Synthesis and Anti-Tumor Activities of 4-Anilinoquinoline Derivatives
Abstract
:1. Introduction
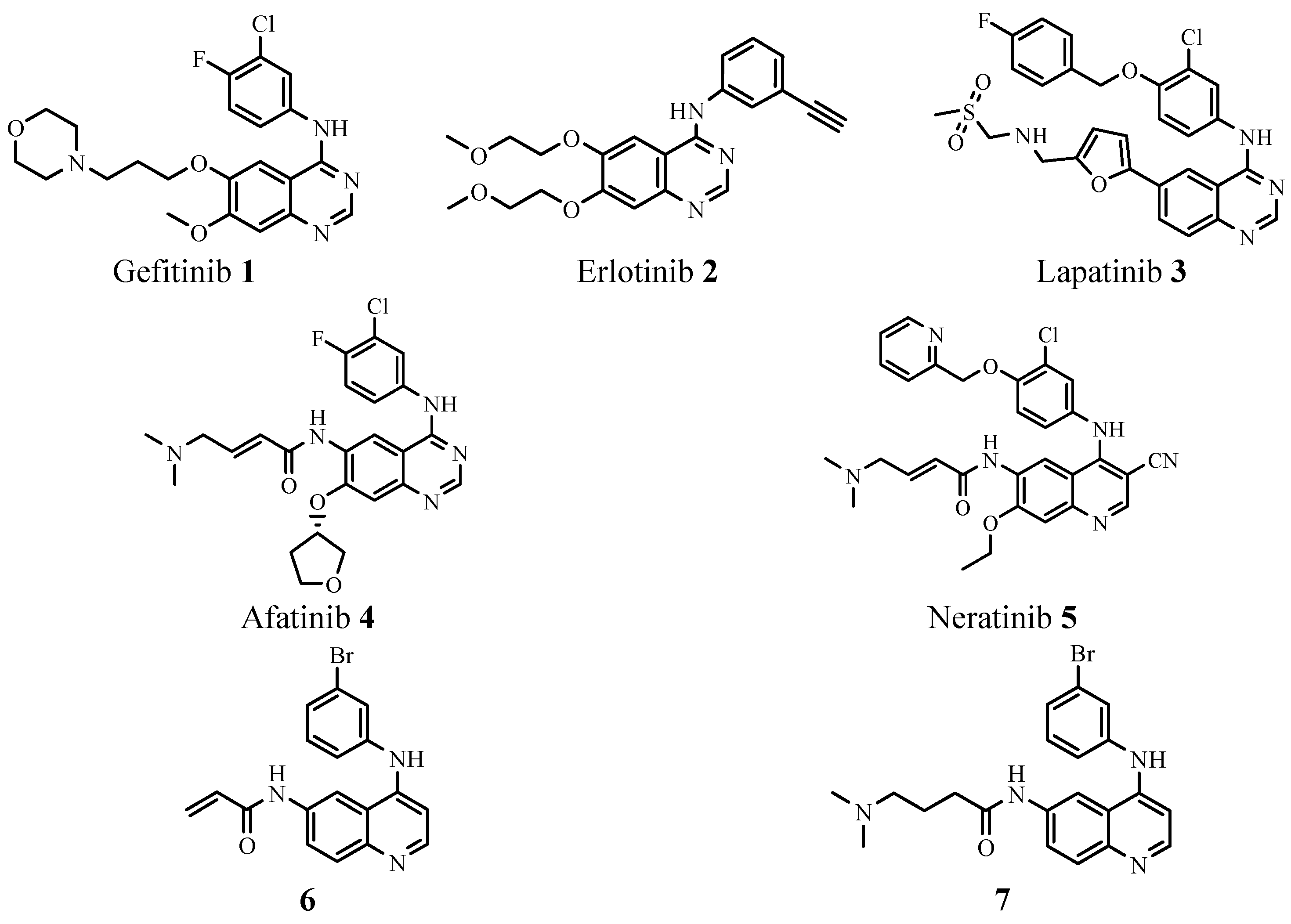
2. Results and Discussion
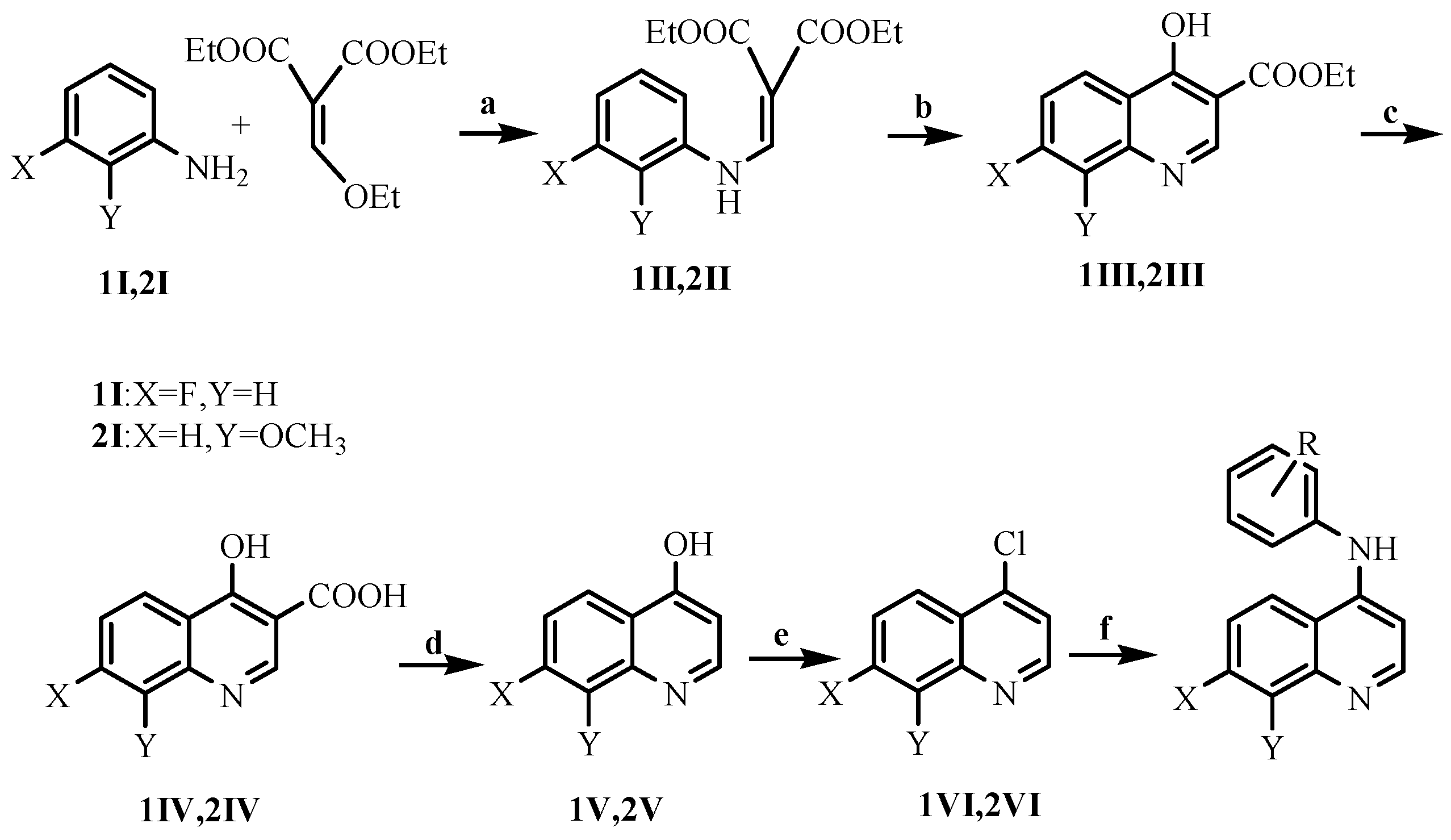
2.1. Chemistry
2.2. Biological Evaluation
| Compound | Substituents | Inhibition Rate % a | IC50 (μM) b | ||
|---|---|---|---|---|---|
| HeLa | BGC-823 | HeLa | BGC-823 | ||
| 1a | X = F, Y = H, R = 3′-Cl | 46.9 | 77.5 | 17.29 | 3.63 |
| 1b | X = F, Y = H, R = 4′-Cl | 40.3 | 63.1 | 20.39 | 7.83 |
| 1c | X = F, Y = H, R = 3′-F | 37.0 | 65.3 | 19.82 | 9.10 |
| 1d | X = F, Y = H, R = 4′-F | 20.4 | 49.5 | 43.25 | 11.10 |
| 1e | X = F, Y = H, R = 3′-Cl,4′-Cl | 40.8 | 56.9 | 36.69 | 8.29 |
| 1f | X = F, Y = H, R = 3′-Cl,4′-F | 46.8 | 67.5 | 10.18 | 8.32 |
| 1g | X = F, Y = H, R = 4′-CH3 | 41.7 | 59.4 | 18.69 | 7.08 |
| 1h | X = F, Y = H, R = 4′-OCH3 | 36.1 | 35.1 | 55.76 | 21.61 |
| 2a | X = H, Y = OCH3, R = 3′-Cl | 35.3 | 19.5 | 66.69 | ˃10 |
| 2b | X = H, Y = OCH3, R = 4′-Cl | 31.5 | 13.1 | ˃10 | ˃10 |
| 2c | X = H, Y = OCH3, R = 3′-F | 31.9 | 48.1 | 75.87 | 11.67 |
| 2d | X = H, Y = OCH3, R = 4′-F | 18.9 | 37.5 | ˃10 | 46.41 |
| 2e | X = H, Y = OCH3, R = 3′-Cl,4′-Cl | 30.8 | 48.7 | 45.87 | 11.45 |
| 2f | X = H, Y = OCH3, R = 3′-Cl,4′-F | 37.2 | 20.2 | 45.84 | ˃10 |
| 2g | X = H, Y = OCH3, R = 4′-CH3 | 30.5 | 31.1 | 99.54 | 61.52 |
| 2h | X = H, Y = OCH3, R = 4′-OCH3 | 21.9 | 32.0 | ˃10 | 74.60 |
| 2i | X = H, Y = OCH3, R = 4′-CH(CH3)2 | 59.1 | 78.4 | 7.15 | 4.65 |
| 2j | X = H, Y = OCH3, R = 3′-Cl,4′-CH3 | 32.7 | 24.5 | 71.26 | ˃10 |
| 2k | X = H, Y = OCH3, R = 2′F, 3′-F, 4′-F | 32.5 | 27.6 | 57.88 | ˃10 |
| 2l | X = H, Y = OCH3, R = 4′-NO2 | 35.8 | 36.8 | 13.48 | ˃10 |
| 2m | X = H, Y = OCH3, R = 4′-OH | 32.7 | 25.6 | 39.58 | ˃10 |
