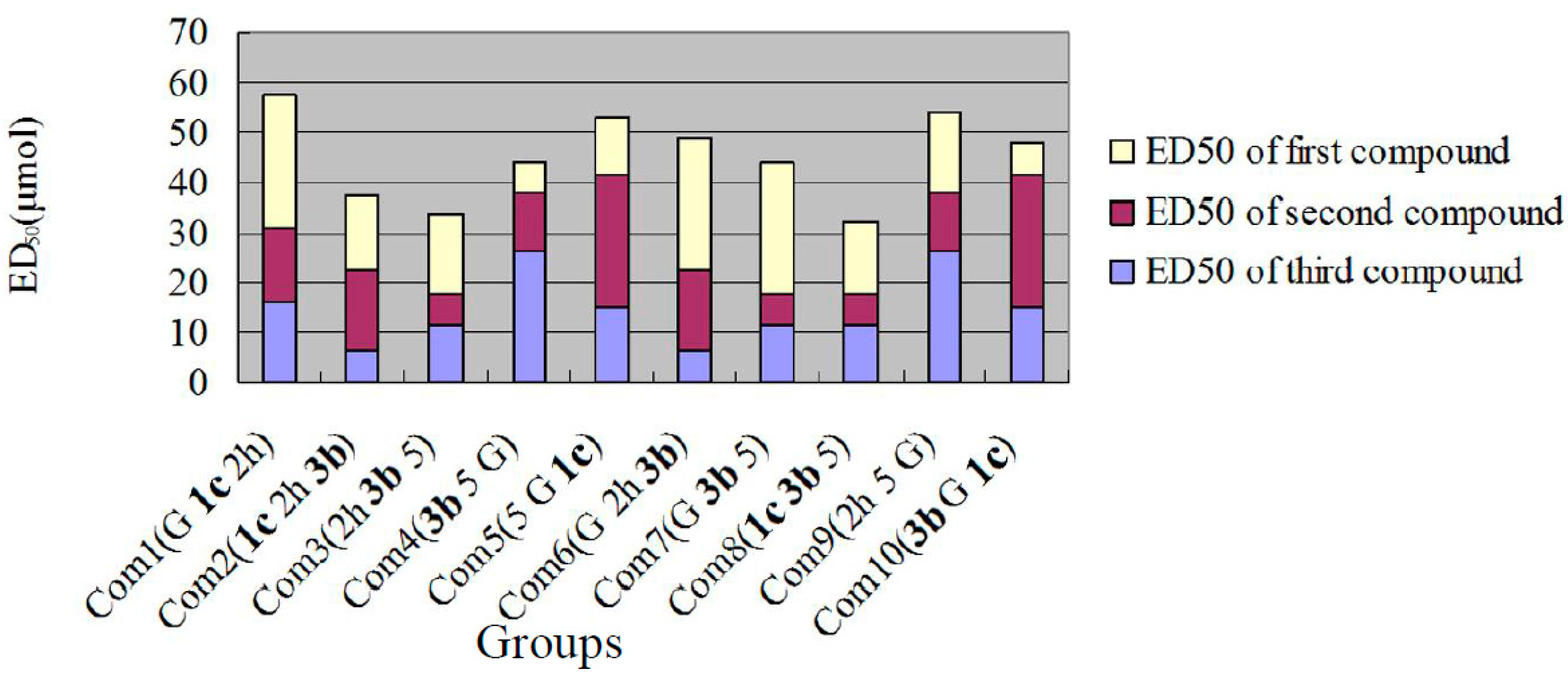
3.2. Chemistry
3.2.1. Synthesis of Compounds 1a–1e (Considering One of the Parental Compounds as an Example)
A mixture of genistein (10.00 g, 0.037 mol) and 25 mL concentrated sulfuric acid was heated to 60 °C under stirring for 0.5 h. The mixture was then poured into 500 mL ice-cold saturated brine. A precipitate formed slowly; the precipitate formed completely after 4 h incubation. The precipitate was filtered and washed with saturated brine until the pH became neutral. The crude material was purified in a 300–400 mesh silica column (water-saturated butanol).
3′-Sodium sulfonate genistein (1a). The product was obtained as a white solid (35.2% yield); m.p. 304~305 °C. 1H-NMR (DMSO-d6) δ (ppm): 12.91 (s, 1H, 5-OH), 10.82 (s, 1H, 7-OH), 10.56 (s, 1H, 4′-OH), 8.36 (s, 1H, H-2), 7.71 (s, 1H, H-2′), 7.37 (d, J = 8.4 Hz, 1H, H-6′), 6.86 (d, J = 8.4 Hz, 1H, H-5′), 6.41 (s, 1H, H-8), 6.24 (s, 1H, H-6). 13C-NMR (DMSO-d6) δ (ppm): 180.50 (s, C-4), 164.82 (s, C-7), 162.42 (s, C-4′), 158.06 (s, C-5), 154.77 (s, C-8a), 153.81 (s, C-2), 132.01 (s, C-2′), 131.08 (s, C-3′), 128.20 (s, C-6′), 122.22 (s, C-1′), 121.55 (s, C-3), 116.84 (s, C-5′), 104.88 (s, C-4a), 99.50 (s, C-6), 94.21 (s, C-8). HRMS (ESI) m/z: 269.01752 [M − SO3Na − H]−, calcd. for C15H9O5 269.04500.
3′,6-Disodium sulfonate genistein (1b). The product was obtained as a white solid (31.2% yield); m.p. 312~313 °C. 1H-NMR (DMSO-d6) δ (ppm): 13.27 (s, 1H, 5-OH), 12.59 (s, 1H, 7-OH), 10.63 (s, 1H, 4′-OH), 8.51 (s, 1H, H-2), 7.74 (s, 1H, H-2′), 7.38 (d, J = 8.4 Hz, 1H, H-6′), 6.85 (d, J = 8.0 Hz, 1H, H-5′), 6.21 (s, 1H, H-8). 13C-NMR (DMSO-d6) δ (ppm): 180.65 (s, C-4), 162.37 (s, C-7), 161.29 (s, C-4′), 155.12 (s, C-5), 154.97 (s, C-8a), 153.94 (s, C-2), 132.25 (s, C-2′), 131.04 (s, C-6′), 128.53 (s, C-3′), 122.66 (s, C-1′), 121.16(s, C-3), 116.87 (s, C-5′), 105.10 (s, C-4a), 101.86 (s, C-6), 99.52 (s, C-8). HRMS (ESI) m/z: 370.98638 [M − SO3Na − H]−, calcd. for C15H8NaO8S 370.98376.
