Antioxidant Activity of Flaxseed Extracts in Lipid Systems
Abstract
:1. Introduction

2. Results and Discussion
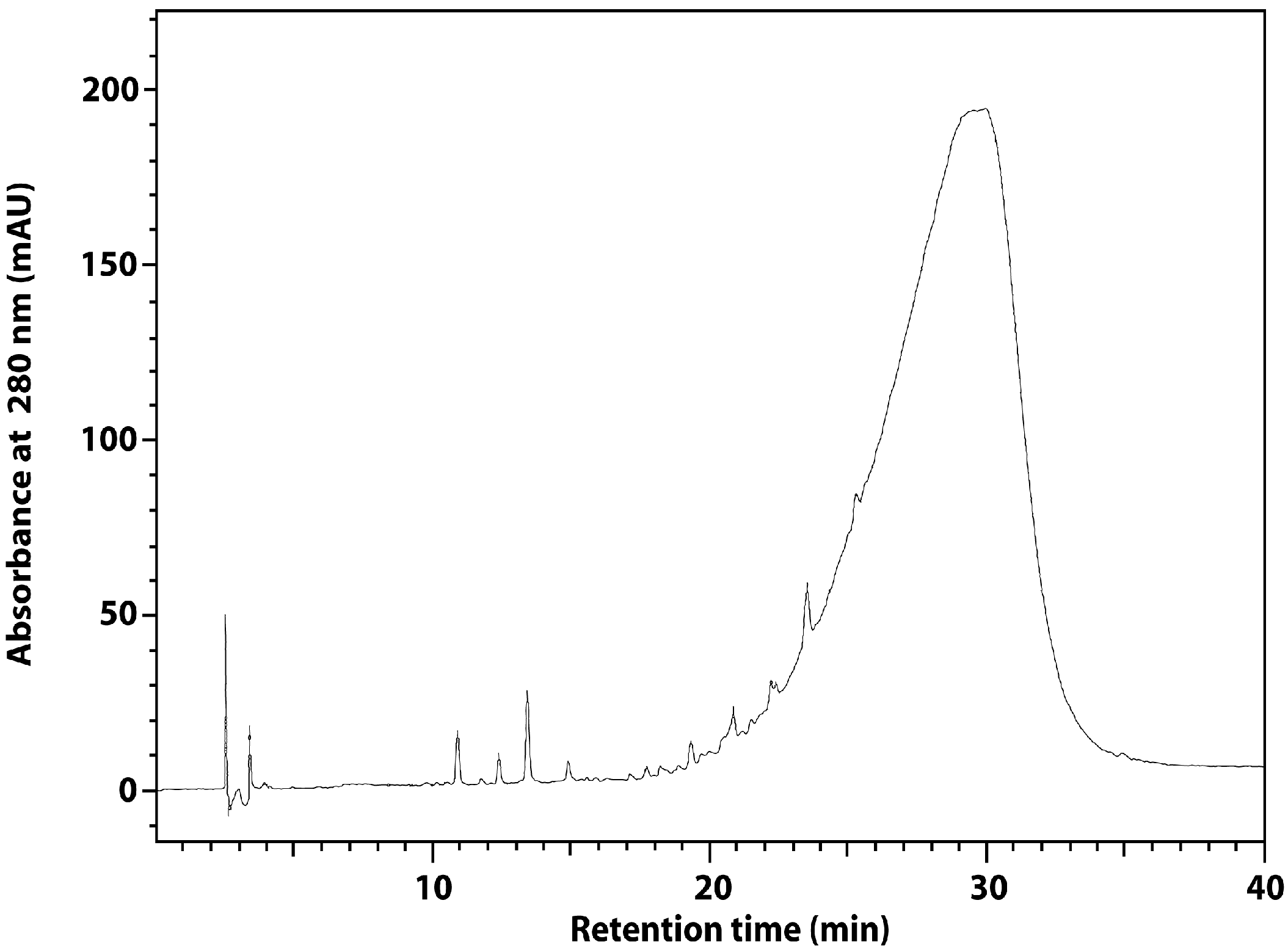
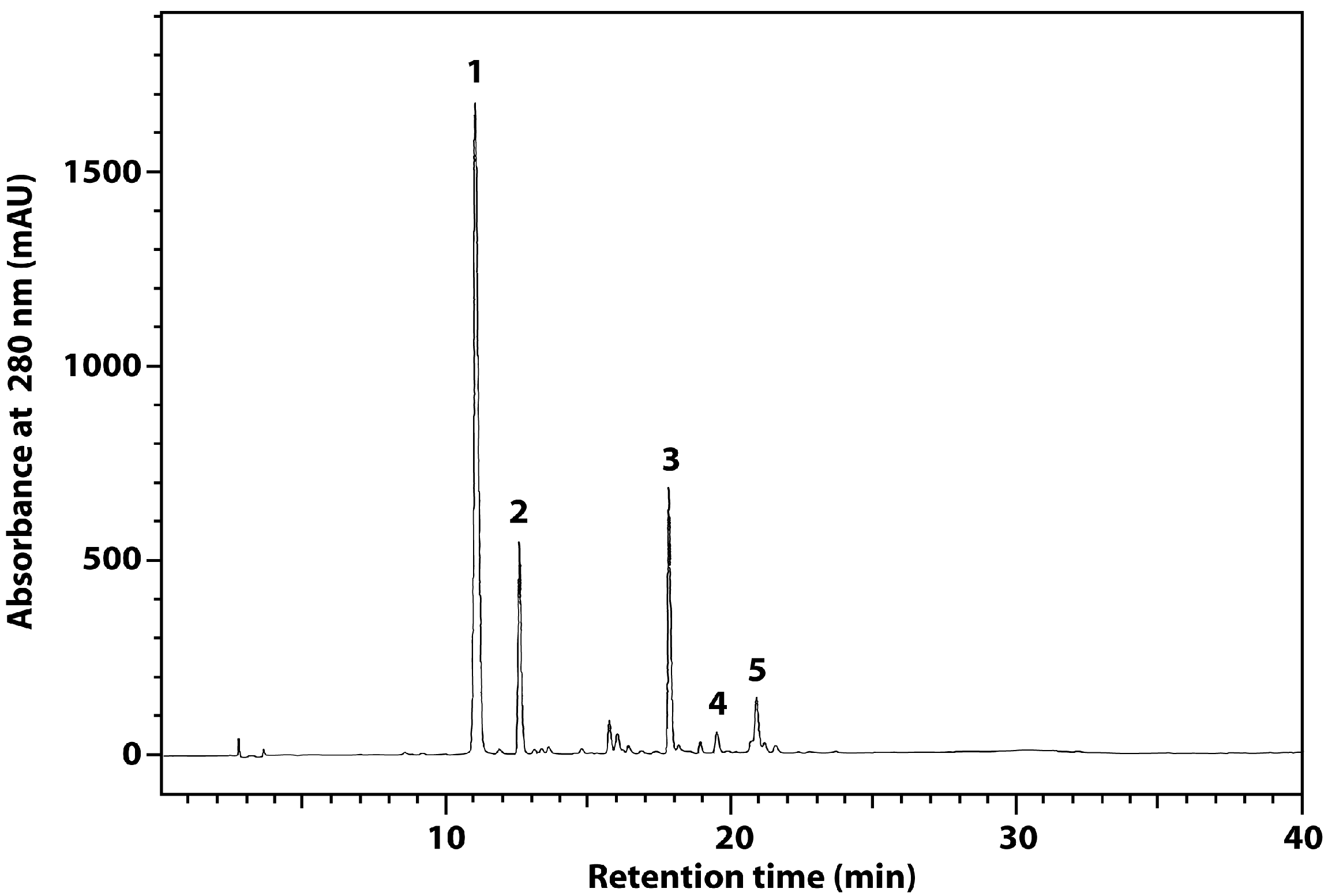
| Material | Total Phenolics (mg CE/g) | SDG (mg/g) | p-Coumaric Acid (mg/g) | Ferulic Acid (mg/g) |
|---|---|---|---|---|
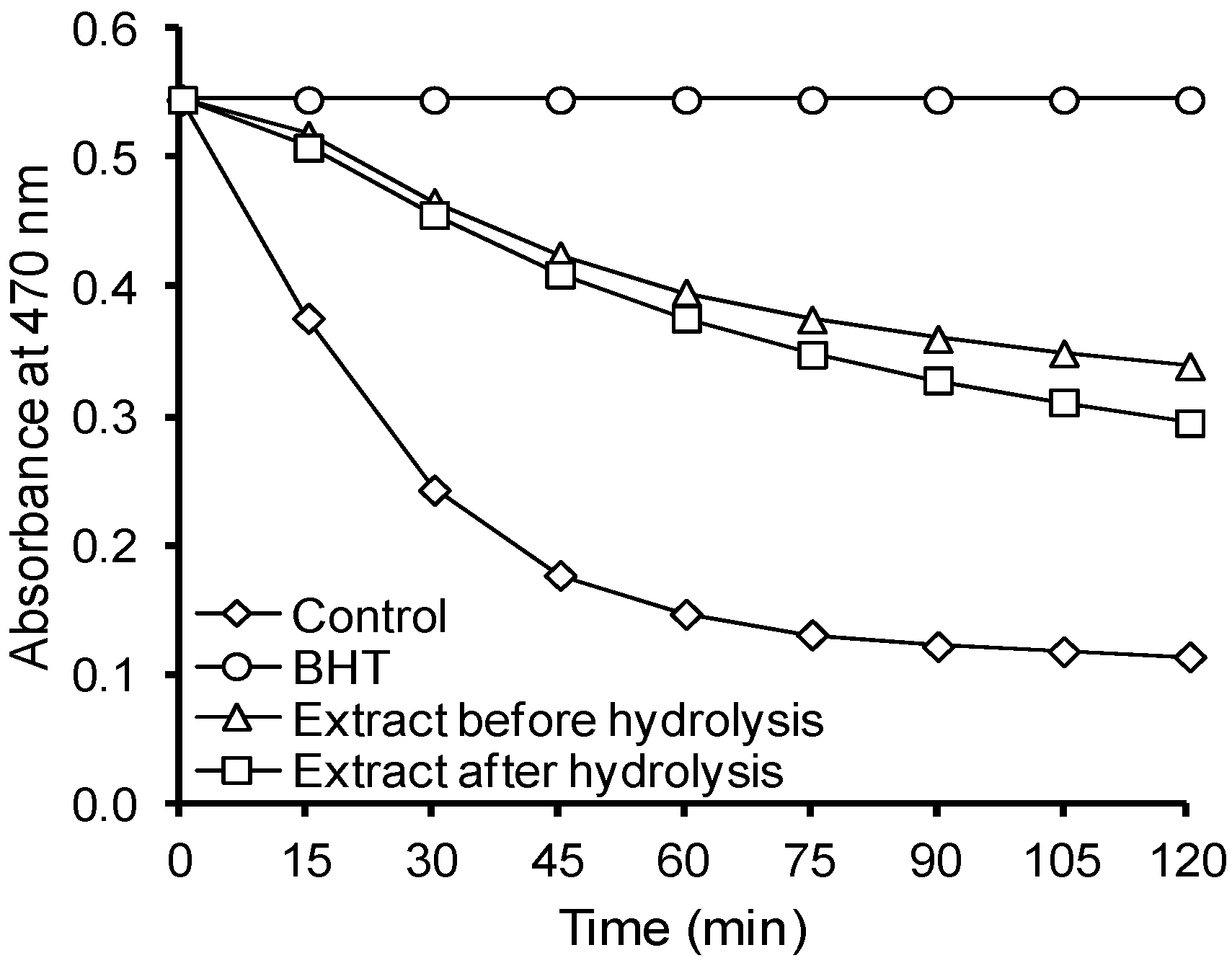
| Extract before hydrolysis | 85 ± 5 | - | - | - |
| Extract after hydrolysis | 96 ± 5 | 333 ± 15 | 3.1 ± 0.2 | 8.5 ± 0.4 |
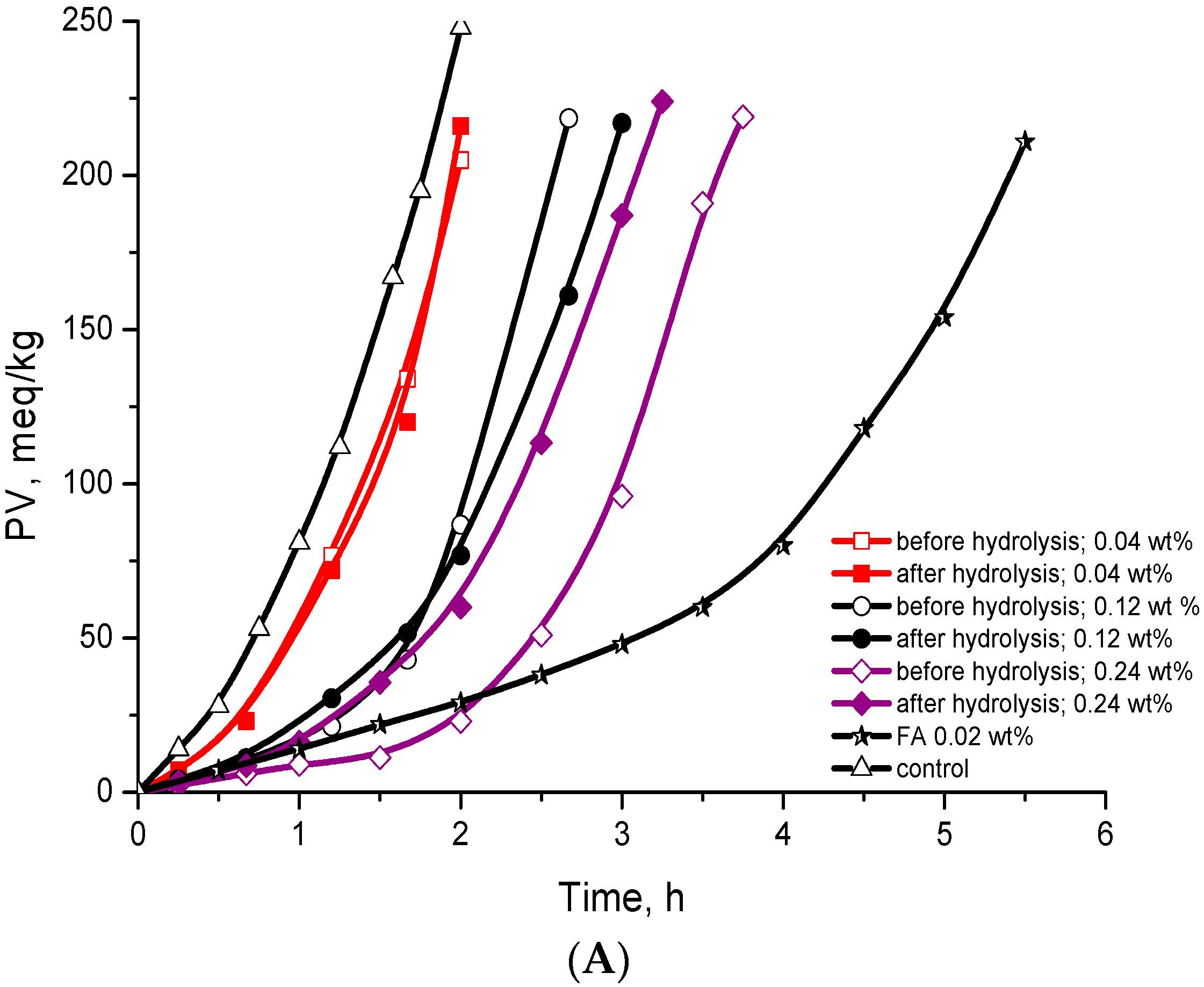

| Addition | wt % | Antioxidant Efficiency | Antioxidant Reactivity | Activity | ||
|---|---|---|---|---|---|---|
| IPA (h) | PF (-) | RA (10−6 M/s) | ID (-) | |||
| Extract before hydrolysis | 0.04 | 1.2 ± 0.2 a | 1.1 | 3.9 ± 0.4 a | 2.0 | No activity |
| 0.12 | 1.7 ± 0.2 a | 1.5 | 2.1 ± 0.3 a | 3.7 | No activity | |
| 0.24 | 2.5 ± 0.3 a | 2.3 | 1.2 ± 0.2 a | 6.5 | Weak | |
| Extract after hydrolysis | 0.04 | 1.4 ± 0.2 a | 1.3 | 3.8 ± 0.4 a | 2.0 | No activity |
| 0.12 | 1.8 ± 0.2 a | 1.6 | 2.3 ± 0.3 a | 3.4 | No activity | |
| 0.24 | 1.9 ± 0.2 a | 1.7 | 1.8 ± 0.2 a | 4.3 | No activity | |
| FA | 0.02 | 4.2 ± 0.5 | 3.2 | 2.0 ± 0.3 | 4.4 | Moderate |
| BHT | 0.02 | 20.5 ± 2.5 | 18.6 | 0.26 ± 0.06 | 30.0 | Strong |
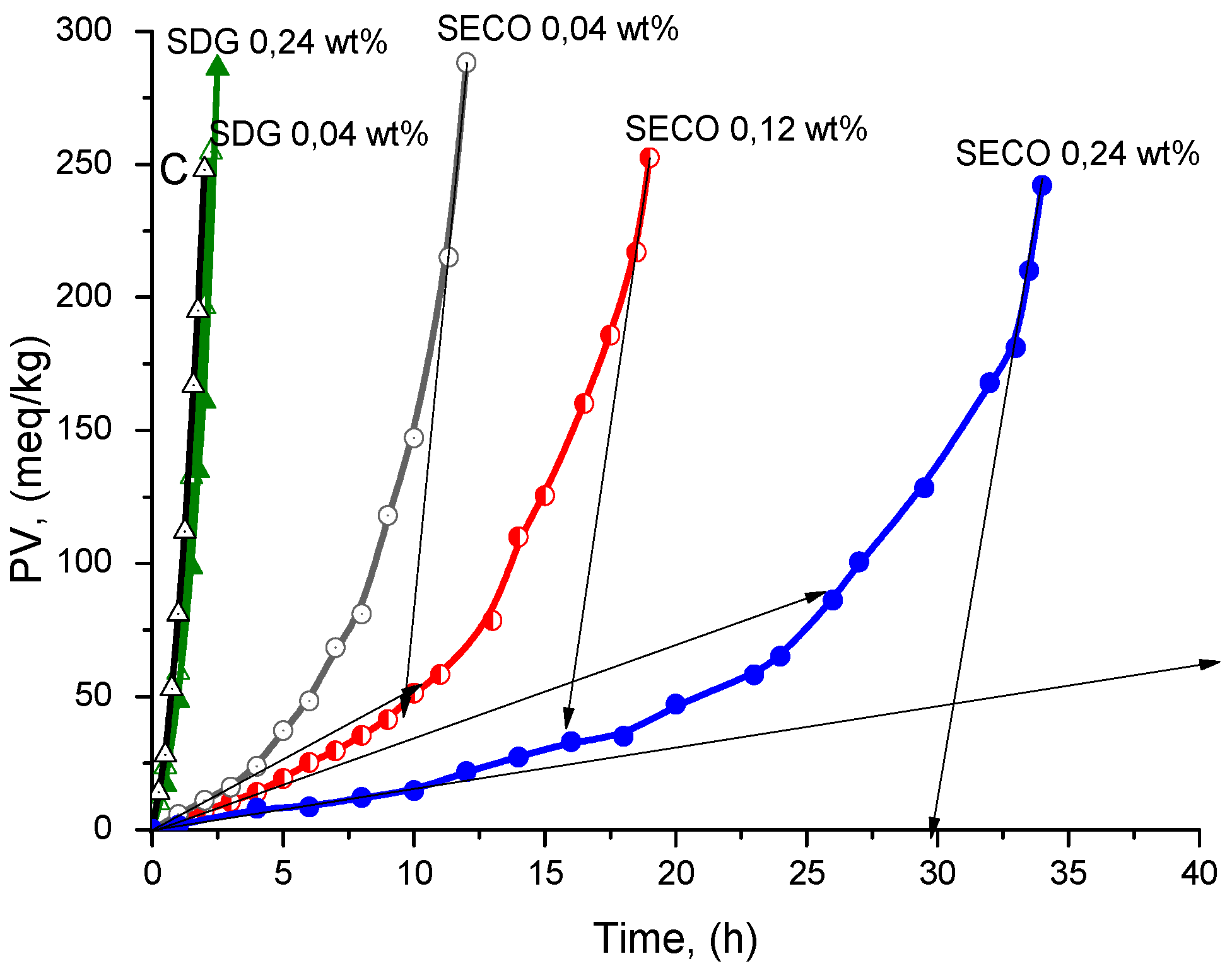