| 2n | X = H, Y = OCH3, R = 3′-CN | 28.6 | 16.0 | 30.61 | ˃10 |
| Gefitinib | 42.6 | 46.0 | 17.12 | 19.27 | |
3. Experimental Section
3.1. Chemistry
3.1.1. Preparation of the Intermediates 1II–1VI
3.1.2. Preparation of the Intermediates 2II–2VI
3.1.3. Preparation of the Title Compounds 1 and 2
3.2. Cell Proliferative Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pao, W.; Miller, V.; Zakowski, M.; Doherty, J.; Politi, K.; Sarkaria, I.; Singh, B.; Heelan, R.; Rusch, V.; Fulton, L.; et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc. Natl. Acad. Sci. USA 2004, 101, 13306–13311. [Google Scholar] [CrossRef] [PubMed]
- Bikker, J.A.; Brooijmans, N.; Wissner, A.; Mansour, T.S. Kinase domain mutations in cancer: Implications for small molecule drug design strategies. J. Med. Chem. 2009, 52, 1493–1509. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.F.; Zeng, C.L. Advance in research for lapatinib, a dual inhibitor of EGFR and ErbB2 tyrosine kinase activity. Chin. New Drugs J. 2007, 16, 1990–1993. [Google Scholar]
- Tao, L.Y.; Fu, L.W. Progress of study on lapatinib. Chin. Pharmacol. Bull. 2008, 24, 1541–1544. [Google Scholar]
- Solca, F.; Dahl, G.; Zoephel, A.; Bader, G.; Sanderson, M.; Klein, C.; Kraemer, O.; Himmelsbach, F.; Haaksma, E.; Adolf, G.R. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J. Pharmacol. Exp. Ther. 2012, 343, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, Q.; Zhu, L.; Pei, D.; Liu, Y.; Yin, Y.; Schuler, M.; Shu, Y. Clinical perspective of afatinib in non-small cell lung cancer. Lung Cancer. 2013, 81, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Rewcastle, G.W.; Denny, W.A.; Bridges, A.J.; Zhou, H.R.; Cody, D.R.; McMichael, A.; Fry, D.W. Tyrosine kinase inhibitors. 