3′-Sodium sulfonate biochanin A (1c). The product was obtained as a buff solid (34.3% yield); m.p. 221~222 °C. 1H-NMR (DMSO-d6) δ (ppm): 12.93 (s, 1H, 5-OH), 10.94 (s, 1H, 7-OH), 8.35 (s, 1H, H-2), 7.89 (s, 1H, H-2′), 7.48 (d, J = 8.4 Hz, 1H, H-6′), 7.02 (d, J = 7.9 Hz, 1H, H-5′), 6.42 (s, 1H, H-8), 6.25 (s, 1H, H-6), 3.79 (s, 3H, -OCH3). 13C-NMR (DMSO-d6) δ (ppm): δ 180.56 (s, C-4), 164.83 (s, C-7), 162.44 (s, C-4′), 158.10 (s, C-5), 156.74 (s, C-8a), 154.76 (s, C-2), 135.92 (s), 131.34 (s), 129.66 (s), 122.58 (s), 121.93 (s), 112.21 (s), 104.90 (s, C-4a), 99.51 (s), 94.23 (s), 56.06 (s, -OCH3). HRMS (ESI) m/z: 283.06832 [M − SO3Na − H]−, calcd. for C16H11O5 283.06065.
3′,6-Disodium sulfonate biochanin A (1d). The product was obtained as a buff solid (32.3% yield); m.p. 228~229 °C. 1H-NMR (DMSO-d6) δ (ppm): 13.28 (s, 1H, 5-OH), 12.59 (s, 1H, 7-OH), 8.50 (s, 1H, H-2), 7.94 (s, 1H, H-2′), 7.47 (d, J = 8.3 Hz, 1H, H-6′), 7.06 (d, J = 8.5 Hz, 1H, H-5′), 6.21 (s, 1H, H-8), 3.82 (s, 3H, -OCH3). 13C-NMR (DMSO-d6) δ (ppm): 180.66 (s, C-4), 162.38 (s, C-7), 161.31 (s, C-4′), 156.73 (s, C-5), 155.15 (s, C-8a), 154.87 (s, C-2), 135.68 (s), 131.39 (s), 129.89 (s), 122.91 (s), 121.73 (s), 112.29 (s), 110.62 (s), 105.09 (s, C-4a), 99.52 (s), 56.19 (s, -OCH3). HRMS (ESI) m/z: 385.01812 [M − SO3Na − H]−, calcd. for C16H10NaO8S 384.99941.
3′-Sodium sulfonate formononetin (1e). The product was obtained as a white solid (31.7% yield); m.p. 263~264 °C. 1H-NMR (DMSO-d6) δ (ppm):10.88 (s, 1H, 7-OH), 8.31 (s, 1H, H-2), 7.98 (d, J = 8.7 Hz, 1H, H-5), 7.89 (d, J = 1.4 Hz, 1H, H-2′), 7.50 (dd, J = 8.4, 1.4 Hz, 1H, H-6′), 7.05 (d, J = 8.5 Hz, 1H, H-5′), 6.96 (dd, J = 8.8, 1.0 Hz, 1H, H-6), 6.91 (s, 1H, H-8), 3.81 (s, 3H, -OCH3). 13C-NMR (DMSO-d6) δ (ppm): 175.03 (s, C-4), 163.10 (s, C-7), 157.95 (s, C-4′), 156.40 (s, C-8a), 153.59 (s, C-2), 135.58 (s), 131.40 (s), 129.53 (s), 127.72 (s), 123.77 (s), 123.37 (s), 117.03 (s), 115.69 (s), 112.12 (s), 102.64 (s), 56.19 (s, -OCH3). HRMS (ESI) m/z: 267.06378 [M − SO3Na − H]−, calcd. for C16H11O4 267.06574.
3.2.2. Synthesis of Compounds 2a–2j (Considering One of the Parental Compounds as an Example)
Compounds
2a–
2j were prepared according to the method described by Xie
et al. [
18]. Formononetin (1.0 g, 3.73 mmol) was dissolved in 16 mL 80% sulfuric acid. After the formononetin was dissolved, isopropyl alcohol (3 mL) was added to the mixture. The mixture was heated to 60 °C under stirring for 3 h, and the mixture was then poured into water (200 mL). White floc formed immediately upon the addition of water, and the precipitate formed completely after 4 h incubation. The precipitate was filtered and washed with water until the pH became neutral. The crude material was separated in a 300–400 mesh silica column (petroleum ether:ethyl acetate, 3:1).
3′,5′-Diisopropyl genistein (2a). The product was obtained as a white solid (22.1% yield); m.p. 284~285 °C. 1H-NMR (DMSO-d6) δ (ppm): 13.01 (s, 1H, 5-OH), 10.89 (s, 1H, 7-OH), 9.31 (s, 1H, 4′-OH), 8.26 (s, 1H, H-2), 7.17 (s, 2H, H-2′,6′), 6.38 (s, 1H, H-8), 6.23 (s, 1H, H-6), 3.33 (dt, J = 13.2, 6.7 Hz, 2H, -CH(CH3)2), 1.18 (d, J = 6.7 Hz, 12H, -CH(CH3)2). 13C-NMR (DMSO-d6) δ (ppm): 180.82 (s, C-4), 164.70 (s, C-7), 162.49 (s, C-4′), 158.05 (s, C-5), 154.53 (s, C-8a), 151.30 (s, C-2), 135.41 (s, C-2′,6′), 124.44 (s, C-3′,5′), 123.45 (s, C-1′), 122.51 (s, C-3), 104.97 (s, C-4a), 99.39 (s, C-6), 94.08 (s, C-8), 26.68 (s, 2C), 23.43 (s, 4C). HRMS (ESI) m/z: 353.09991 [M − H]−, calcd. for C21H21O5 353.13890.
3′,5′,6,8-Tetraisopropyl genistein (2b). The product was obtained as a white solid (21.1% yield); m.p. 278~279 °C. 1H-NMR (DMSO-d6) δ (ppm): 1H-NMR (500 MHz, DMSO) δ 13.61 (s, 1H, 5-OH), 10.99 (s, 1H, 7-OH), 9.46 (s, 1H, 4′-OH), 8.24 (s, 1H, H-2), 7.17 (s, 2H, H-2′,6′), 3.58 (m, J = 14.0, 6.9 Hz, 1H, -CH(CH3)2), 3.50 (dt, J = 13.9, 7.1 Hz, 1H, -CH(CH3)2), 3.34–3.26 (m, 2H, -CH(CH3)2), 1.34 (d, J = 7.4 Hz, 6H, -CH(CH3)2), 1.31 (d, J = 6.8 Hz, 6H, -CH(CH3)2), 1.18 (d, J = 6.7 Hz, 12H, -CH(CH3)2). 13C-NMR (DMSO-d6) δ (ppm): 181.58 (s, C-4), 158.91 (s, C-7), 158.18 (s, C-4′), 154.34 (s, C-5), 153.90 (s, C-8a), 151.18 (s, C-2), 135.39 (s,C-2′,6′), 125.39 (s, C-1′), 124.41 (s, C-3′,5′), 122.83 (s, C-3), 117.74 (s, C-4a), 112.68 (s, C-6), 105.59 (s, C-8), 30.88 (s, 2C), 26.65 (s, 1C), 24.45 (s, 1C), 23.46 (s, 4C), 21.45 (s, 2C), 20.69 (s, 2C). HRMS (ESI) m/z: 437.23114 [M − H]−, calcd. for C27H33O5 437.23280.