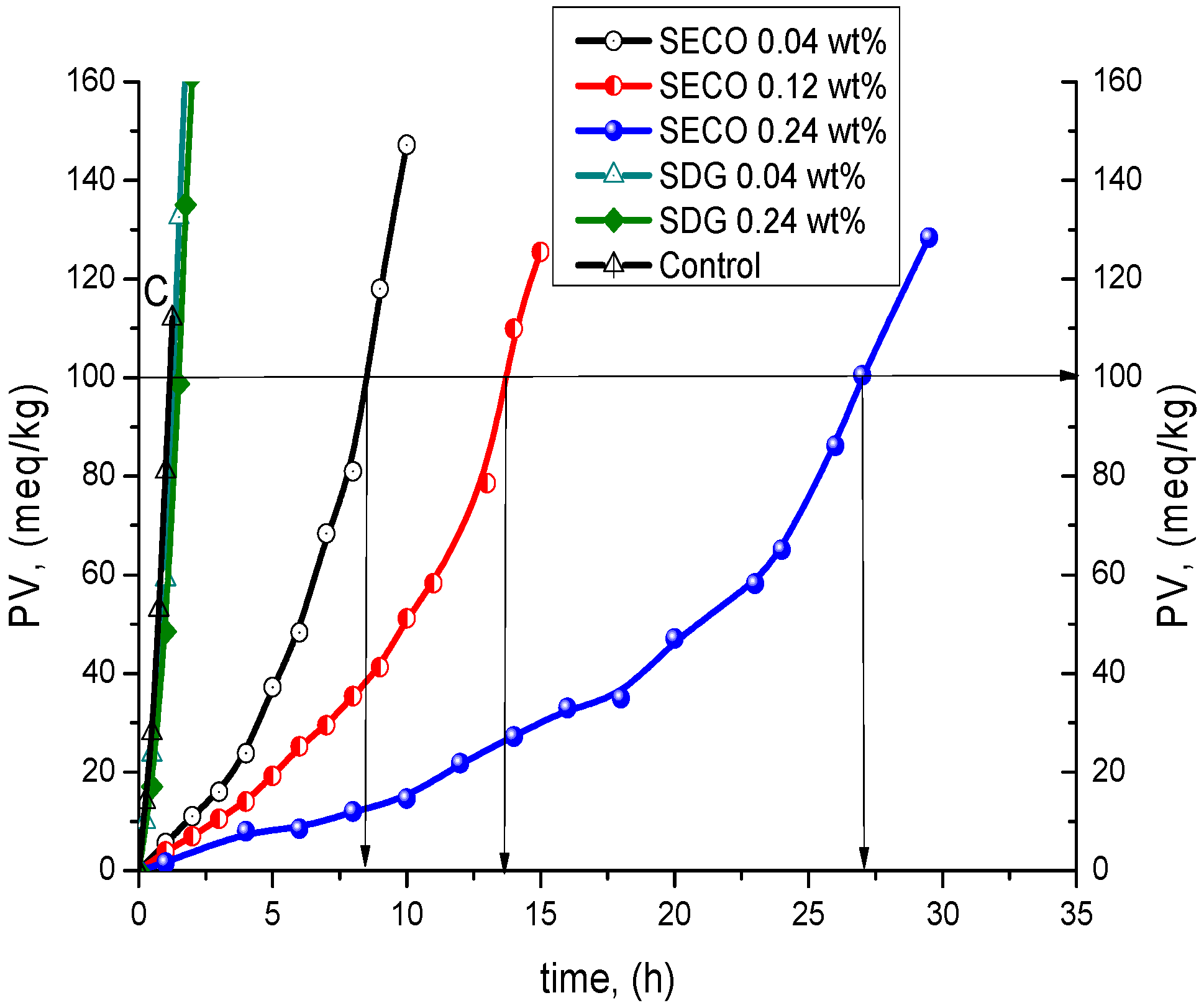
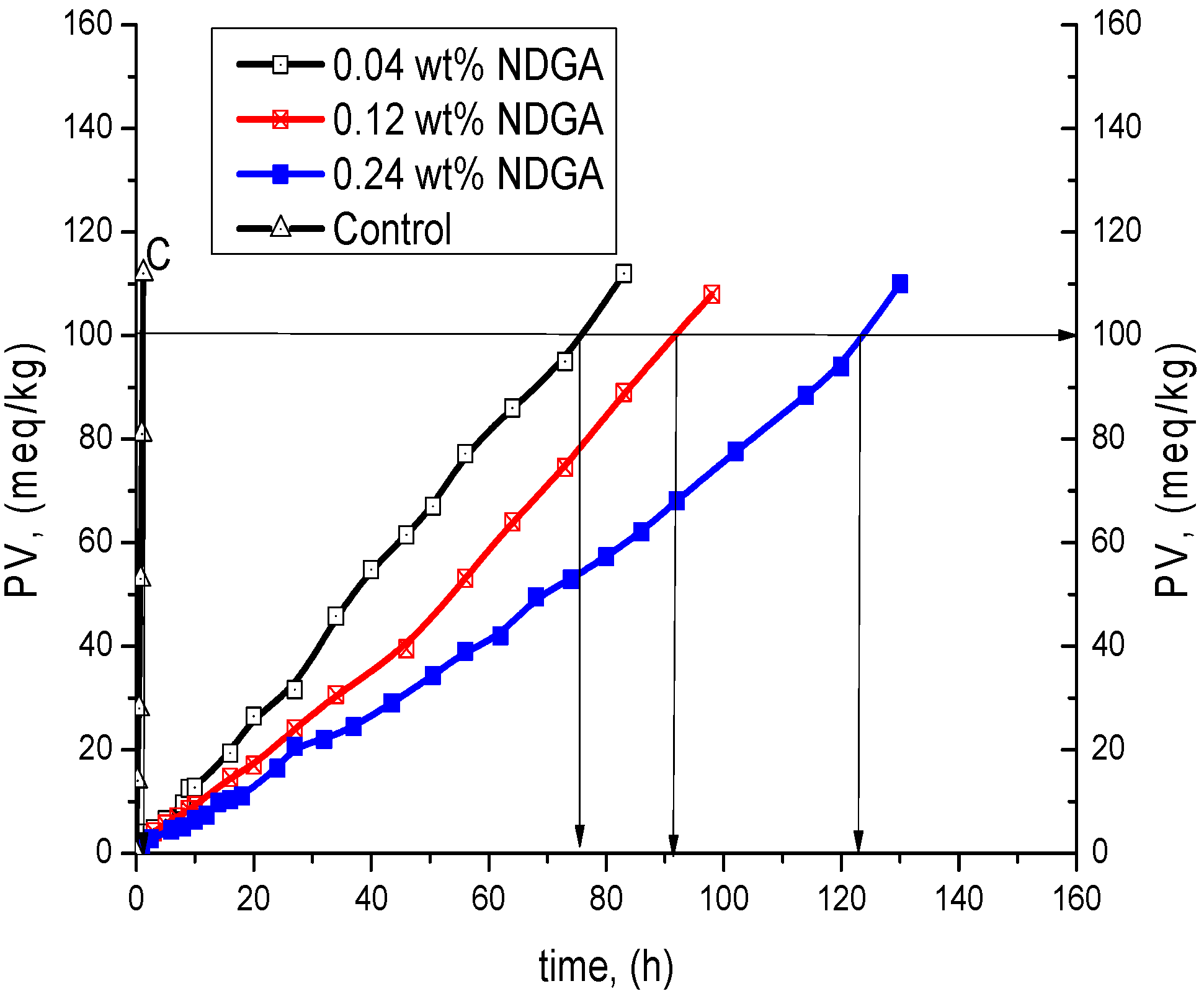
| Compound | wt % | Antioxidant Efficiency | Antioxidant Efficiency at PV = 100 meq/kg | Antioxidant Reactivity | |||
|---|---|---|---|---|---|---|---|
| IPA (h) | PF (-) | IPA100 (h) | PF100 (-) | RA (10−7 M/s) | ID (-) | ||
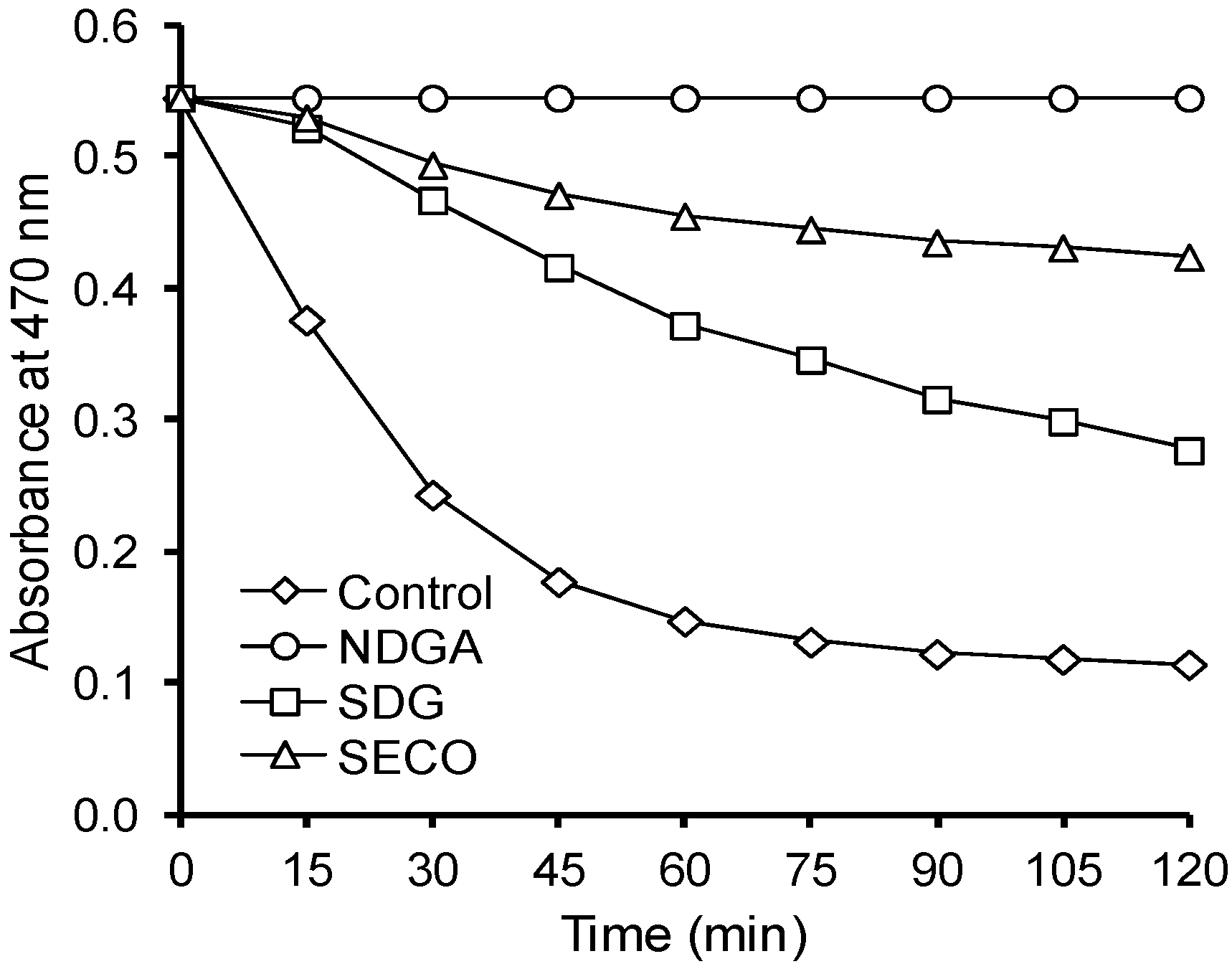
| SDG | 0.04 | 1.4 ± 0.2 | 1.3 | 1.3 ± 0.2 | 1.2 | 7.8 ± 1.0 | 1.0 |
| 0.24 | 1.6 ± 0.2 | 1.5 | 1.5 ± 0.2 | 1.4 | 7.8 ± 1.0 | 1.0 | |
| SECO | 0.04 | 9.6 ± 0.9 | 8.7 | 8.5 ± 0.8 | 7.7 | 7.5 ± 0.5 | 10.4 |
| 0.12 | 16.1 ± 1.5 | 14.6 | 13.7 ± 1.9 | 12.5 | 4.9 ± 0.5 | 15.9 | |
| 0.24 | 30.7 ± 1.9 | 27.9 | 27.1 ± 1.7 | 24.6 | 2.1 ± 0.3 | 37.1 | |
| NDGA | 0.04 | - | - | 76 ± 4 | 69 | 1.9 ± 0.3 | 41 |
| 0.12 | - | - | 91 ± 5 | 8.3 | 1.3 ± 0.3 | 60 | |
| 0.24 | - | - | 123 ± 6 | 112 | 1.0 ± 0.3 | 78 | |
3. Experimental Section
3.1. Chemicals
3.2. Plant Material
3.3. Extract Preparation
3.4. Extract Purification
3.5. Extract Alkaline Hydrolysis
3.6. Determination of Total Phenolic Content
3.7. RP-HPLC
3.8. SDG and SECO Separation
3.9. Preparation of Triacylglycerols
3.10. Lipid Autoxidation
3.11. Kinetic Parameters of the Studied Extracts and Pure Compounds
3.12. Antioxidant Activity in a β-Carotene-Linoleate Model System
