2756–2774. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, I.; Scartoni, V. 8-Azapurine nucleus: A versatile scaffold for different targets. Mini Rev. Med. Chem. 2009, 9, 1367–1378. [Google Scholar] [CrossRef] [PubMed]
- Wierzchowski, J.; Wielgus-Kutrowska, B.; Shugar, D. Fluorescence emission properties of 8-azapurines and their nucleosides, and application to the kinetics of the reverse synthetic reaction of PNP. Biochim. Biophys. Acta 1996, 1290, 9–17. [Google Scholar] [CrossRef]
- Stachelska-Wierzchowska, A.; Wierzchowski, J.; Wielgus-Kutrowska, B.; Mikleušević, G. Enzymatic synthesis of highly fluorescent 8-azapurine ribosides using purine-nucleoside phosphorylase reverse reaction: Variable ribosylation sites. Molecules 2013, 18, 12587–12598. [Google Scholar] [CrossRef] [PubMed]
- Wierzchowski, J.; Stachelska-Wierzchowska, A.; Wielgus-Kutrowska, B.; Mikleušević, G. Two fluorogenic substrates for purine-nucleoside phosphorylase, selective for mammalian and bacterial forms of the enzyme. Anal. Biochem. 2014, 446, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Stoeckler, J.D.; Poirot, A.F.; Smith, R.M.; Parks, R.E., Jr.; Ealick, S.E.; Takabayashi, K.; Erion, M.D. Purine nucleoside phosphorylase. 3. Reversal of purine base specificity by site-directed mutagenesis. Biochemistry 1997, 36, 11749–11756. [Google Scholar] [CrossRef] [PubMed]
- Mikleušević, G.; Štefanić, Z.; Narczyk, M.; Wielgus-Kutrowska, B.; Bzowska, A.; Luić, M. Validation of the catalytic mechanism of Escherichia coli purine nucleoside phosphorylase by structural and kinetic studies. Biochimie 2011, 93, 1610–1622. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, J.A.; Shortnacy, A.T.; Secrist, J.A., III. Synthesis and biological evaluation of 2-fluoro-8-azaadenosine and related compounds. J. Med. Chem. 1983, 26, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Seela, F.; Lampe, S. 8-Aza-2′-deoxyguanosine and related 1,2,3-triazolo[4,5-d]pyrimidine 2′-deoxyribofuranosides. Helv. Chim. Acta 1993, 72, 2388–2397. [Google Scholar] [CrossRef]
- Elliott, R.D.; Montgomery, J.A. Analogues of 8-azaguanosine. J. Med. Chem. 1976, 19, 1186–1191. [Google Scholar] [CrossRef]
- Ye, W.; Paul, D.; Gao, L.; Seckute, J.; Sangaiah, R.; Jayaraj, K.; Zhang, Z.; Kaminski, P.A.; Ealick, S.E.; Gold, A.; et al. Ethenoguanines undergo glycosylation by nucleoside 2′-deoxyribosyltransferases at non-natural sites. PLoS ONE 2014, 9, e115082. [Google Scholar] [CrossRef] [PubMed]
- Luić, M.; Koellner, G.; Shugar, D.; Saenger, W.; Bzowska, A. Calf spleen purine nucleoside phosphorylase: Structure of its ternary complex with an N(7)-acycloguanosine inhibitor and a phosphate anion. Acta Crystallogr. D57 2001, 57, 30–36. [Google Scholar] [CrossRef]
- Kierdaszuk, B.; Modrak-Wójcik, A.; Wierzchowski, J.; Shugar, D. Formycin A and its N-methyl analogues, specific inhibitors of E. coli purine nucleoside phosphorylase: Induced tautomeric shift on binding to enzyme, and enzyme → ligand fluorescence resonance energy transfer. Biochim. Biophys. Acta 2000, 1476, 109–128. [Google Scholar] [CrossRef]
- Pyrka, M.; Maciejczyk, M. Theoretical study of tautomeric equillibria of 2,6–diamino-8-azapurine and 8-aza-isoguanine. Chem. Phys. Lett. 2015, 627, 30–35. [Google Scholar] [CrossRef]
- Wierzchowski, J.; Bzowska, A.; Stępniak, K.; Shugar, D. Interactions of calf spleen purine nucleoside phosphorylase with 8-azaguanine, and a bisubstrate analogue inhibitor: Implications for the reaction mechanism. Z. Naturforsch. 2004, 59, 713–725. [Google Scholar] [CrossRef]
- Gasik, Z.; Shugar, D.; Antosiewicz, J.M. Resolving differences in substrate specificities between human and parasite phosphoribosyltransferases via analysis of functional groups of substrates and receptors. Curr. Pharm. Des. 2013, 19, 4226–4240. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Szeker, K.; Jiao, L.Y.; Oestreich, M.; Mikhailopulo, I.A.; Neubauer, P. Synthesis of 2,6-dihalogenated purine nucleosides by thermostable nucleoside phosphorylases. Adv. Synth. Catal. 2015, 357, 1237–1244. [Google Scholar] [CrossRef]
- Fateev, I.V.; Kharitonova, M.I.; Antonov, K.V.; Konstantinova, I.D.; Stepanenko, V.N.; Esipov, R.S.; Seela, F.; Temburnikar, K.W.; Seley-Radtke, K.L.; Stepchenko, V.A.; et al. Recognition of Artificial Nucleobases by E. coli Purine Nucleoside Phosphorylase versus its Ser90Ala Mutant in the Synthesis of Base-Modified Nucleosides. Chem. Eur. J. 2015, 21, 13401–13419. [Google Scholar] [CrossRef] [PubMed]