2′,5′,6,8-Tetraisopropyl genistein (2c). The product was obtained as a white solid (20.3% yield); m.p. 273~274 °C. 1H-NMR (DMSO-d6) δ (ppm): 13.48 (s, 1H, 5-OH), 11.01 (s, 1H, 7-OH), 9.51 (s, 1H, 4′-OH), 8.26 (s, 1H, H-2), 7.07 (s, 1H, H-6′), 6.70 (s, 1H, H-3′), 3.72 (m, J = 5.2 Hz, 1H, -CH(CH3)2), 3.62–3.54 (m, 1H, -CH(CH3)2), 3.49 (d, J = 13.7 Hz, 2H, -CH(CH3)2), 1.23(d, J = 17.4 Hz, 24H, -CH(CH3)2). 13C-NMR (DMSO-d6) δ (ppm): 172.78 (s, C-4), 159.13 (s, C-7), 158.07 (s, C-4′), 155.39 (s, C-5), 155.11 (s, C-8a), 154.06 (s, C-2), 130.11 (s, C-2′, 6′), 129.78 (s, C-1′), 128.91 (s, C-3), 128.24 (s, C-3′,5′), 105.30 (s, C-4a), 100.36 (s, C-6), 97.40 (s, C-8), 29.62 (s, 2C), 27.08 (s, 2C), 25.68 (s, 1C), 24.46 (s, 1C), 22.68 (s, 1C), 22.46 (s, 1C), 21.48 (s, 2C), 20.66 (s, 2C). HRMS (ESI) m/z: 437.23457 [M − H]−, calcd. for C27H33O5 437.23280.
2′,6′,6,8-Tetraisopropyl genistein (2d). The product was obtained as a white solid (19.5% yield); m.p. 289~290 °C. 1H-NMR (DMSO-d6) δ (ppm): 13.52 (s, 1H, 5-OH), 11.07 (s, 1H, 7-OH), 9.32 (s, 1H, 4′-OH), 8.25 (s, 1H, H-2), 6.86 (s, 2H, H-3′,5′), 3.63–3.52 (m, 1H, -CH(CH3)2), 3.53–3.43 (m, 1H, -CH(CH3)2), 3.16 (dt, J = 13.3, 6.8 Hz, 1H, -CH(CH3)2), 2.66 (dt, J = 13.3, 6.6 Hz, 1H, -CH(CH3)2), 1.37–1.32 (m, 12H), 1.30 (d, J = 6.7 Hz, 6H), 1.15 (d, J = 6.2 Hz, 6H, -CH(CH3)2). 13C-NMR (DMSO-d6) δ (ppm): 182.16 (s, C-4), 159.08 (s, C-7), 158.08 (s, C-4′), 155.45 (s, C-5), 155.08 (s, C-8a), 154.07 (s, C-2), 140.08 (s, C-2′,6′), 128.98 (s, C-1′), 125.32 (C-3′,5′), 121.60 (s, C-3), 105.33 (s, C-4a), 96.86 (s, C-6), 93.18 (s, C-8), 34.86 (s, 1C), 30.88 (s, 2C), 30.45 (s, 1C), 26.66 (s, 1C), 24.46 (s, 1C), 22.96 (s, 2C), 21.48 (s, 2C), 20.66 (s, 2C). HRMS (ESI) m/z: 437.23080 [M − H]−, calcd. for C27H33O5 437.23280.
3′,5′-Diisopropyl biochanin A (2e). The product was obtained as a buff solid (21.2% yield); m.p. 216~217 °C. 1H-NMR (DMSO-d6) δ (ppm): 12.94 (s, 1H, 5-OH), 10.90 (s, 1H, 7-OH), 8.37 (s, 1H, H-2), 7.30 (s, 2H, H-2′,6′), 6.41 (d, J = 1.5 Hz, 1H, H-8), 6.25 (d, J = 1.5 Hz, 1H, H-6), 3.70 (s, 3H, -OCH3), 3.29 (dt, J = 13.7, 6.9 Hz, 2H, -CH(CH3)2), 1.22 (d, J = 6.9 Hz, 12H, -CH(CH3)2). 13C-NMR (DMSO-d6) δ (ppm): 180.49 (s, C-4) 165.19 (s, C-7), 162.46 (s, C-4′), 158.08 (s, C-5), 155.12 (s, C-8a), 154.57 (s, C-2), 141.38 (s, C-2′,6′), 127.38 (s, C-1′), 125.41 (s, C-3′,5′), 122.89 (s, C-3), 104.81 (s, C-4a), 99.60 (s, C-6), 94.24 (s, C-8), 62.44 (s, -OCH3), 26.52 (s, 2C), 24.34 (s, 4C). HRMS (ESI) m/z: 367.15324 [M − H]−, calcd. for C22H23O5 367.15455.
3′,5′,8-Triisopropyl biochanin A (2f). The product was obtained as a buff solid (19.2% yield); m.p. 211~212 °C. 1H-NMR (DMSO-d6) δ (ppm): 12.99 (s, 1H, 5-OH), 10.87 (s, 1H, 7-OH), 8.45 (s, 1H, H-2), 7.32 (s, 2H, H-2′,6′), 6.33 (s, 1H, H-8), 3.69 (s, 3H, -OCH3), 3.28 (dt, J = 13.4, 6.6 Hz, 3H, -CH(CH3)2), 1.21 (d, J = 6.7 Hz, 18H, -CH(CH3)2). 13C-NMR (DMSO-d6) δ (ppm): 180.94 (s, C-2), 162.54 (s, C-7), 159.94 (s, C-4′), 155.55 (s, C-5), 155.08 (s, C-8a), 154.51 (s, C-2), 141.35 (s, C-2′,6′), 127.48(s, C-1′), 125.40 (s, C-3′,5′), 122.34 (s, C-3), 105.61 (s, C-4a), 99.42 (s, C-6), 93.70 (s, C-8), 62.41 (s, -OCH3), 26.51 (s, 2C), 25.60 (s, 1C), 24.33 (s, 6C). HRMS (ESI) m/z: 409.20875 [M − H]−, calcd. for C25H29O5 409.20150.
3′,5′,6,8-Tetraisopropyl biochanin A (2g). The product was obtained as a buff solid (23.4% yield); m.p. 208~209 °C. 1H-NMR (DMSO-d6) δ (ppm): 13.55 (s, 1H, 5-OH), 9.50 (s, 1H, 7-OH), 8.47 (s, 1H, H-2), 7.31 (s, 2H, H-2′,6′), 3.70 (s, 3H,-OCH3), 3.62–3.54 (m, 1H, -CH(CH3)2), 3.53–3.46 (m, 1H, -CH(CH3)2), 3.32–3.23 (m, 2H, -CH(CH3)2), 1.40–1.13 (m, 24H, -CH(CH3)2). 13C-NMR (DMSO-d6) δ (ppm): 181.33 (s, C-4), 159.04 (s, C-7), 158.21 (s, C-4′), 154.89 (s, C-5), 154.40 (s, C-8a), 153.90 (s, C-2), 141.35 (s, C-2′,6′), 127.60 (s, C-1′), 125.38 (s, C-3′,5′), 122.32 (s, C-3), 117.91 (s, C-4a), 112.81 (C-6), 105.59 (s, C-8), 62.44 (s, -OCH3), 30.89 (s, 1C), 26.52 (s, 2C), 24.47 (s, 1C), 24.34 (s, 4C), 21.44 (s, 2C), 20.68 (s, 2C). HRMS (ESI) m/z: 451.24680 [M − H]−, calcd. for C28H35O5 451.24845.