3.13. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Touré, A.; Xueming, X. Flaxseed lignans: Sources, biosynthesis, metabolism, antioxidant activity, bio-active components, and health benefit. Compr. Rev. Food Sci. Food Saft. 2010, 9, 261–269. [Google Scholar] [CrossRef]
- Eldin-Kamal, A.; Peerlkamp, N.; Johnsson, P.; Andersson, R.; Andersson, R.E.; Lundgren, L.N.; Åman, P. An oligomer from flaxseed composed of secoisolariciresinoldiglucoside and 3-hydroxy-3-methyl glutaric acid residues. Phytochemistry 2001, 58, 587–590. [Google Scholar] [CrossRef]
- Borriello, S.P.; Setchell, K.D.; Axelson, M.; Lawson, A.M. Production and metabolism of lignans by the human faceal flora. J. Appl. Bacteriol. 1985, 58, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt, P.; Thompson, L. Human metabolism of mammalian lignans precursors in raw and processed flaxseed. Am. J. Clin. Nutr. 1999, 69, 549–555. [Google Scholar] [PubMed]
- Jenab, M.; Thompson, L.U. The influence of flaxseed and lignans on colon carcinogenesis and β-glucuronidase activity. Carcinogenesis 1996, 17, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Adlercreutz, H. Phytoestrogens and cancer. Lancet Oncol. 2002, 3, 364–373. [Google Scholar] [CrossRef]
