Spectroscopic Study on the Interaction between Naphthalimide-Polyamine Conjugates and Bovine Serum Albumin (BSA)
Abstract
:1. Introduction
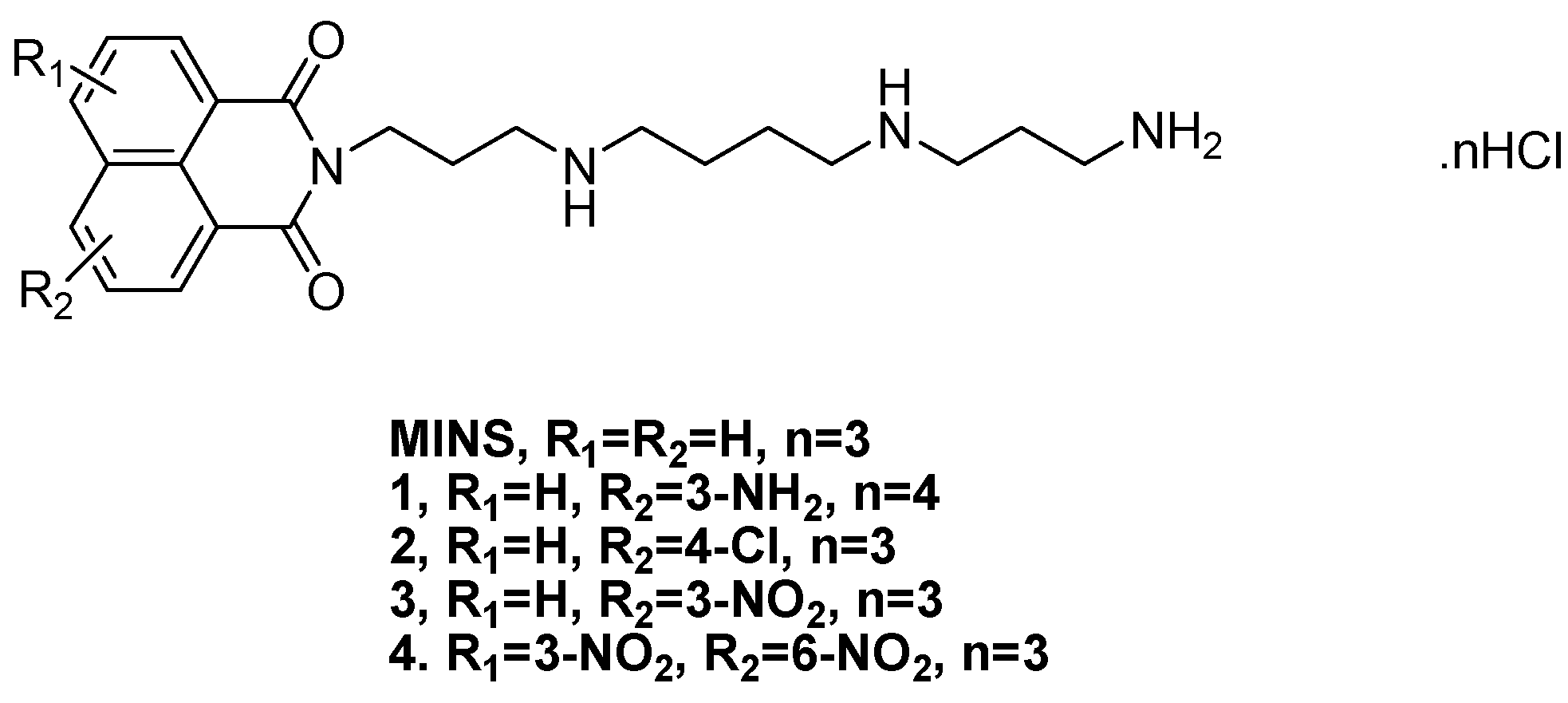
2. Results and Discussion
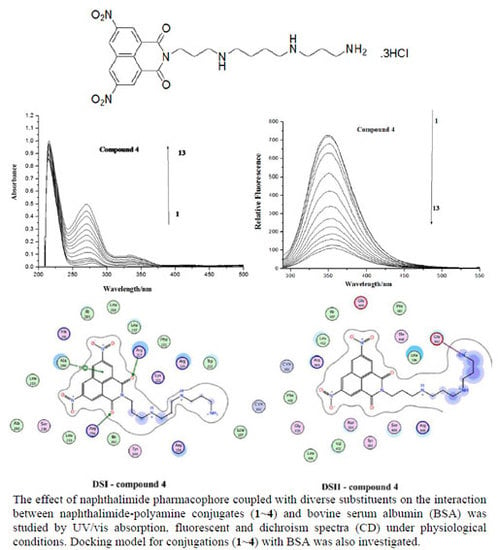
2.1. UV Spectroscopic Characteristics
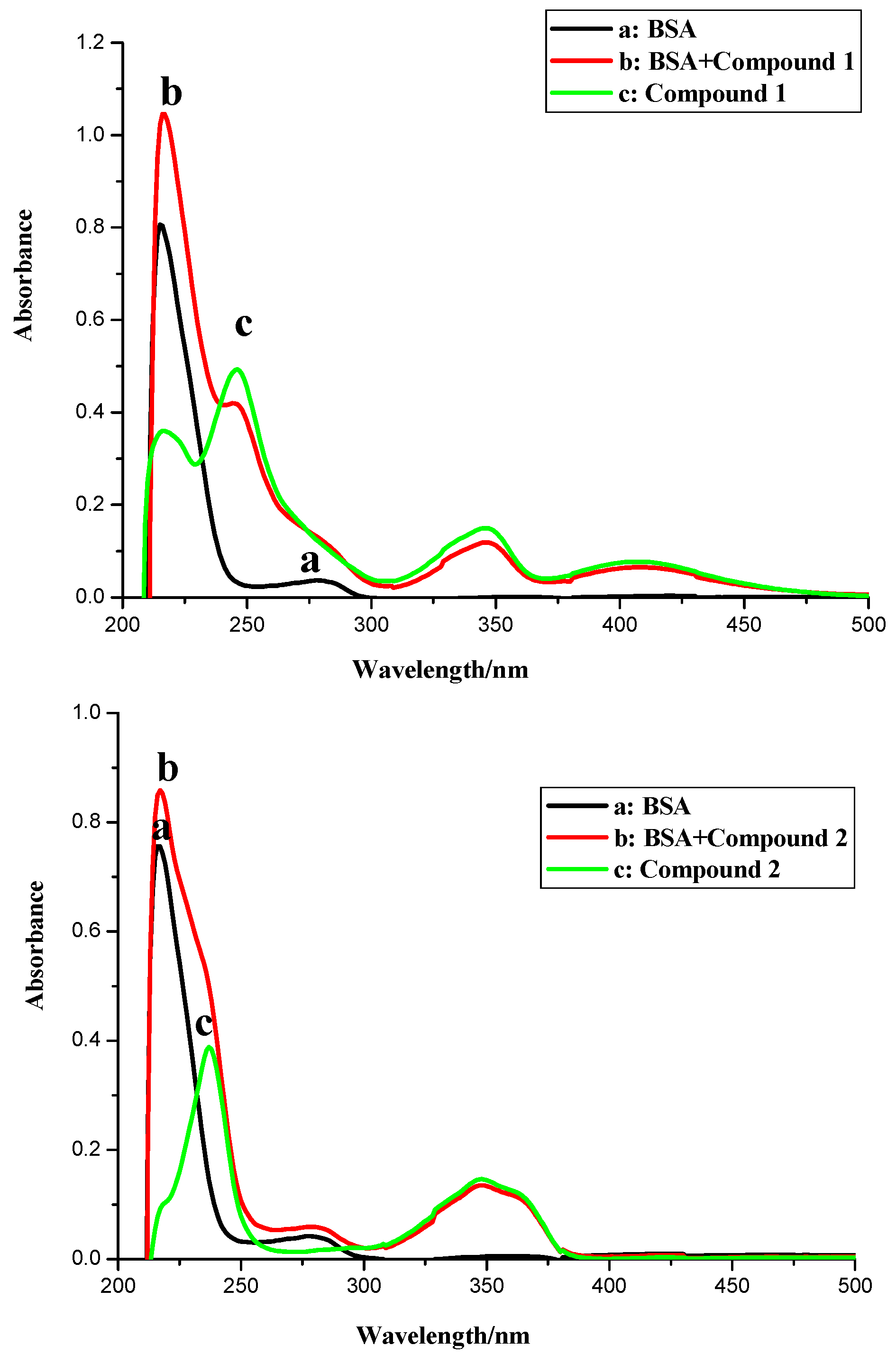
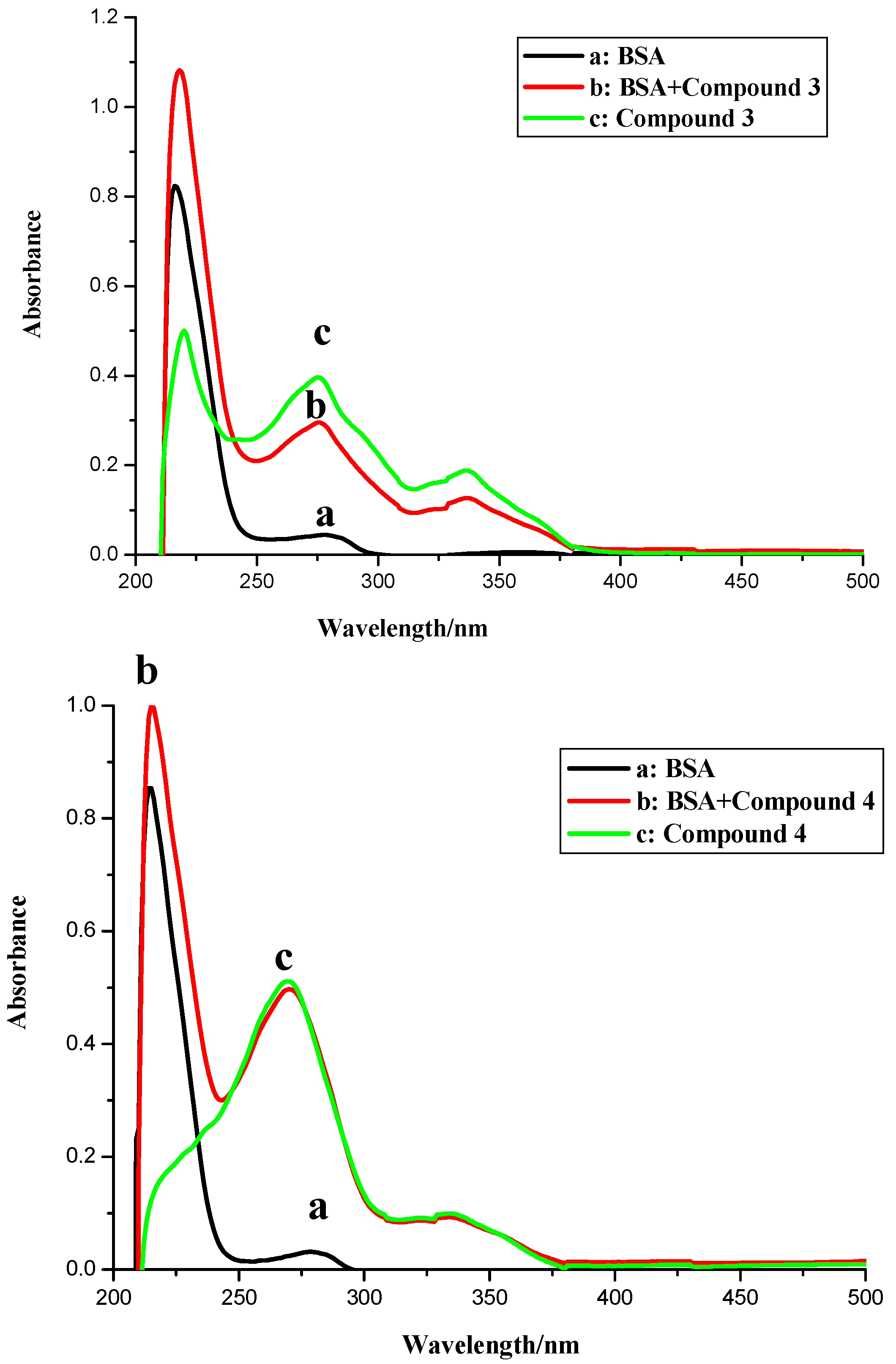
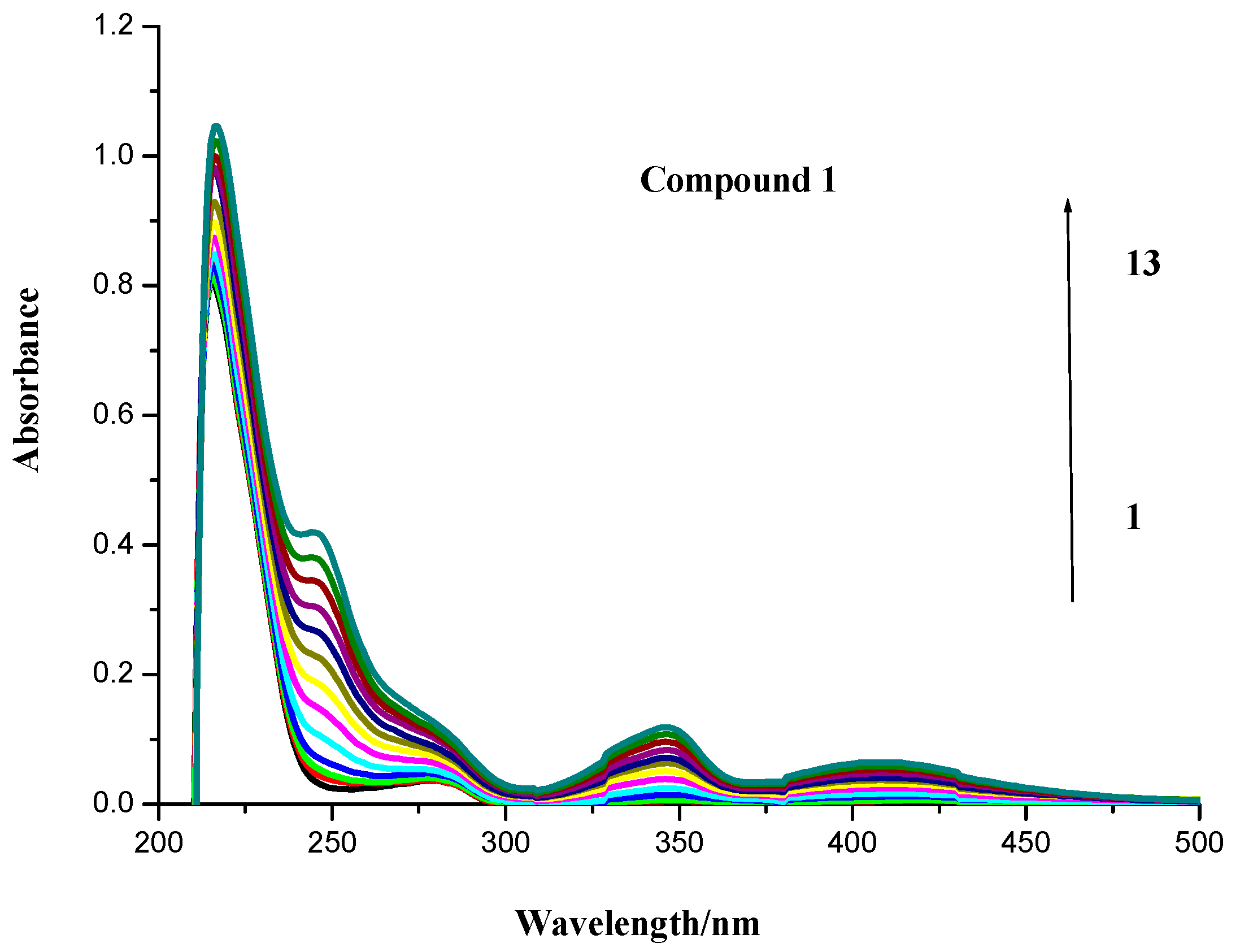
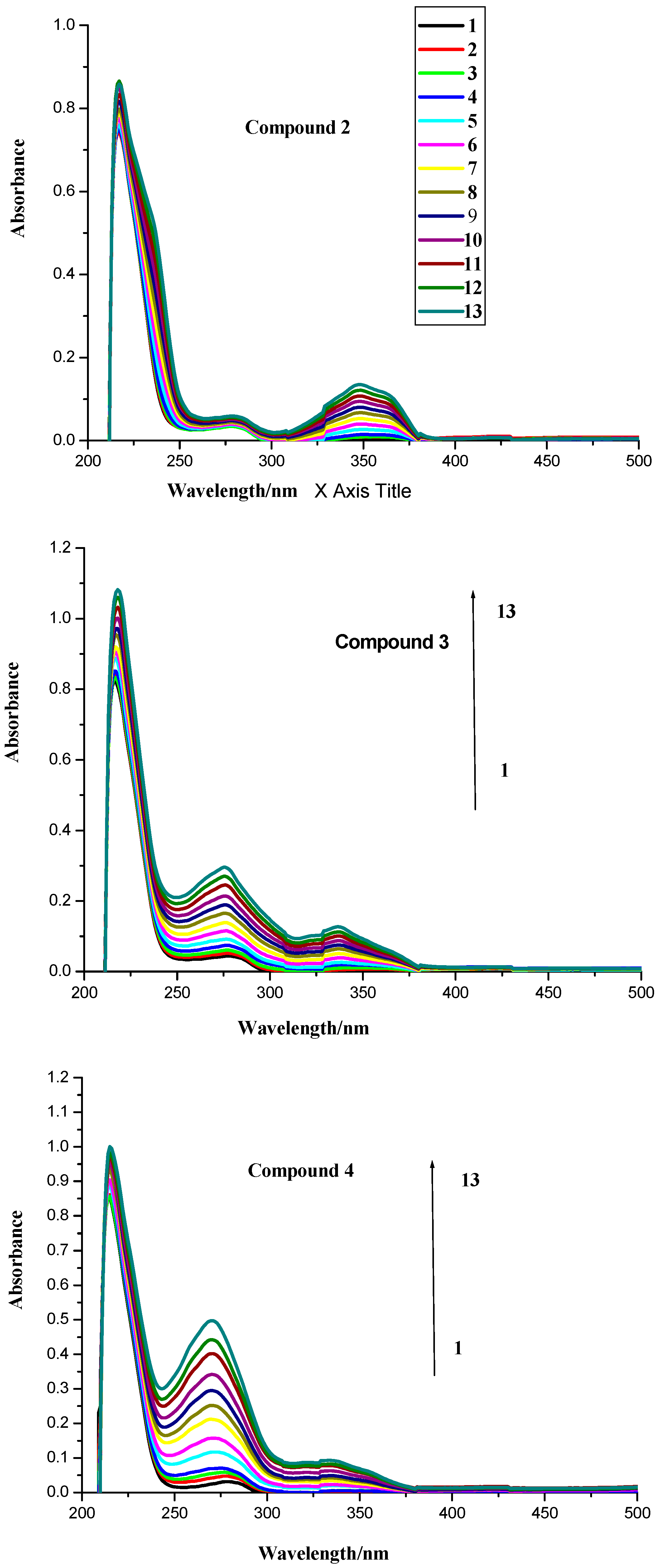
2.2. Fluorescence Spectroscopy
2.2.1. Fluorescence Quenching
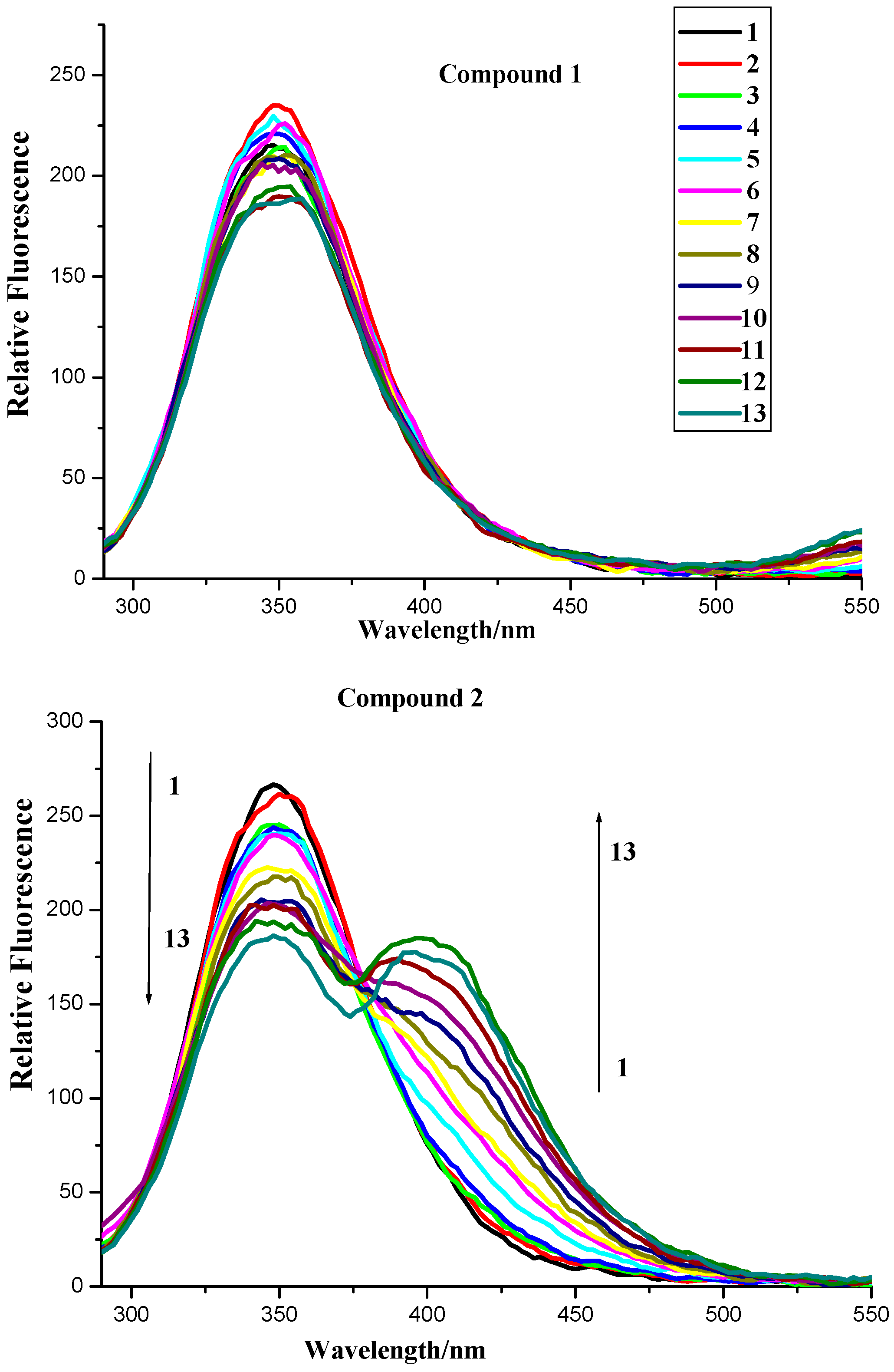




2.2.2. Fluorescence Quenching Mechanism
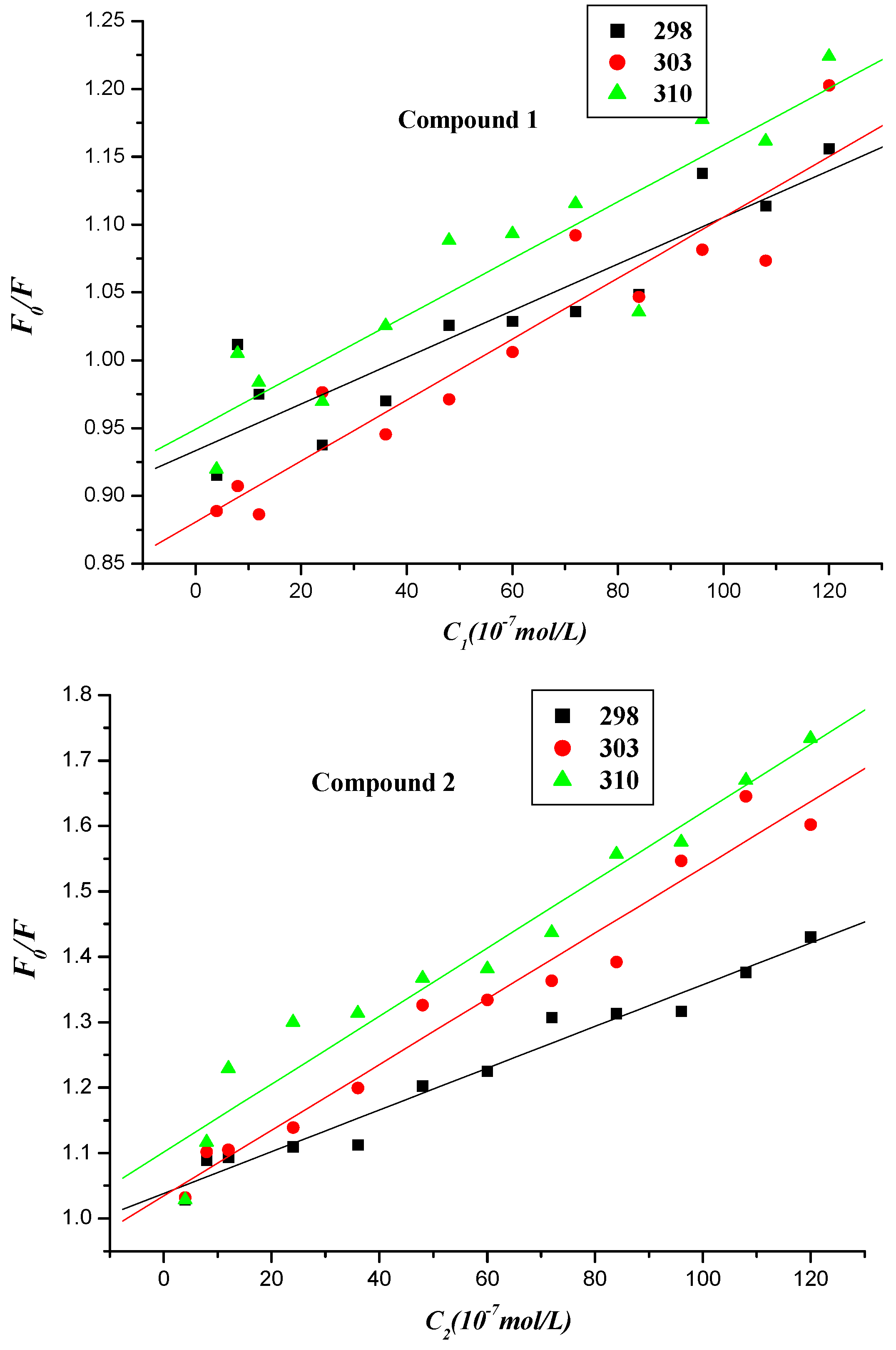
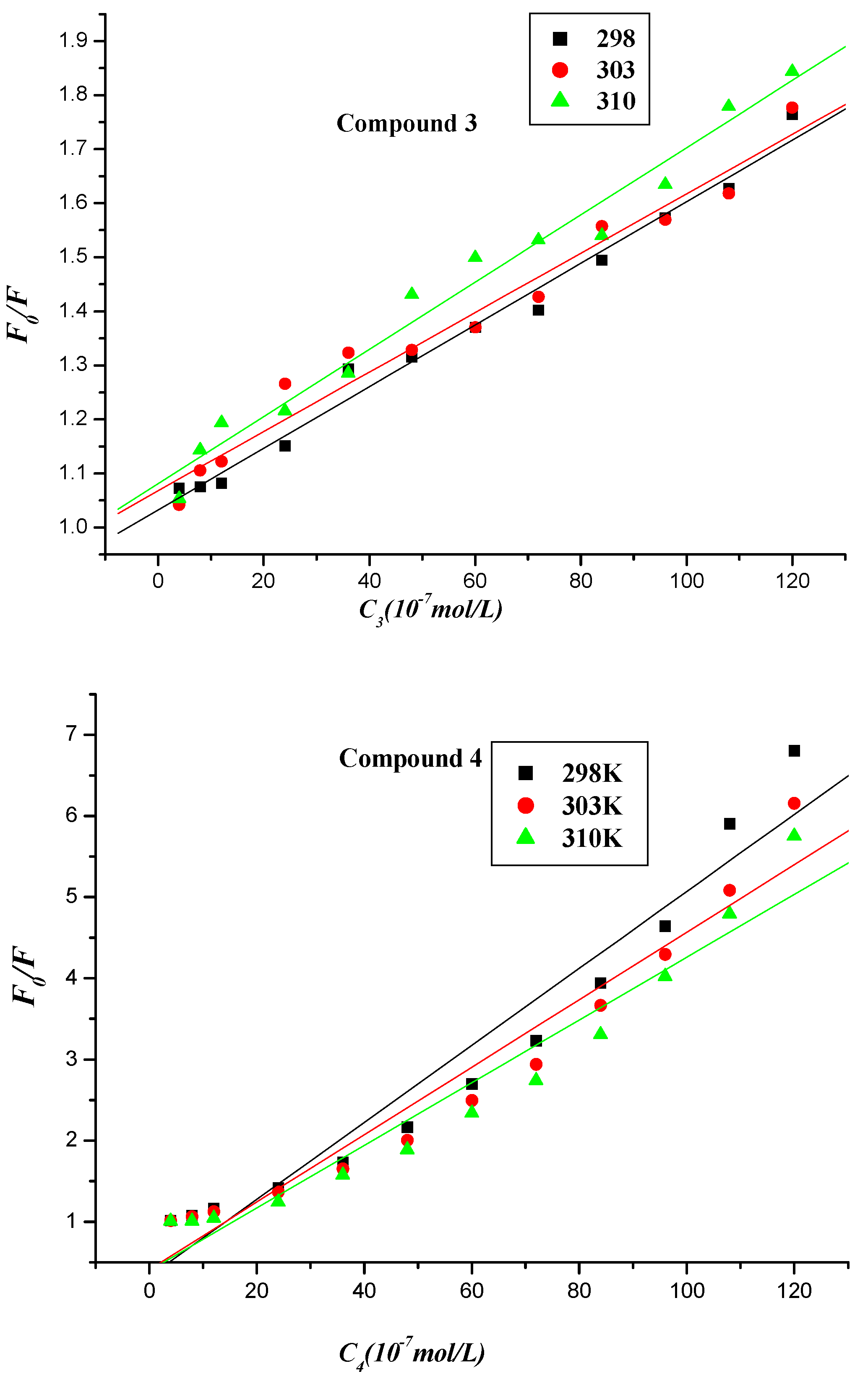
| Compound | T (K) | Ksv (L∙mol−1) | Kq (1012 L∙mol−1) | r |
|---|---|---|---|---|
| 1 | 298 303 310 | (1.720 ± 0.243) × 104 (2.250 ± 0.252) × 104 (2.090 ± 0.295) × 104 | (2.895 ± 0.409) × 1012 | 0.9133 0.9424 0.9137 |