5′-Isopropyl biochanin A (2h). The product was obtained as a buff solid (21.2% yield); m.p. 202~203 °C. 1H-NMR (DMSO-d6) δ (ppm): 12.94 (s, 1H, 5-OH), 10.76 (s, 1H, 7-OH), 8.37 (s, 1H, H-2), 7.30 (s, 1H, H-6′), 7.21(d, J = 7.7 Hz, 1H, H-2′), 6.88 (d, J = 7.8 Hz, 1H, H-3′), 6.42 (s, 1H, H-8), 6.24 (s, 1H, H-6), 3.70 (s, 3H, -OCH3), 3.28 (m, J = 13.8, 6.9 Hz, 1H, -CH(CH3)2), 1.36 (d, J = 6.9 Hz, 6H, -CH(CH3)2). 13C-NMR (DMSO-d6) δ (ppm): 180.53 (s, C-4), 164.96 (s, C-7), 162.48 (s, C-4′), 158.07 (s, C-5), 155.16 (s, C-8a), 154.60 (s, C-2), 141.39 (s, C-2′,6′), 128.51 (s, C-1′), 125.41 (s, C-3′,5′), 122.93 (s, C-3), 104.91 (s, C-4a), 99.54 (s, C-6), 94.21 (s, C-8), 62.44 (s, -OCH3), 26.53 (s, 1C), 24.34 (s, 2C). HRMS (ESI) m/z: 325.10345 [M − H]−, calcd. for C19H17O5 325.10760.
3′,5′-Diisopropyl formononetin (2i). The product was obtained as a white solid (23.1% yield); m.p. 250~251 °C. 1H-NMR (DMSO-d6) δ (ppm): 10.83 (s, 1H, 7-OH), 8.35 (s, 1H, H-2), 7.97 (d, J = 11.2 Hz, 1H, H-5), 7.30 (s, 2H, H-2′,6′), 6.95 (d, J = 8.7 Hz, 1H, H-6), 6.88 (s, 1H, H-8), 3.69 (s, 3H, -OCH3), 3.28 (dt, J = 13.5, 6.7 Hz, 2H, -CH(CH3)2), 1.26–1.12 (m, 12H, two -CH(CH3)2).13C-NMR (DMSO-d6) δ (ppm): 175.04 (s, C-4), 163.06 (s, C-7), 157.91 (s, C-4′), 154.36 (s, C-8a), 154.04 (s, C-2), 141.19 (s, C-2′,6′), 128.60 (s, C-5), 127.80 (s, C-1′), 125.31 (s, C-3′,5′), 124.14 (s, C-3), 117.13 (s, C-6), 115.68 (s, C-8), 102.59 (s, C-4a), 62.42 (s, -OCH3), 26.50 (s, 2C), 24.36 (s, 4C). HRMS (ESI) m/z: 351.15826 [M − H]−, calcd. for C22H23O4 351.15964.
3′,5′,8-Triisopropyl formononetin (2j). The product was obtained as a white solid (24.3% yield); m.p. 242~243 °C. 1H-NMR (DMSO-d6) δ (ppm): 10.62 (s, 1H, 7-OH), 8.43 (s, 1H, H-2), 7.85 (d, J = 8.6 Hz, 1H, H-5), 7.33 (s, 2H, H-2′,6′), 6.99 (d, J = 8.7 Hz, 1H, H-6), 3.74–3.70 (m, 1H, -CH(CH3)2), 3.70 (s, 3H, -OCH3), 3.28 (dt, J = 13.4, 6.6 Hz, 2H, -CH(CH3)2), 1.37 (d, J = 6.8 Hz, 6H, -CH(CH3)2), 1.22 (d, J = 6.6 Hz, 12H, -CH(CH3)2). 13C-NMR (DMSO-d6) δ (ppm): 175.40 (s, C-4), 160.33 (s, C-7), 155.96 (s, C-4′), 154.29 (s, C-8a), 153.92 (s, C-2), 141.16 (s, C-2′,6′), 128.71 (s, C-5), 125.28 (s, C-3′,5′), 124.62 (s, C-1′), 123.47 (s, C-3), 120.69 (s, C-6), 117.51 (s, C-8), 115.21 (s, C-4a), 62.41 (s, -OCH3), 26.49 (s, 2C), 24.44 (s, 4C), 24.28 (s, 1C), 20.92 (s, 2C). HRMS (ESI) m/z: 393.20514 [M − H]−, calcd. for C25H29O4 393.20659.
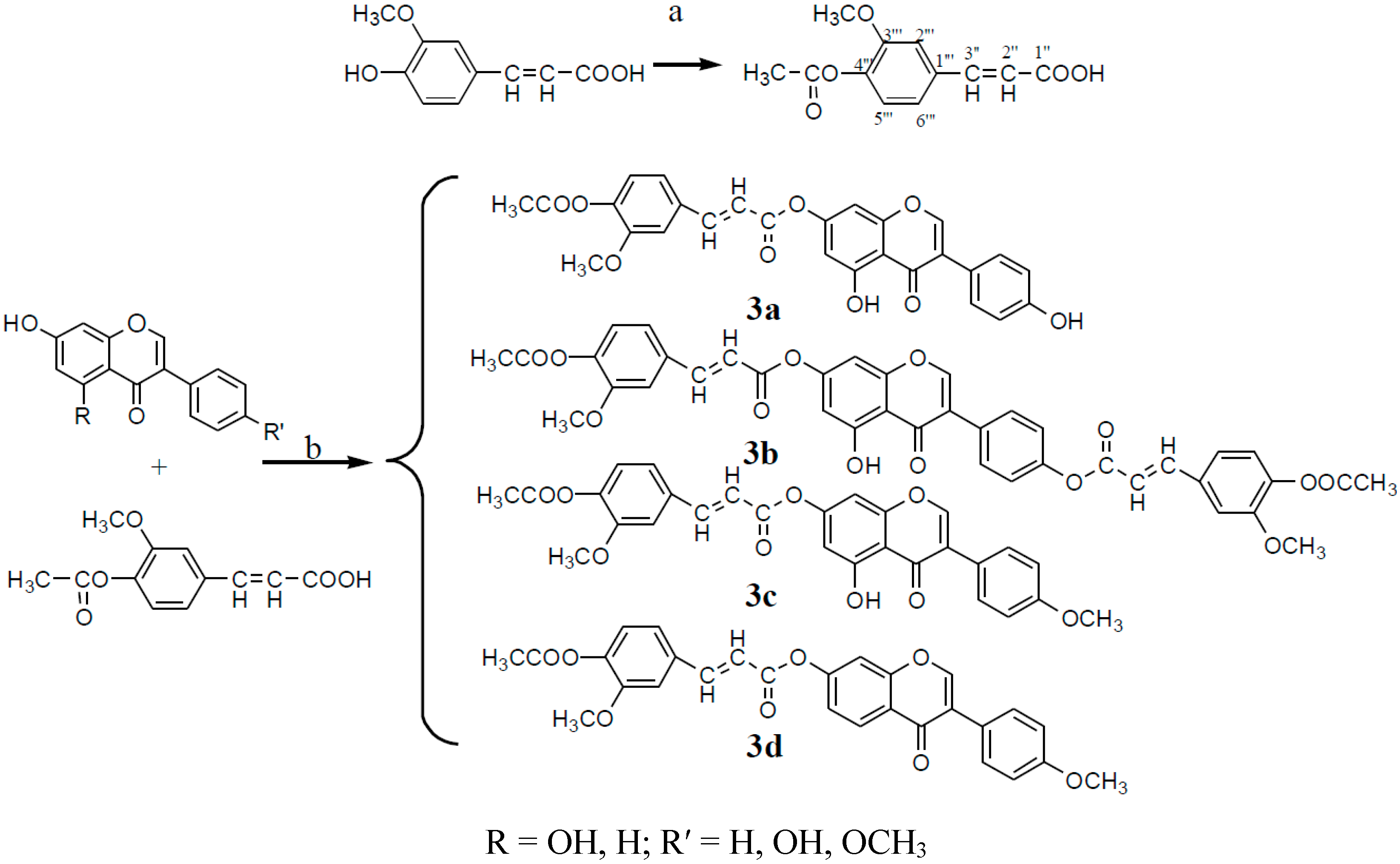
3.2.3. Synthesis of Compounds 3a–3d (Considering One of the Parental Compounds as an Example)
Treatment of acetic anhydride [
19]: P
2O
5 was added to acetic anhydride until the P
2O
5 became a powder. The mixture was filtered to remove the P
2O
5. The filtrate was distilled at 90 °C and 0.096 MPa; fractions were collected at a constant temperature and saved in a dryer.