5. Synthesis and structure-activity relationships for 4-[(phenylmethyl)amino]- and 4-(phenylamino) quinazolines as potent adenosine 5′-triphosphate binding site inhibitors of the tyrosine kinase domain of the epidermal growth factor receptor. J. Med. Chem. 1995, 38, 3482–3487. [Google Scholar] [PubMed]
- Wissner, A.; Berger, D.M.; Boschelli, D.H.; Floyd, M.B.; Greenberger, L.M.; Gruber, B.C.; Johnson, B.D.; Mamuya, N.; Nilakantan, R.; Reich, M.F.; et al. 4-Anilino-6,7-dialkoxyquinolime-3-carbonitrile inhibitors of epidermal growth factor receptor kinase and their bioisosteric relationship to the 4-anilino-6,7-dialkoxyquinazoline inhibitors. J. Med. Chem. 2000, 3, 3244–3256. [Google Scholar] [CrossRef]
- Barbosa, M.L.D.C.; Lima, L.M.; Tesch, R.; Sant’Anna, C.M.R.; Totzke, F.; Kubbutat, M.H.G.; Schächtele, C.; Laufer, S.A.; Barreiro, E.J. Novel 2-chloro-4-anilino-quinazoline derivatives as EGFR and VEGFR-2 dual inhibitors. Eur. J. Med. Chem. 2014, 71, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Zhai, X.; Zhao, Y.F.; Liu, Y.J.; Qi, B.H.; Tao, H.Y.; Gong, P. Synthesis and biological evaluation of novel 2-(2-arylmethylene) hydrazinyl-4-aminoquinazoline derivatives as potent antitumor agents. Eur. J. Med. Chem. 2012, 54, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Minami, Y.; Shimamura, T.; Shah, K.; LaFramboise, T.; Glatt, K.A.; Liniker, E.; Borgman, C.L.; Haringsma, H.J.; Feng, W.; Weir, B.A.; et al. The major lung cancer-derived mutants of ERBB2 are oncogenic and are associated with sensitivity to the irreversible EGFR/ERBB2 inhibitor HKI-272. Oncogene 2007, 26, 5023–5027. [Google Scholar] [CrossRef] [PubMed]