| 2 | 298 303 310 | (3.190 ± 0.179) × 104 (5.020 ± 0.304) × 104 (5.200 ± 0.364) × 104 | (5.369 ± 0.301) × 1012 | 0.9846 0.9822 0.9763 |
| 3 | 298 303 310 | (5.700 ± 0.223) × 104 (5.500 ± 0.318) × 104 (6.220 ± 0.304) × 104 | (9.594 ± 0.375) × 1012 | 0.9924 0.9837 0.9883 |
| 4 | 298 303 310 | (4.745 ± 0.354) × 105 (4.159 ± 0.302) × 105 (3.864 ± 0.293) × 105 | (7.987 ± 0.596) × 1013 | 0.9735 0.9747 0.9724 |
2.2.3. Interaction Mode between Compounds 1–4 and BSA
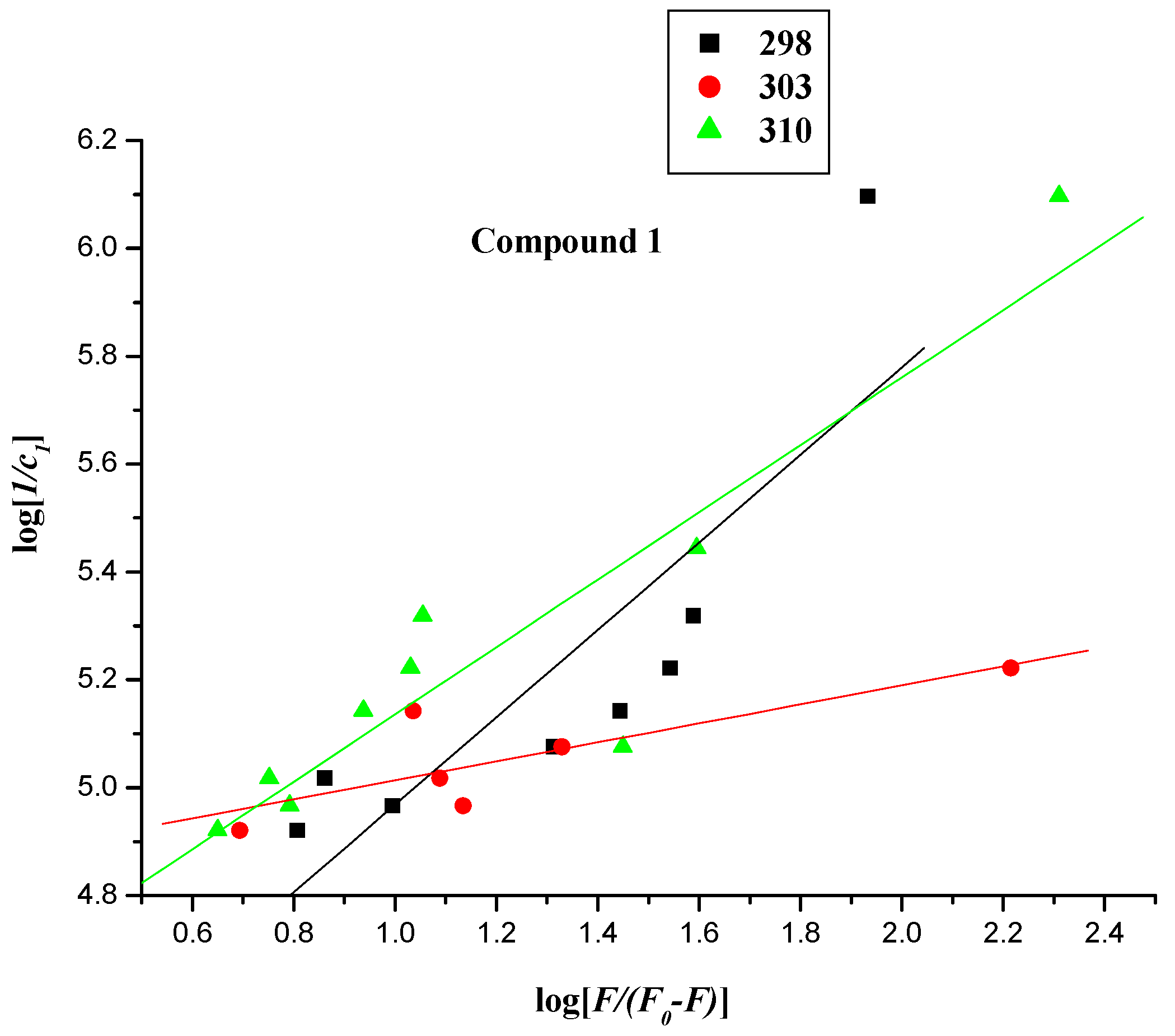
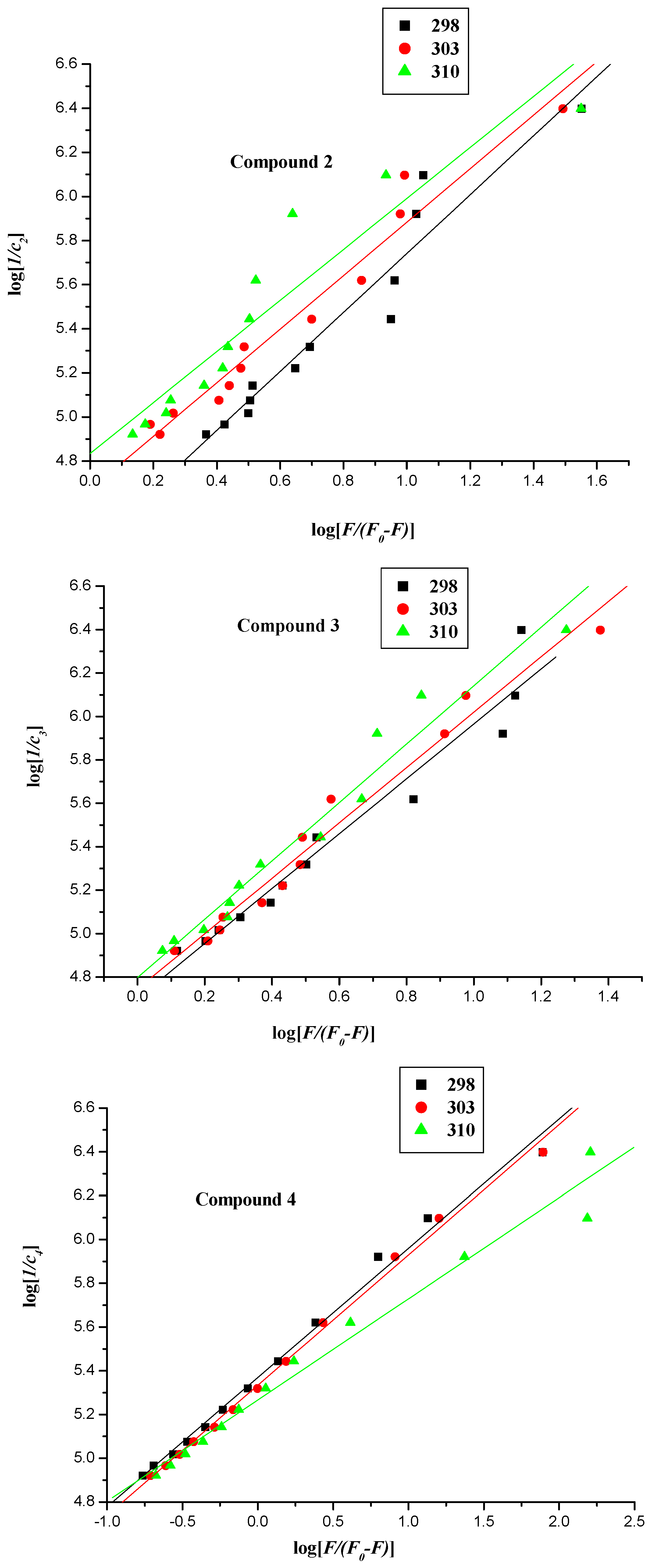
| Compound | T (K) | Kb (L∙mol−1) | ∆G° (L∙mol−1) | ∆H° (kJ∙mol−1) | ∆S° (kJ∙mol−1) | r |
|---|---|---|---|---|---|---|
| 1 | 298 | 1.438 × 104 ± 1.923 | −23.718 | 52.076 | 0.2543 | 0.8464 |
| 303 | 6.879 × 104 ± 1.217 | −28.060 | 52.076 | 0.2543 | 0.8093 | |
| 310 | 3.244 × 104 ± 1.361 | −26.771 | 52.076 | 0.2543 | 0.9137 | |
| 2 | 298 | 2.541 × 104 ± 1.246 | −25.913 | 91.697 | 0.932 | 0.9652 |
| 303 | 4.680 × 104 ± 1.134 | −27.090 | 91.697 | 0.932 | 0.9813 | |