Treatment of pyridine: KOH was added to pyridine and refluxed for 5 h until the KOH became pasty. Then, the reflux was stopped to distill the solution at a lower pressure. Fractions were collected at 68 °C until the pressure reached 0.085 MPa, and the pyridine was finally sealed with wax.
Synthesis of acetyl ferulic acid: Ferulic acid (19.4 g, 0.1 mol) was dissolved in acetic anhydride (40 mL) and anhydrous pyridine (10 drops). The mixture was stirred at 120 °C for 4 h, and was then cooled to room temperature. A large quantity of precipitate formed immediately. The precipitate was filtered and washed with water and anhydrous ethanol. The resulting solid was finally re-crystallized with anhydrous ethanol, producing the product as a white solid (67.2%); m.p. 190–191 °C.
Synthesis of target compounds 3a–3d: Acetyl ferulic acid (0.4 g, 1.69 mmol), genistein (0.5 g, 1.85 mmol), and DCC (0.6 g, 2.90 mmol) were dissolved in tetrahydrofuran (20 mL), and anhydrous pyridine (1 mL) was then added to the mixture. The mixture was then stirred at 80 °C for 4 h. The resulting mixture was filtered to remove the DCU, and the crude material was then purified in a 300–400 mesh silica column (petroleum ether:ethyl acetate:methanol, 6:2:1).
Genistein 7-acetylferulic acid (3a). The product was obtained as a white solid (33.2%); m.p. 257~258 °C. 1H-NMR (DMSO-d6) δ (ppm): 13.02 (s, 1H, 5-OH), 9.63 (s, 1H, 4′-OH), 8.52 (s, 1H, H-2), 7.92 (d, 1H, J = 15.4 Hz, H-3′′), 7.63 (m, 1H, H-2′′′), 7.45 (d, 2H, J = 8.3 Hz, H-2′,6′), 7.17 (m, 1H, H-6′′′), 7.13 (m, 1H, H-5′′′), 6.97 (d, 1H, J = 15.4Hz, H-2′′), 6.83 (d, 2H, J = 8.3 Hz, H-3′,5′), 6.81 (s, 1H, H-6), 6.77 (s, 1H, H-8), 3.86 (s, 3H, -OCH3), 2.33 (s, 3H, -COCH3); 13C-NMR (DMSO-d6) δ (ppm): 180.7 (s, C-4), 168.2 (s, C-1′′), 164.2 (s, -COO-), 161.1 (s, C-5), 157.8 (s, C-8a), 156.6 (s, C-7), 155.7 (s, C-4′), 155.6 (s, C-2), 151.8 (s, C-3′′′), 146.1 (s, C-3′′), 140.3 (s, C-4′′′), 132.9 (s, C-1′′′), 131.2 (s, C-2′,6′), 123.4 (s, C-1′), 123.1 (s, C-5′′′), 122.0 (s, C-3), 120.4 (s, C-6′′′), 116.3 (s, C-2′′′), 115.3 (s, C-3′, 5′), 112.2 (s, C-2′′′), 108.4 (s, C-4a), 104.2 (s, C-6), 101.1 (s, C-8), 56.4 (s, -OCH3), 20.5 (s, -CH3). HRMS (ESI) m/z: 489.10428 [M + H]+, calcd. for C27H21O9 489.11856.
Genistein 7,4′-diacetylferulic acid (3b). The product was obtained as a white solid (31.7%); m.p. 234–235 °C. 1H-NMR (DMSO-d6) δ (ppm): 12.95 (s, 1H, 5-OH), 8.73 (s, 1H, H-2), 7.97 (d, 2H, J = 15.64 Hz, H-3′′), 7.75 (m, 2H, H-2′′′), 7.71 (d, 2H, J = 8.35 Hz, H-2′,6′), 7.43 (d, 2H, J = 8.35 Hz, H-3′,5′), 7.42 (m, 2H, H-6′′′), 7.22 (m, 2H, H-5′′′), 7.05 (d, 2H, J = 15.65 Hz, H-2′′), 6.85 (s, 1H, H-6), 6.84 (s, 1H, H-8), 3.91 (s, 6H, -OCH3), 2.33 (s, 6H, -CH3); 13C-NMR (DMSO-d6) δ (ppm): 180.1 (s, C-4), 167.5 (s), 167.7 (s, C-1′′), 164.2 (s), 164.2 (s, 2-COO-), 156.2 (s, C-5), 155.7 (s, C-4′), 152.4 (s, C-7), 150.5 (s, C-8a), 150.5 (s), 150.6 (s, C-3′′′), 150.2 (s, C-2), 145.3 (s), 145.6 (s, C-3′′), 141.3 (s), 141.3 (s, C-4′′′), 131.6 (s), 132.1 (s, C-1′′′), 129.2 (s, C-2′,6′), 122.6 (s, C-1′), 122.3 (s), 122.3 (s, C-5′′′), 120.8 (s, C-3), 120.5 (s), 120.6 (s, C-6′′′), 116.4 (s), 116.6 (s, C-2′′), 112.4 (s, C-3′,5′), 110.6 (s), 110.7 (s, C-2′′′), 104.7 (s, C-4a), 100.1 (s, C-6), 95.3 (s, C-8), 55.1, 55.1 (s, -OCH3), 19.4 (s), 19.4 (-CH3). HRMS (ESI) m/z: 707.15763 [M + H]+, calcd. for C39H31O13 707.17647.