- Boccardo, F.; Lunardi, G.; Guglielmini, P.; Parodi, M.; Murialdo, R.; Schettini, G.; Rubagotti, A. Serum enterolactone levels and the risk of breast cancer in woman with palpable cysts. Eur. J. Cancer 2004, 40, 84–89. [Google Scholar] [CrossRef]
- Lin, X.; Gingrich, J.R.; Bao, W.; Li, J.; Haroon, Z.A.; Demark-Wahnefried, W. Effect of flaxseed supplementation on prostatic carcinoma in transgenic mice. Urology 2002, 60, 919–924. [Google Scholar] [CrossRef]
- Amarowicz, R.; Wanasundara, U.; Wanasundara, J.; Shahidi, F. Antioxidant activity of ethanolic extracts of flaxseed in a β-carotene-linoleate model system. J. Food Lipids 1993, 1, 111–117. [Google Scholar] [CrossRef]
- Amarowicz, R.; Karamać, M.; Wanasundara, J.P.D.; Shahidi, F. Antioxdant activity of hydrophobic fractions of flaxseed. Nahrung-Food 1997, 41, 178–180. [Google Scholar] [CrossRef]
- Niemeyer, H.B.; Metzler, M. Differences in the antioxidant activity of plant and mammalian lignans. J. Food Eng. 2003, 56, 255–256. [Google Scholar] [CrossRef]
- Hu, C.; Yuan, Y.V.; Kitts, D.D. Antioxidant activities of the flaxseed lignin secoisolariciresinol diglucoside, its aglycone secoisolariciresinol and the mammalian lignans enterodiol and enterolactone in vitro. Food Chem. Toxicol. 2007, 45, 2219–2227. [Google Scholar] [CrossRef] [PubMed]
- Hosseinian, F.S.; Muir, A.D.; Westcott, N.D.; Krol, E.S. Antioxidant capacity of flaxseed lignans in two model systems. J. Am. Oil. Chem. Soc. 2006, 83, 835–840. [Google Scholar] [CrossRef]