| 310 | 6.823 × 104 ± 1.208 | −28.687 | 91.697 | 0.932 | 0.9435 | |
| 3 | 298 | 5.040 × 104 ± 1.146 | −26.827 | 14.915 | 0.140 | 0.9769 |
| 303 | 5.567 × 104 ± 1.101 | −27.527 | 14.915 | 0.140 | 0.9873 | |
| 310 | 6.281 × 104 ± 1.115 | −28.474 | 14.915 | 0.140 | 0.9820 | |
| 4 | 298 | 2.341 × 105 ± 1.030 | −30.361 | −12.221 | 0.143 | 0.9961 |
| 303 | 2.158 × 105 ± 1.022 | −30.940 | −12.221 | 0.143 | 0.9980 | |
| 310 | 1.847 × 105 ± 1.056 | −31.254 | −12.221 | 0.143 | 0.9884 |
2.2.4. Synchronous Fluorescence Spectroscopy
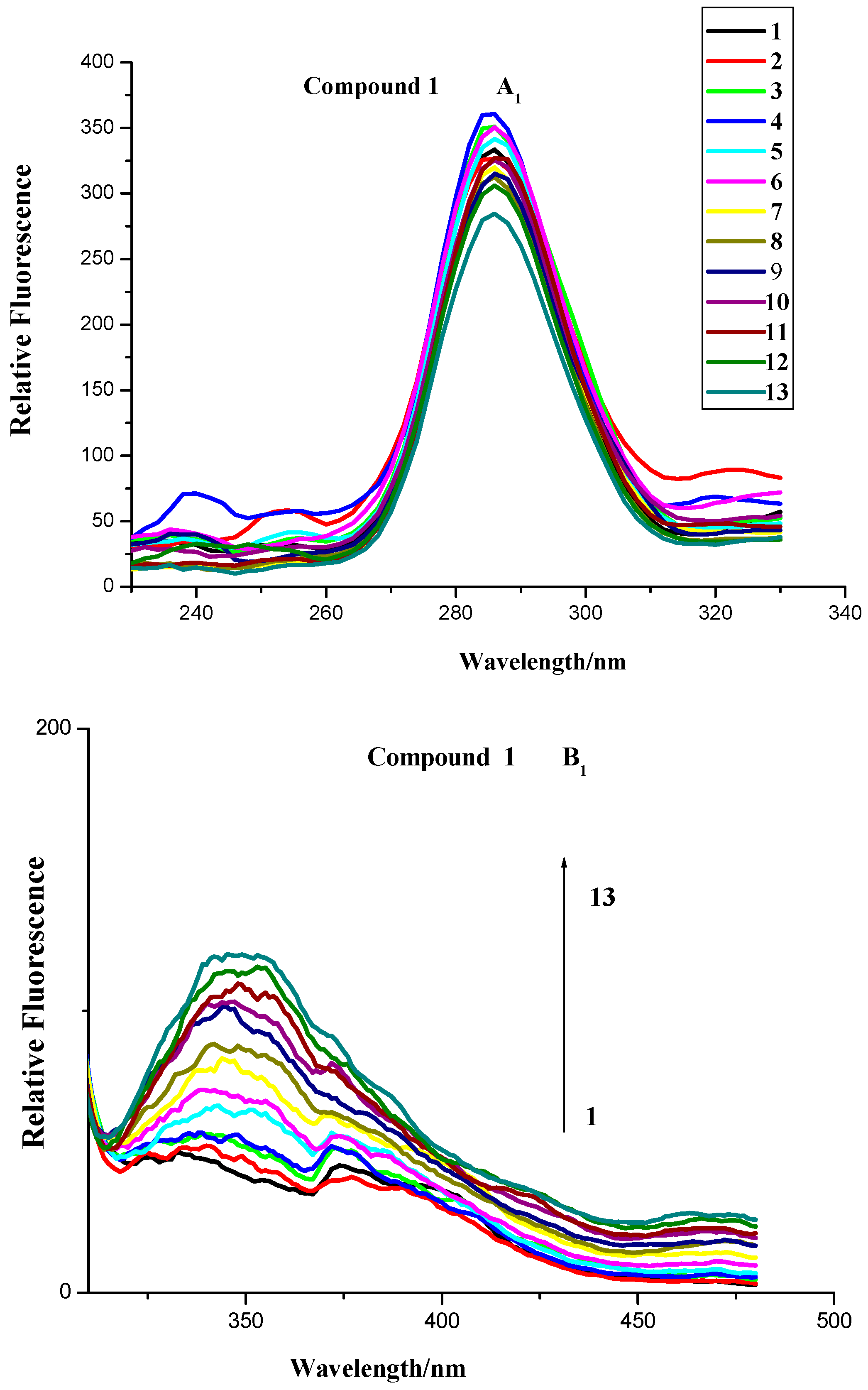


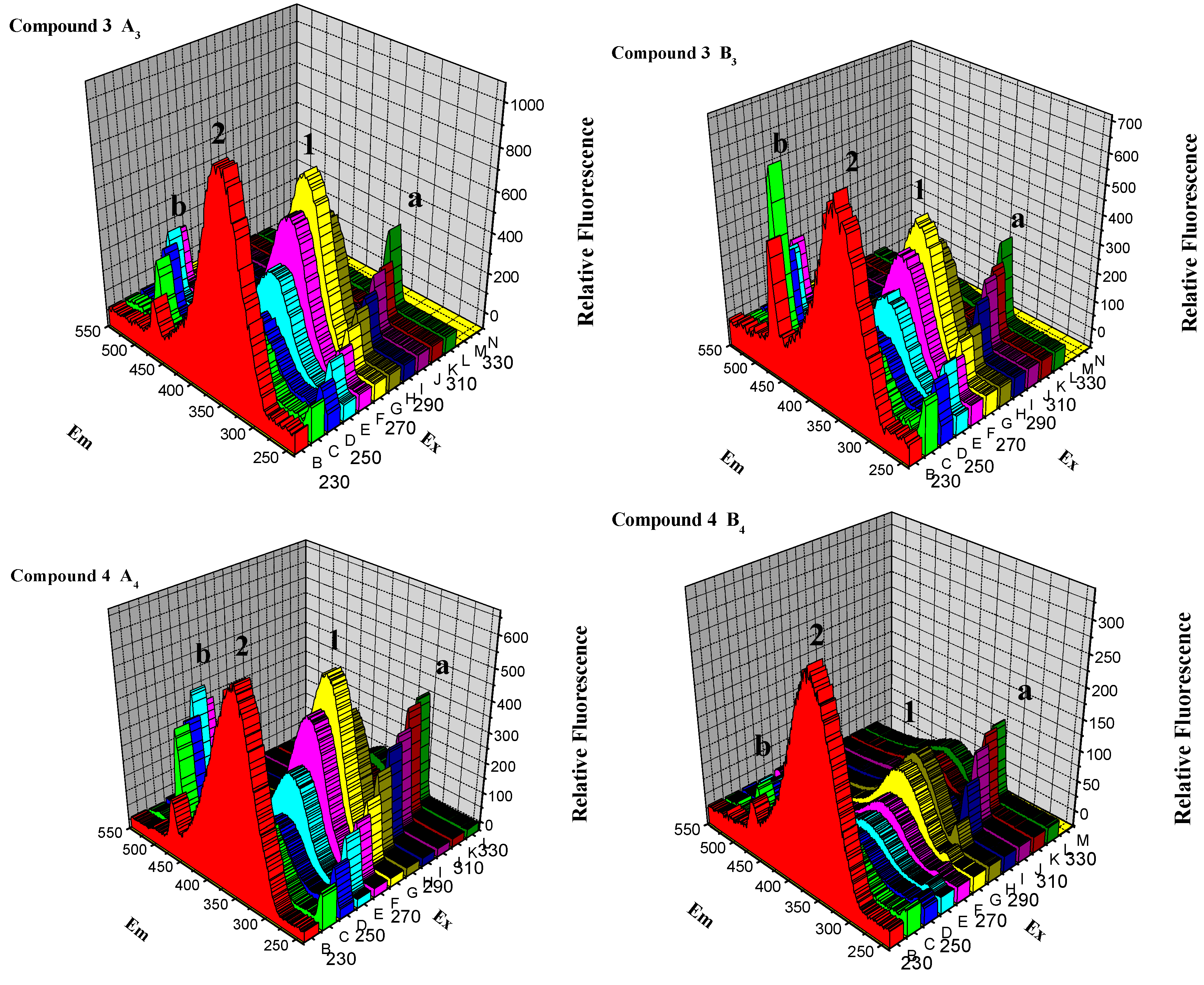
2.2.5. Three-Dimensional Fluorescence Spectroscopy Analysis

2.3. CD Measurement
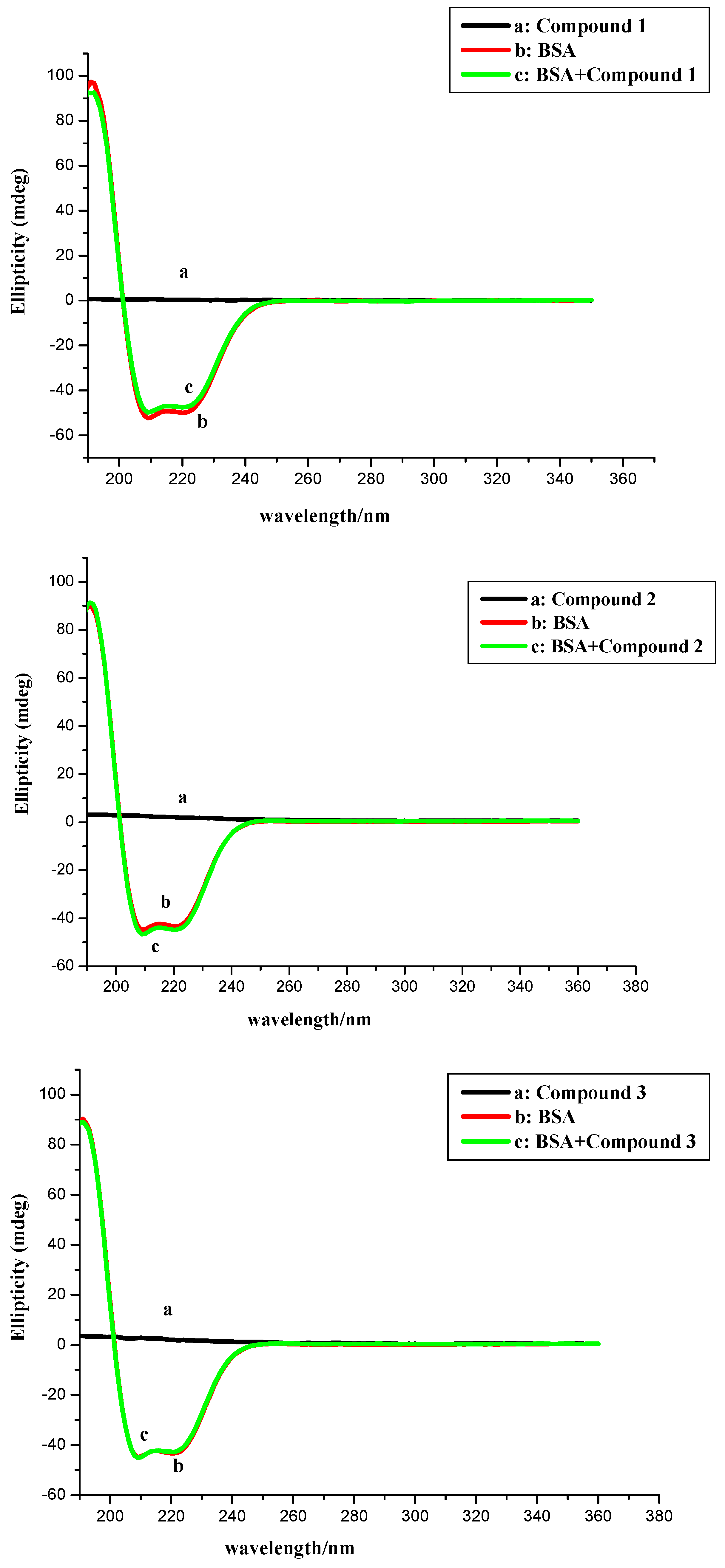
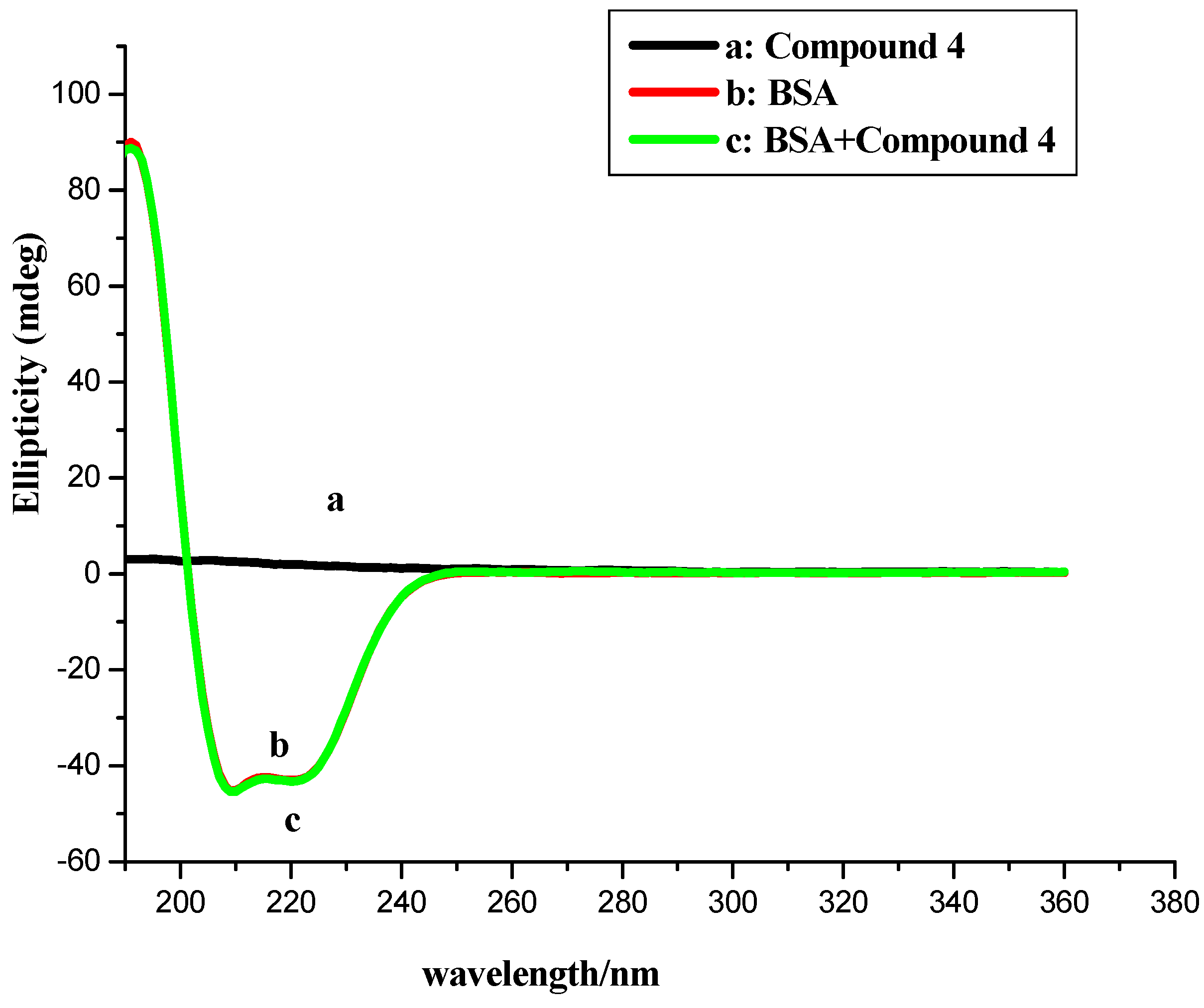
| Compounds 1–4 Concentration (μM) | α-Helix | Antiparallel | Parallel | β-Turn | Random Coil | Total Sum |
|---|---|---|---|---|---|---|