Biochanin A 7-acetylferulic acid ester (3c). The product was obtained as a buff solid (38.3%); m.p. 192~193 °C. 1H-NMR (DMSO-d6) δ (ppm): 12.86 (s, 1H, 5-OH), 8.46 (s, 1H, H-2), 7.61 (d, J = 8.4 Hz, 2H, H-2′,6′), 7.38 (d, J = 8.4 Hz, 1H, H-6′′′), 7.21 (d, J = 8.4 Hz, 2H, H-3′,5′), 7.05–6.97 (d, J = 16.4 Hz, 1H, H-3′′), 6.88 (s, 1H, H-2′′′), 6.82 (d, J = 8.4 Hz, 1H, H-5′′′), 6.75–6.66 (d, J = 16.1 Hz, 1H, H-2′′), 6.41 (s, 1H, H-8), 6.25 (s, 1H, H-6), 3.80 (s, 3H, -OCH3), 3.93 (s, 3H, -OCH3), 2.30 (s, 3H, CH3COO-). 13C-NMR (DMSO-d6) δ (ppm): 180.28 (s, C-4), 169.69 (s, C-1′′), 165.14 (s, -COO-), 162.45 (s, C-5), 158.09 (s, C-8a), 156.69 (s, C-7), 155.50 (s, C-4′), 154.45 (s, C-2), 151.94 (s, C-3′′′), 150.78 (s, C-3′′), 139.66 (s, C-4′′′), 133.3 (s, C-1′′′), 130.59 (s, C-2′,6′), 128.85 (s, C-1′), 125.38 (s, C-5′′′), 122.74 (s, C-3), 122.17 (s, C-6′′′), 115.60 (s, C-2′′), 115.53 (s, C-3′,5′), 111.74 (s, C-2′′′), 106.01(s, C-4a), 99.67 (s, C-6), 94.34 (s, C-8), 57.44 (s, -OCH3), 49.94 (s, -OCH3), 21.93 (s, -CH3). HRMS (ESI) m/z: 503.11478 [M + H]+, calcd. for C28H23O9 503.13421.
Formononetin 7-acetylferulic acid ester (3d). The product was obtained as a white solid (30.8%); m.p. 236~237 °C. 1H-NMR (DMSO-d6) δ (ppm): 8.44 (s, 1H, H-2), 8.04 (d, J = 8.8 Hz, 1H, H-5), 7.66 (d, J = 16.3 Hz, 1H, H-3′′), 7.62 (s, 1H, H-2′′′), 7.54 (d, J = 6.2 Hz, 2H, H-2′,6′),7.37 (d, J = 8.0 Hz, 1H, H-6′′′), 7.32 (d, J = 8.4 Hz, 1H, H-5′′′), 7.08 (d, J = 8.9 Hz, 1H, H-6), 7.01 (d, J = 7.3 Hz, 2H, H-3′,5′), 6.88 (s, 1H, H-8), 6.73 (d, J = 16.3 Hz, 1H, H-2′′), 3.87 (s, 3H, -OCH3), 3.79 (s, 3H, -OCH3), 2.30 (d, J = 8.5 Hz, 3H, -COCH3).13C-NMR (DMSO-d6) δ (ppm): 180.91 (s, C-4), 168.63 (s, C-1′′), 164.91 (s, -COO-), 162.10 (s, C-7), 157.93 (s, C-4′), 156.27 (s, C-8a), 152.03 (s, C-2), 150.36 (s, C-3′′′), 146.21 (s, C-1′′), 142.04 (s, C-4′′′), 132.50 (s, C-1′′′), 130.66 (s, C-2′,6′), 128.62 (s, C-5), 125.39 (s, C-1′), 124.80 (s, C-3), 123.70 (s, C-5′′′), 119.96 (s, C-6′′′), 117.95 (s, C-2′′′), 116.60 (s, C-6), 114.92 (s, C-8), 114.09 (s, C-3′,5′), 112.34 (s, C-2′′′), 101.43 (C-4a), 64.71 (s, -OCH3), 56.61 (s, -OCH3), 21.89 (s, -CH3). HRMS (ESI) m/z: 509.12245 [M + Na]+, calcd. for C28H22NaO8 509.12124.
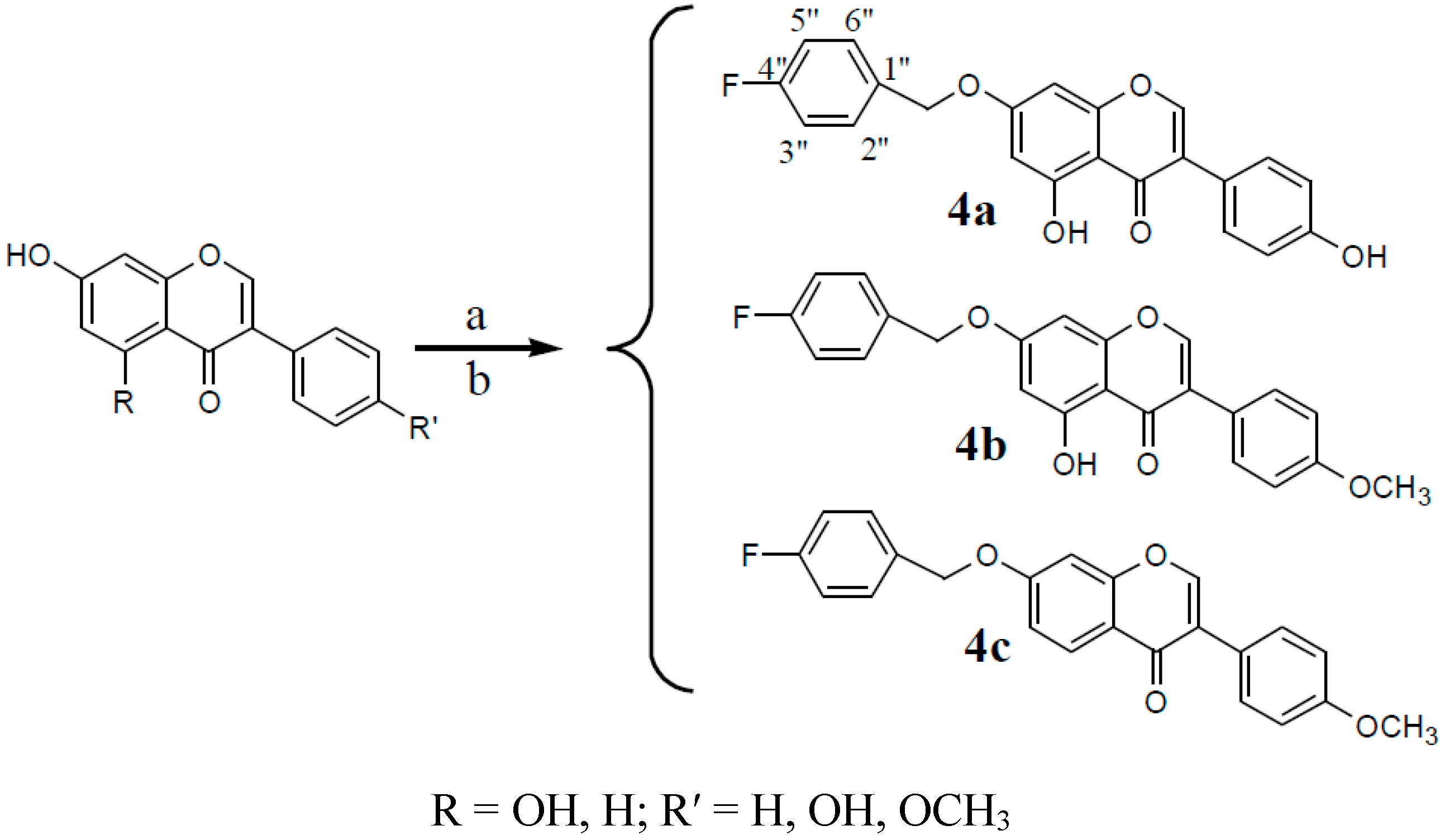
3.2.4. Synthesis of Compounds 4a–4e (Take One of the Parental Compounds as an Example)
Formononetin (0.6 g, 2.1 mmol) was dissolved in anhydrous methanol (60 mL), and CH3ONa-CH3OH (5 mL) was then added to the mixture. 4-fluorobenzyl bromide (0.47 g, 2.5 mmol) was dissolved in dichloromethane (10 mL) and then added to the mixture. The solution was stirred under reflux for 20 h. The resulting product was filtered and separated in a 300–400 mesh silica column (petroleum ether:ethyl acetate:methanol, 6:2:1).