- Hosseinian, F.S.; Muir, A.D.; Westcott, N.D.; Krol, E.S. AAPH mediated antioxidant reactions of secoisolariciresinol and SDG. Org. Biomol. Chem. 2007, 5, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Eklund, P.C.; Langvik, O.K.; Warna, J.P.; Salmi, T.O.; Willfort, S.M.; Sjoholm, R.E. Chemical studies on antioxidant mechanism and free radical scavenging properties of lignans. Org. Biomol. Chem. 2005, 3, 3336–3347. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K. Antioxidant activity of secoisolariciresinol diglucoside-derived metabolites, secoisolariciresinol, enterodiol, and enterolactone. Int. J. Angiol. 2000, 9, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K. Hydroxyl radical-scavenging property of secoisolariciresinol diglucoside (SDG) isolated from flax. Mol. Cell Biochem. 1997, 168, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Kitts, D.D.; Yuan, Y.V.; Wijewickreme, A.N.; Thompson, L.U. Antioxidant activity of the flaxseed lignan of secoisolariciresinol diglycoside and its mammalian metabolites enterodiol and enterolactone. Mol. Cell Biochem. 1999, 202, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, P.; Peerlkamp, N.; Kamal-Eldin, A.; Andersson, R.E.; Andersson, R.; Lundgren, L.N.; Åman, P. Polymeric fractions containing phenol glucosides in flaxseed. Food Chem. 2002, 76, 207–212. [Google Scholar] [CrossRef]