- Calleri, E.; Cattaneo, G.; Rabuffetti, M.; Serra, I.; Bavaro, T.; Massolini, G.; Speranza, G.; Ubiali, D. Flow-Synthesis of Nucleosides Catalyzed by an Immobilized Purine Nucleoside Phosphorylase from Aeromonas hydrophila: Integrated Systems of Reaction Control and Product Purification. Adv. Synth. Catal. 2015, 357, 2520–2528. [Google Scholar] [CrossRef]
- Calleri, E. Immobilized purine nucleoside phosphorylase from Aeromonas hydrophila as an on-line enzyme reactor for biocatalytic applications. J. Chromatogr. B 2014, 968, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Štefanić, Z.; Mikleušević, G.; Narczyk, M.; Wielgus-Kutrowska, B.; Bzowska, A.; Luić, M. Still a long way to fully understanding the molecular mechanism of Escherichia coli Purine Nucleoside Phosphorylase. Croat. Chem. Acta 2013, 86, 117–127. [Google Scholar] [CrossRef]
- Sinkeldam, R.W.; Greco, N.J.; Tor, Y. Fluorescent analogs of biomolecular building blocks: Design, properties, and applications. Chem. Rev. 2010, 110, 2579–2619. [Google Scholar] [CrossRef] [PubMed]
- Pollum, M.; Martínez-Fernández, L.; Crespo-Hernández, C.E. Photochemistry of nucleic acid bases and their thio- and aza-analogues in solution. Top. Curr. Chem. 2015, 355, 245–355. [Google Scholar] [PubMed]
- Liu, L.; Cottrell, J.W.; Scott, L.G.; Fedor, M.J. Direct measurement of the ionization state of an essential guanine in the hairpin ribozyme. Nat. Chem. Biol. 2009, 5, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, J.W.; Scott, L.G.; Fedor, M.J. The pH dependence of hairpin ribozyme catalysis reflects ionization of an active site adenine. J. Biol. Chem. 2011, 286, 17658–17664. [Google Scholar] [CrossRef] [PubMed]
- Wierzchowski, J.; Mędza, G.; Szabelski, M.; Stachelska-Wierzchowska, A. Properties of 2,6-diamino-8-azapurine, a highly fluorescent purine analog and its N-alkyl derivatives: Tautomerism and excited-state proton transfer reactions. J. Photochem. Photobiol. A 2013, 265, 49–57. [Google Scholar] [CrossRef]
- Wierzchowski, J.; Ogiela, M.; Iwańska, B.; Shugar, D. Selective fluorescent and fluorogenic substrates for purine-nucleoside phosphorylases from various sources, and direct fluorimetric determination of enzyme levels in human and animal blood. Anal. Chim. Acta 2002, 472, 63–74. [Google Scholar] [CrossRef]
- Breer, K.; Girstun, A.; Wielgus-Kutrowska, B.; Staron, K.; Bzowska, A. Overexpression, purification and characterization of functional calf purine nucleoside phosphorylase (PNP). Protein Expr. Purif. 2008, 61, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Krenitsky, T.A.; Koszalka, G.W.; Tuttle, J.V. Purine nucleoside synthesis, an efficient method employing nucleoside phosphorylases. Biochemistry 1981, 20, 3615–3621. [Google Scholar] [CrossRef] [PubMed]
- Kulikowska, E.; Bzowska, A.; Wierzchowski, J.; Shugar, D. Properties of two unusual, and fluorescent substrates of purine-nucleoside phosphorylase: 7-methylguanosine and 7-methylinosine. Biochim. Biophys. Acta 1986, 874, 355–366. [Google Scholar] [CrossRef]
- Wielgus-Kutrowska, B. Division of Biophysics, Institute of Experimental Physics; University of Warsaw: Warsaw, Poland, Unpublished Work.
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stachelska-Wierzchowska, A.; Wierzchowski, J.; Bzowska, A.; Wielgus-Kutrowska, B. Site-Selective Ribosylation of Fluorescent Nucleobase Analogs Using Purine-Nucleoside Phosphorylase as a Catalyst: Effects of Point Mutations. Molecules 2016, 21, 44. https://doi.org/10.3390/molecules21010044
Stachelska-Wierzchowska A, Wierzchowski J, Bzowska A, Wielgus-Kutrowska B. Site-Selective Ribosylation of Fluorescent Nucleobase Analogs Using Purine-Nucleoside Phosphorylase as a Catalyst: Effects of Point Mutations. Molecules. 2016; 21(1):44. https://doi.org/10.3390/molecules21010044
Chicago/Turabian StyleStachelska-Wierzchowska, Alicja, Jacek Wierzchowski, Agnieszka Bzowska, and Beata Wielgus-Kutrowska. 2016. "Site-Selective Ribosylation of Fluorescent Nucleobase Analogs Using Purine-Nucleoside Phosphorylase as a Catalyst: Effects of Point Mutations" Molecules 21, no. 1: 44. https://doi.org/10.3390/molecules21010044