7-(4-Fluorine benzyl)-O-genistein (4a). The product was obtained as a white solid (30.1%); m.p. 243~244 °C. 1H-NMR (DMSO-d6) δ (ppm): 12.95 (s, 1H, 5-OH), 9.63 (s, 1H, 4′-OH), 8.45 (s, 1H, H-2), 7.57–7.49 (m, 2H, H-2′′,6′′), 7.40 (d, J = 8.0 Hz, 2H, H-2′,6′), 7.25 (d, J = 8.5 Hz, 2H, H-3′′,5′′), 6.83 (d, J = 7.9 Hz, 2H, H-3′,5′), 6.76 (s, 1H, H-8), 6.50 (s, 1H, H-6), 5.19 (s, 2H, -CH2-). 13C-NMR (DMSO-d6) δ (ppm): 180.89 (s, C-4), 164.56 (s, C-7), 163.39 (s, C-4′′), 162.24 (s, C-4′), 157.93 (s, C-5), 157.90 (s, C-8a) 154.96 (s, C-2), 132.80 (s, C-1′′), 130.83 (s, C-2′′), 130.76 (s, C-6′′), 130.66 (s, C-2′,6′), 122.99 (s, C-1′), 121.49 (s, C-3), 115.96 (s, C-3′′), 115.78 (s, C-5′′), 115.55 (s, C-3′,5′), 106.02 (s, C-4a), 99.14 (s, C-6), 93.77 (s, C-8), 69.77 (s, -CH2O-). HRMS (ESI) m/z: 377.09124 [M − H]−, calcd. for C22H14FO5 377.08253.
7-(4-Fluorine benzyl)-O-biochanin (4b). A The product was obtained as a buff solid (26.8%); m.p. 175~176 °C. 1H-NMR (DMSO-d6) δ (ppm): 12.94 (s, 1H, 5-OH), 8.48 (s, 1H, H-2), 7.54 (dd, J = 13.6, 7.4 Hz, 4H, H-2′,6′; H-2′′,6′′), 7.26 (t, J = 8.3 Hz, 2H, H-3′′,5′′), 7.02 (d, J = 7.6 Hz, 2H, H-3′,5′), 6.78 (s, 1H, H-8), 6.52 (s, 1H, H-6), 5.23 (s, 2H, -CH2-), 3.80 (s, 3H, -OCH3). 13C-NMR (DMSO-d6) δ (ppm): 180.81 (s, C-4), 167.45 (s, C-4′′), 164.62 (s, C-7), 162.24 (s, C-4′), 159.69 (s, C-5), 157.92 (s, C-8a), 155.30 (s, C-2), 132.02 (s, C-1′′), 130.84 (s, C-2′′), 130.78 (s, C-6′′), 130.66 (s, C-2′,6′), 123.21 (s, C-1′), 122.67 (s, C-3), 115.96 (s, C-3′′), 115.79 (s, C-5′′), 114.21 (s, C-3′,5′), 106.02 (s, C-10), 99.21 (s, C-6), 93.84 (s, C-8), 69.79 (s, -CH2O-), 55.65 (s, CH3O-). HRMS (ESI) m/z: 391.11194 [M − H]–, calcd. for C23H16FO5 391.09818.
7-(4-Fluorine benzyl)-O-formononetin (4c). The product was obtained as a white solid (32.3%); m.p. 193~194 °C. 1H-NMR (DMSO-d6) δ (ppm): 8.44 (s, 1H, H-2), 8.05 (d, J = 8.8 Hz, 1H, H-5), 7.62–7.48 (m, 4H, H-2′,6′; H-2′′,6′′), 7.23–7.33 (m, 3H, H-3′′,5′′;H-8), 7.16 (d, J = 8.8 Hz, 1H, H-6), 7.01 (d, J = 7.6 Hz, 2H, H-3′,5′), 5.26 (s, 2H, -CH2-), 3.80 (s, 3H, -OCH3). 13C-NMR (DMSO-d6) δ (ppm): 175.09 (s, C-4), 167.44 (s, C-4′′), 163.06 (s, C-7), 159.48(s, C-4′), 157.81 (s, C-8a), 154.03 (s, C-2), 132.02 (s, C-1′′), 130.89 (s, C-2′′), 130.82 (s, C-6′′), 130.56 (s, C-2′, 6′), 127.50 (s, C-5), 124.50 (s, C-1′), 123.85 (s,C-3), 118.23 (s, C-6), 115.97 (s, C-8),115.80 (s, C-3′′), 115.73(s, C-5′′), 114.10 (s, C-3′,5′), 102.06 (s, C-4a), 69.82 (s, -CH2O-), 55.62 (s, CH3O-). HRMS (ESI) m/z: 399.11270 [M + Na]+, calcd. for C23H17FNaO4 399.10085.
3.2.5. Synthesis of Compound 5
Biochanin A (0.81 g, 2.8 mmol) was dissolved in anhydrous ethanol (30 mL). The mixture was heated to 60 °C. After the biochanin A dissolved, (CH3COO)3Cr (0.34 g, 1.5 mmol) was added to the solution, and the mixture was stirred under reflux for 18 h. The mixture was then cooled to room temperature. A green precipitate formed and was filtered. The precipitate was washed with DI H2O and anhydrous ethanol until the biochanin A and (CH3COO)3Cr were removed. The resulting product was purified in a 300–400 mesh silica column (petroleum ether:ethyl acetate:methanol, 6:2:1).
(1) The physicochemical properties and element analysis
The biochanin A chromium complex was in the form of a grass-green powder that could be dissolved in DMSO, dimethyl formamide (DMF), and tetrahydrofuran (THF), that was slightly soluble in methanol or acetone and that was insoluble in water and CCl
4. The C and H contents of the biochanin A chromium complex were measured using a Vario EL element analyzer. The chromium content was measured via EDTA titration. The molecular weight was analyzed using a Thermo LQT Orbitrap XL LC-MS. The results are shown in
Table 2.