- Struijs, K.; Vincken, J.P.; Verhoef, R.; Voragen, A.G.J.; Gruppen, H. Hydrocinnamic acids are ester-linkeddirectly to glucosyl moieties within the lignan macromolecule from flaxseed hulls. Phytochemistry 2008, 69, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- Amarowicz, R.; Fornal, J.; Karamać, M.; Shahidi, F. Antioxidant activity of extracts of phenolic compounds from rapeseed oil cakes. J. Food Lipids 2001, 28, 525–530. [Google Scholar] [CrossRef]
- Wanasundara, U.N.; Amarowicz, R.; Shahidi, F. Partial characterization of natural antioxidants in canola meal. Food Res. Int. 1996, 28, 525–530. [Google Scholar] [CrossRef]
- Srivastava, A.; Greenspan, P.; Hartle, D.K.; James, L.; Hargrove, J.L.; Amarowicz, R.; Pegg, R.B. Antioxidant and anti-inflammatory activities of polyphenolics from southeastern U.S. range blackberry cultivars. J. Agric. Food Chem. 2010, 58, 6102–6109. [Google Scholar] [CrossRef] [PubMed]
- Kancheva, V.D.; Taskova, R.; Totseva, I.; Handjieva, N. Antioxidant activity of extracts, fractions and flavonoid contents from Carthamus lanatus L. Riv. Ital. Sostanze Grasse 2007, 84, 77–86. [Google Scholar]