| Free BSA | 65.3% | 3.4% | 3.4% | 12.4% | 16.1% | 100.5% |
| compound 1-BSA | 67.8% | 3.1% | 3.1% | 12.1% | 15.0% | 101.1% |
| compound 2-BSA | 66.8% | 3.2% | 3.2% | 12.2% | 15.4% | 100.9% |
| compound 3-BSA | 61.0% | 3.8% | 3.9% | 13.0% | 17.9% | 99.6% |
| compound 4-BSA | 61.7% | 3.7% | 3.8% | 12.9% | 17.6% | 99.7% |
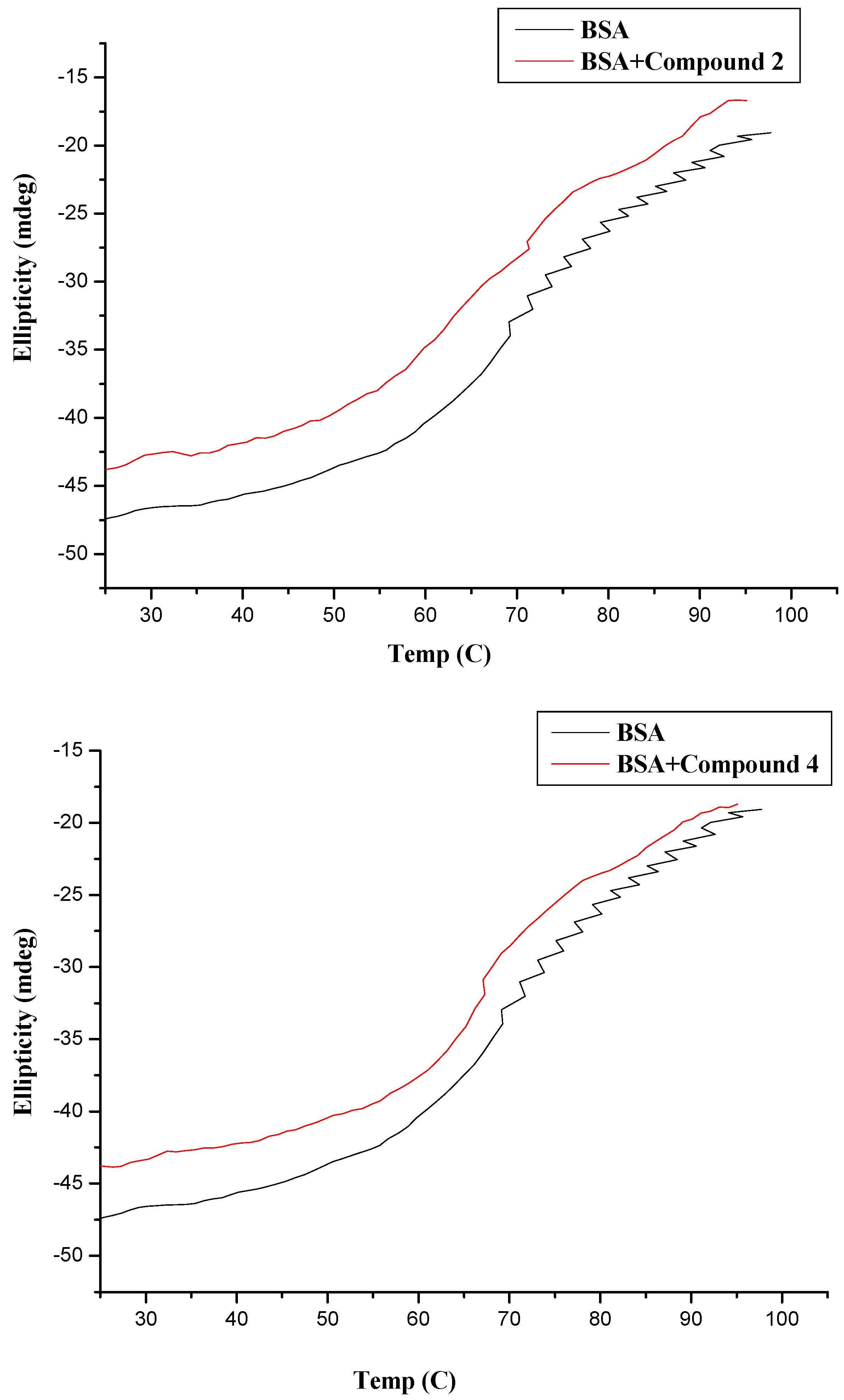
2.4. Docking Analysis
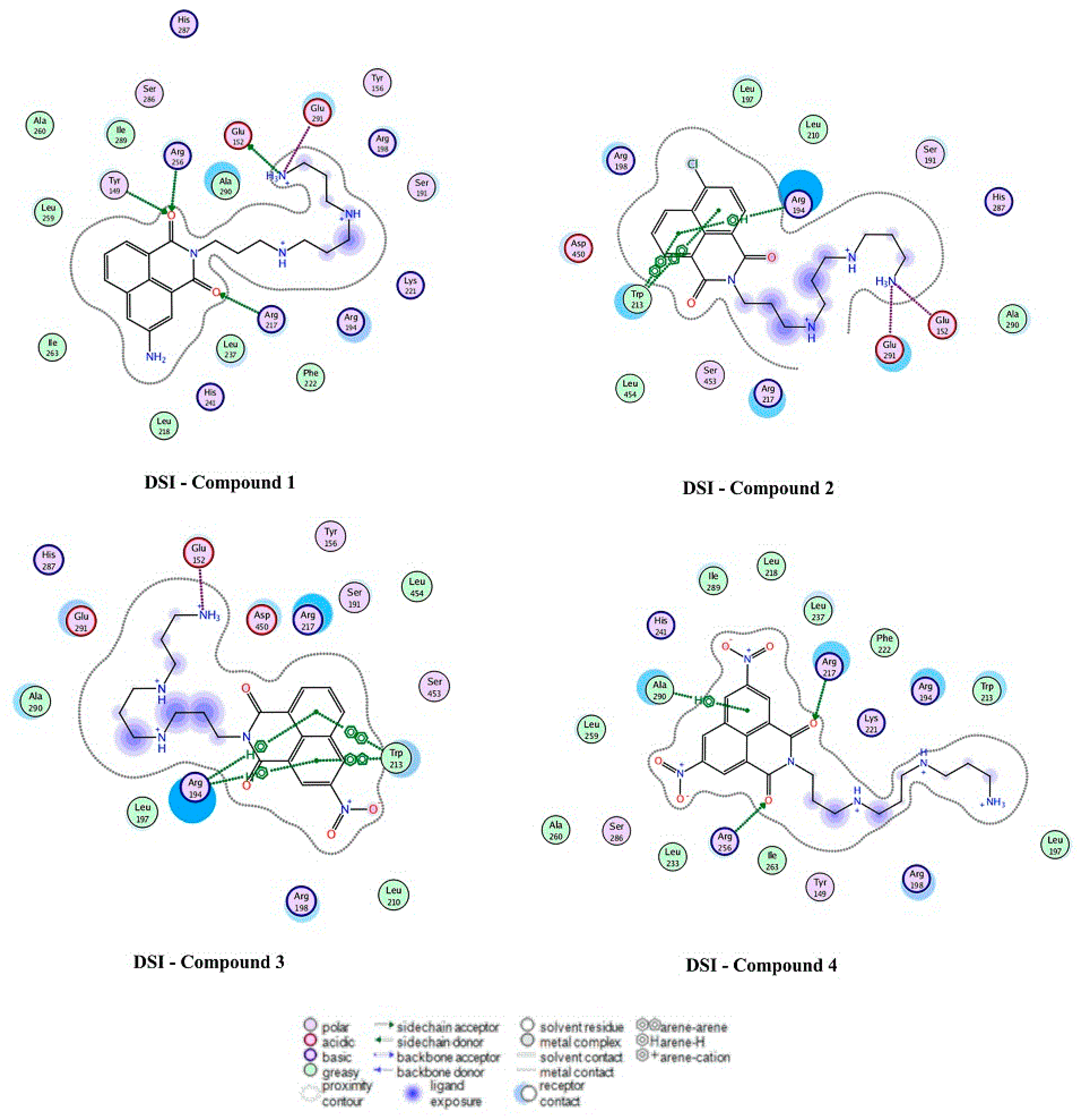

| System | Affinity ∆G Scoring | System | Affinity ∆G Scoring |
|---|---|---|---|
| DSI–Compound 1 | –6.18 | DSII–Compound 1 | –6.28 |
| DSI–Compound 2 | –5.12 | DSII–Compound 2 | –6.45 |
| DSI–Compound 3 | –5.39 | DSII–Compound 3 | –7.41 |
| DSI–Compound 4 | –7.45 | DSII–Compound 4 | –8.49 |
3. Experimental Section
3.1. Apparatus
3.2. Materials
3.3. Procedures
3.3.1. UV-Vis Measurements
3.3.2. Fluorescence Measurement
Interaction of Compounds 1–4 with BSA
Synchronous and Three-Dimensional Fluorescence Spectroscopy