- Wissner, A.; Mansour, T.S. The development of HKI-272 andrelated compounds for the treatment of cancer. Arch. Pharm. 2008, 341, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Pawar, V.G.; Sos, M.L.; Rode, H.B.; Rabiller, M.; Heynck, S.; Otterlo, W.A.L.V.; Thomas, R.K.; Rauh, D. Synthesis and biological evaluation of 4-anilinoquinolines as potent inhibitors of epidermal growth factor receptor. J. Med. Chem. 2010, 53, 2892–2901. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R., Jr. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol. Res. 2014, 79, 34–74. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.Z.; Li, X.H.; Zhang, Y.X. Methylation patterns of the human EGFR gene promoter region. Chin. J. Biochem. Mol. Biol. 2010, 26, 568–574. [Google Scholar]
- Long, H.; Li, H.; Wu, Q.M. RNA interference targeting EGFR on proliferation and apoptosis of human gastric carcinama cell line BCG823. China J. Mod. Med. 2012, 22, 59–62. [Google Scholar]
- Ray, A.W.; Joseph, C.M.; Victor, K.E.; Phil, J.G.; George, S.R. Quinoline, Quinazoline, and Cinnoline Fungicides. E.P. Patent 0,326,330(A2), 2 August 1989. [Google Scholar]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Luan, T.; Kong, J.; Zhang, Y.; Wang, H.-F. Synthesis and Anti-Tumor Activities of 4-Anilinoquinoline Derivatives. Molecules 2016, 21, 21. https://doi.org/10.3390/molecules21010021
Liu D, Luan T, Kong J, Zhang Y, Wang H-F. Synthesis and Anti-Tumor Activities of 4-Anilinoquinoline Derivatives. Molecules. 2016; 21(1):21. https://doi.org/10.3390/molecules21010021
Chicago/Turabian StyleLiu, Dan, Tian Luan, Jian Kong, Ying Zhang, and Hai-Feng Wang. 2016. "Synthesis and Anti-Tumor Activities of 4-Anilinoquinoline Derivatives" Molecules 21, no. 1: 21. https://doi.org/10.3390/molecules21010021