- Hopia, A.; Heinonen, M. Antioxidant activity of flavonoid aglycones and their glycosides in methyl linoleate. J. Am. Oil Chem. Soc. 1999, 76, 139–144. [Google Scholar] [CrossRef]
- Kancheva, V.D.; Kasaikina, O.T. Lipid oxidation in homogeneous and micro-heterogeneous media in prewsence of prooxidants, antioxidants and surfactants. In Lipid Peroxidation; Catala, Ed.; InTech: Rijeka, Croatia, 2012; pp. 31–62. [Google Scholar]
- Pokorny, J.; Yanishlieva, N.; Gordon, M.H. Measuring antioxidant activity. In Antioxidants in Food—Practical Applications; CRC Press: Boca Raton, FL, USA, 2001; pp. 71–84. [Google Scholar]
- Roeding-Pennman, A.; Gordon, M.H. Antioxidant properties of myricetin and quercetin in oil and emulsions. J. Am. Oil Chem. Soc. 1998, 75, 169–180. [Google Scholar] [CrossRef]
- Kancheva, V.D. Oxidative stress and lipid oxidation- noninhibited and inhibited. In Reactive Oxygen Species, Lipid Peroxidation and Protein Oxidation; Catala, E., Ed.; Nova Sci. Publ. Inc.: New York, NY, USA, 2014; pp. 1–42. [Google Scholar]
- Janiak, M.; Slavova-Kazakova, A.; Kancheva, V.; Amarowicz, R. Sephadex LH-20 column chromatography of the hydrolysed lignan macromolecule of flaxseed. Bulg. Chem. Comm. 2014, 46, 640–644. [Google Scholar]