3.3.3. Fluorescence Lifetime Measurement
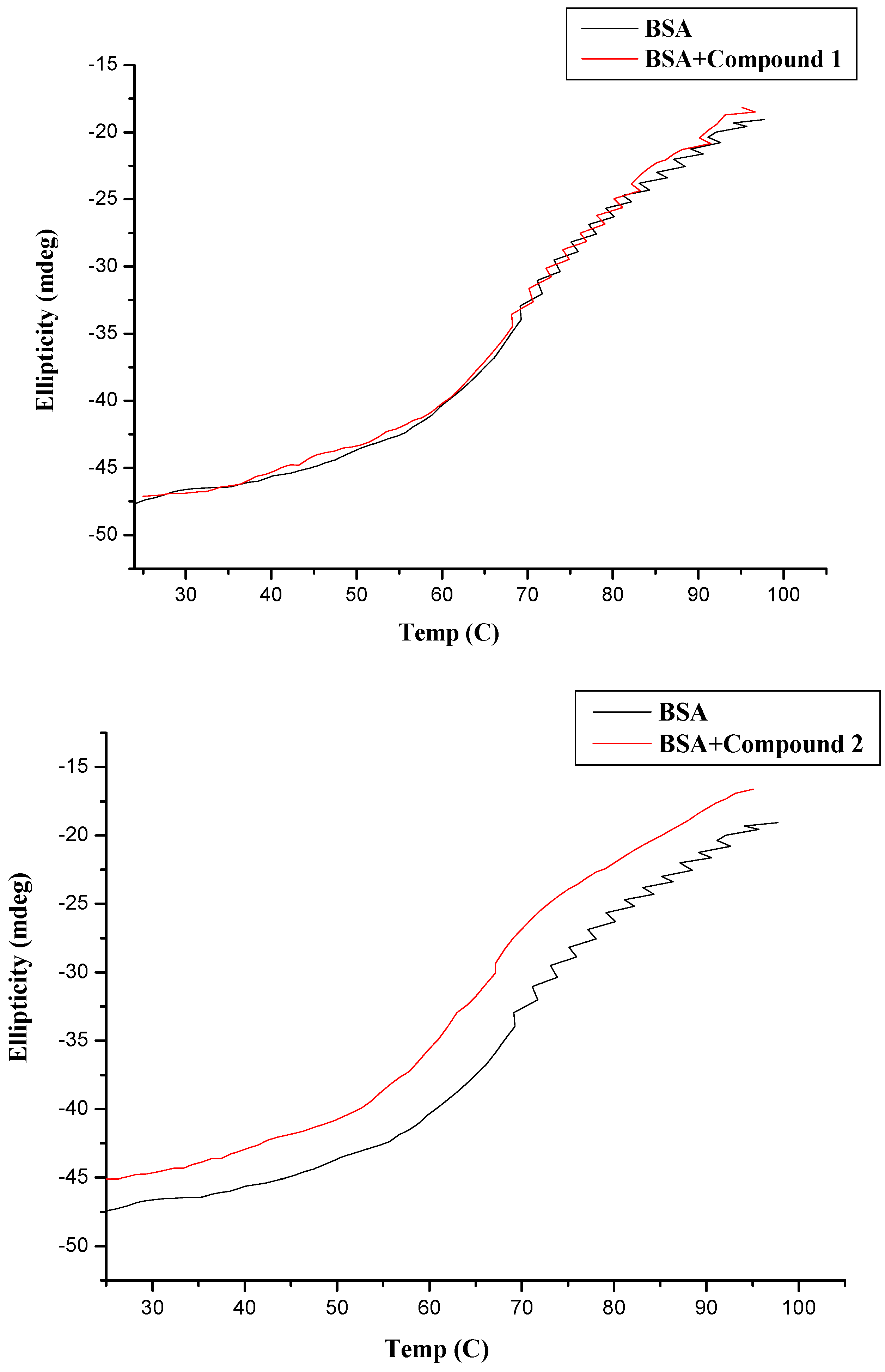
3.3.4. CD Measurement
3.3.5. Molecular Modeling Study
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cui, F.L.; Fan, J.; Li, J.P.; Hu, Z.D. Interactions between 1-benzoyl-4-p-chlorophenyl thiosemicarbazide and serum albumin: Investigation by fluorescence spectroscopy. Bioorg. Med. Chem. 2004, 12, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wei, S.; Yin, C.; Liu, L.S.; Hu, C.M.; Zhao, Y.Y.; Ye, Y.X.; Hu, X.Y.; Fan, J. Synthesis and spectroscopic characterization of 4-butoxyethoxy-N-octadecyl-1,8-naphthalimide as a new fluorescent probe for the determination of proteins. Bioorg. Med. Chem. Lett. 2011, 21, 3798–3804. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.S.; Sun, J.; Hu, Y.Y. Determination of protein by hydroxypropyl-β-cyclodextrin sensitized fluorescence quenching method with erythrosine sodium as a fluorescence