(2) Thermogravimetry (TG) and differential thermal analysis (DTA)
The thermal spectrum of the complex was measured under airflow at a heating rate of 10 °C/min. Two stages of weight loss can be observed in the TG-DTA spectrum. The first stage of weight loss was at approximately 75 °C, corresponding to a weight loss rate of 3.94% and a considerable loss of 2 water molecules (the theoretical weight loss rate was 4.05%). The corresponding absorption peak in the DTA curve was very small and showed that the complex contained 2 crystal water molecules. The second stage included the range from 320 °C to 420 °C, and the weight loss rate was 90.60%, corresponding to the considerable loss of 3 ligand molecules (the theoretical weight loss rate was 90.62%). The corresponding absorption peak, which was an oxidation-decomposition peak of the complex in the DTA curve, was very large. Chromium was oxidized in air, and the remnant of the decomposition was Cr2O3.
Table 2.
Elemental analysis of the complex.
Table 2.
Elemental analysis of the complex.
| C % | H % | Cr % | Molecular |
|---|
| test | theory | test | theory | test | theory | test | theory [M − 2H2O − H]− |
| 61.43 | 61.47 | 3.93 | 3.95 | 5.53 | 5.55 | 900.12354 | 900.11463 |
(3) Infrared spectrum analysis
From 400–4000 cm
−1, the IR spectra of the ligand and complex were measured using a Thermo-Nicolet nexus of a Fourier transform IR spectrometer (KBr pellets), as shown in
Figure 7. The primary spectral data are listed in
Table 3.
A comparison of the IR spectral data of the ligand and chromium complex is shown in
Table 3. Biochanin A exhibited an absorption band (OH) at 3388 cm
−1, which shifted to 3432 cm
−1 in the IR spectrum of the complex. Biochanin A exhibited a very strong absorption band at 1653 cm
−1 due to the stretching vibration of the 4-carbonyl, which shifted to 1627 cm
−1 in the IR spectrum of its complex. Therefore, coordination of the 4-carbonyl is involved in the coordination of the ligands.
Table 3.
The primary IR spectral data of genistein and the chromium complexes (cm−1).
Table 3.
The primary IR spectral data of genistein and the chromium complexes (cm−1).
| Compound | V(O-H) | V(C=O) | V(C=C) | V(C-O-C) |
|---|
| Biochanin A (L) | 3388 | 1653 | 1623 | 1249 |
| CrL3·2H2O | 3432 | 1627 | 1612 | 1249 |
Figure 7.
IR spectra analysis.
Figure 7.
IR spectra analysis.
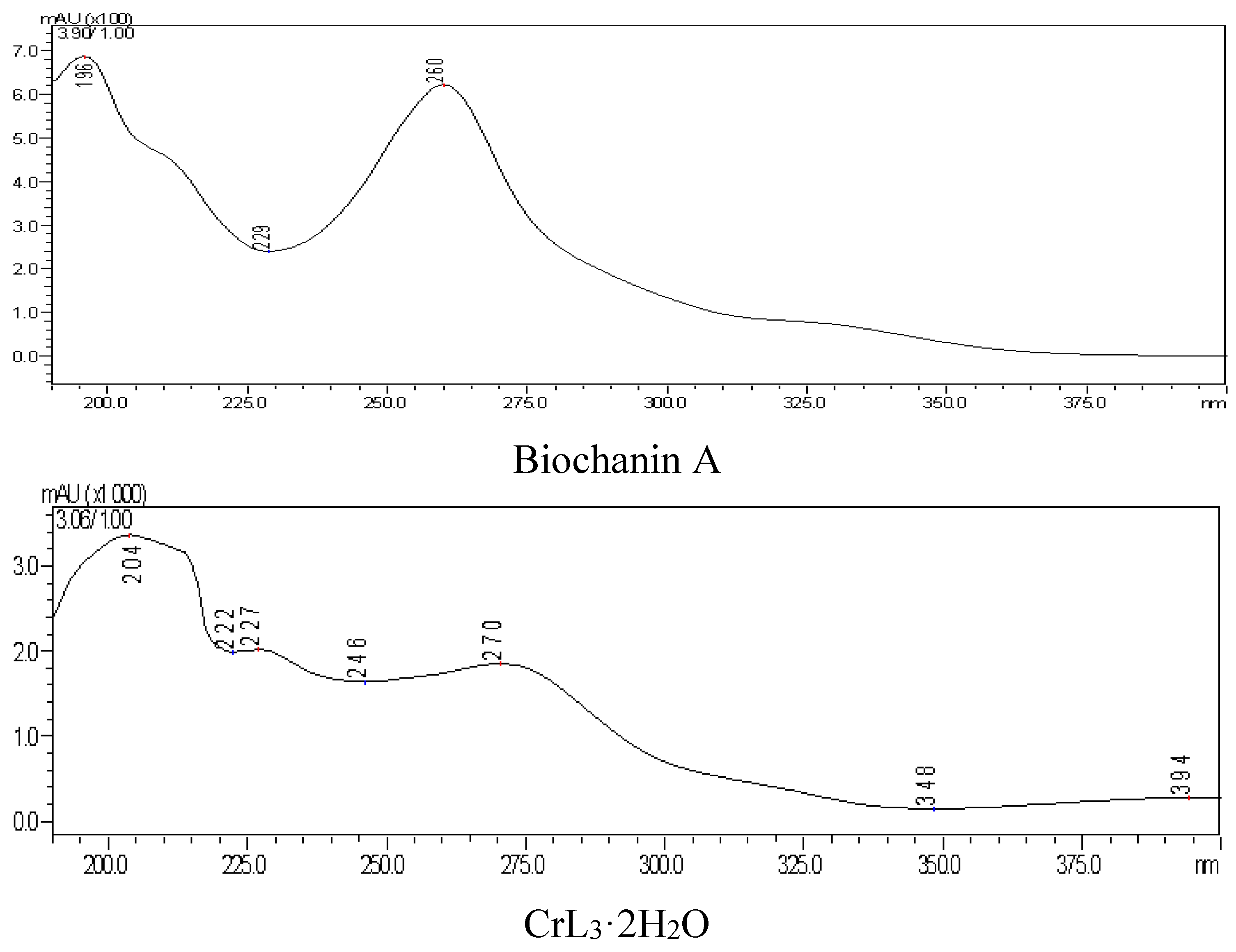
(4) Ultraviolet spectrum
There were two characteristic UV absorption peaks at 260 nm (band II) and 330 nm (band I, weak) in DMSO:CH3OH = 1:9 solvent. After complex formation, the two peaks red shifted to 270 nm and 394 nm (weak). Because the B ring could not conjugate with the unsaturated carbonyl of the C ring in biochanin A, the absorption intensity of the band I was therefore weakened. However, the band I was red-shifted in the chromium complex, and the intensity of this band increased due to the enhanced plane type of molecule. The conjugated system of complex was larger, which was attributed to the 4-carbonyl and 5-hydroxyl of the three biochanin A molecules coordinated with chromium, as shown in
Figure 8.
Figure 8.
UV spectra analysis.
Figure 8.
UV spectra analysis.