- Kancheva, V.; Slavova-Kazakova, A.; Fabbri, D.; Dettori, M.A.; Delogu, G.; Janiak, M.; Amarowicz, R. Protective effects of equimolar mixtures of monomer and dimer of dehydrozingerone with α-tocopherol and/or ascorbyl palmitate during bulk lipid autoxidation. Food Chem. 2014, 157, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Kancheva, V.D. Phenolic antioxidants - Radical-scavenging and chain-breaking antioxidant activity: A comparative study. Eur. J. Lipid Sci. Technol. 2009, 111, 1072–1089. [Google Scholar] [CrossRef]
- Miller, H.E. A simplified method for the evaluation of antioxidants. J. Am. Oil Chem. Soc. 1971, 48, 91. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the SDG and SECO are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slavova-Kazakova, A.; Karamać, M.; Kancheva, V.; Amarowicz, R. Antioxidant Activity of Flaxseed Extracts in Lipid Systems. Molecules 2016, 21, 17. https://doi.org/10.3390/molecules21010017
Slavova-Kazakova A, Karamać M, Kancheva V, Amarowicz R. Antioxidant Activity of Flaxseed Extracts in Lipid Systems. Molecules. 2016; 21(1):17. https://doi.org/10.3390/molecules21010017
Chicago/Turabian StyleSlavova-Kazakova, Adriana, Magdalena Karamać, Vessela Kancheva, and Ryszard Amarowicz. 2016. "Antioxidant Activity of Flaxseed Extracts in Lipid Systems" Molecules 21, no. 1: 17. https://doi.org/10.3390/molecules21010017