probe. Anal. Chim. Acta 2007, 596, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Shrivastaw, K.P.; Singh, S.; Sharma, S.B. Quantitation of protein content by biuret method during production of yellow fever vaccine. Biologicals 1995, 23, 299–300. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wang, X.X.; Shen, H.X.; Hao, Y.M. Resonance light scattering of protein-amaranth system and its analytical application. Chin. J. Anal. Chem. 2000, 28, 1388–1390. [Google Scholar]
- Guo, C.Y.; Wu, X.; Yang, J.H.; Wang, F.; Jia, Z.; Ran, D.H.; Zheng, J.H. Determination of proteins using fluorescence enhancement of Tb3+-benzoyl-acetone-sodium dodecyl benzene sulfonate-protein system. J. Photochem. Photobiol. A 2006, 181, 50–51. [Google Scholar] [CrossRef]
- Flores, R. A rapidand reproducible for quantitative estimation Bromphenol Blue. Anal. Biochem. 1978, 88, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Braña, M.F.; Ramos, A. Naphthalimides as anticancer agents: Synthesis and biological activity. Curr. Med. Chem. Anti-Cancer Agents 2001, 1, 237–255. [Google Scholar] [CrossRef] [PubMed]
- Braña, M.F.; Cacho, M.; Gradillas, A.; de Pascual-Teresa, B.; Ramos, A. Intercalators as anticancer drugs. Curr. Pharm. Des. 2001, 7, 1745–1780. [Google Scholar] [CrossRef] [PubMed]
- Beauchemin, R.; N’soukpoe-Kossi, C.N.; Thomas, T.; Thomas, T.J.; Carpentier, R.; Tajmir-Riahi, H.A. Polyamine analogues bind human serum albumin. Biomacromolecules 2007, 8, 3177–3183. [Google Scholar] [CrossRef] [PubMed]
- Ouameur, A.A.; Mangier, E.; Diamantoglou, S.; Rouillon, R.; Carpentier, R.; Tajmir-Riahi, H.A. Effects of organic and inorganic polyamine cations on the structure of human serum albumin. Biopolymers 2004, 73, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Essemine, J.; Hasni, I.; Carpentier, R.; Thomas, T.J.; Tajmir-Riahi, H.A. Binding of biogenic and synthetic polyamines to β-lactoglobulin. Int. J. Biol. Macromol. 2011, 49, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Dubeau, S.; Bourassa, P.; Thomas, T.J.; Tajmir-Riahi, H.A. Biogenic and synthetic polyamines bind bovine serum albumin. Biomacromolecules 2010, 11, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.H.; Zhao, J.; Zhu, Y.Q.; Tian, Z.Y.; Wang, C.J. Reactive oxygen species (ROS) accumulation induced by mononaphthalimide-spermidine leads to intrinsic and AIF-mediated apoptosis in HeLa cells. Oncol. Rep. 2011, 25, 1099–1107. [Google Scholar] [PubMed]
- Tian, Z.Y.; Su, L.P.; Xie, S.Q.; Zhao, J.; Wang, C.J. Synthesis, biological activitity and fluorescence spectroscopy of naphthalimide-polyamine conjugates. Chin. J. Org. Chem. 2013, 33, 1514–1521. [Google Scholar] [CrossRef]
- Tian, Z.Y.; Li, J.H.; Li, Q.; Zang, F.L.; Zhao, Z.H.; Wang, C.J. Study on the Synthesis, biological activity and spectroscopy of naphthalimide-diamine conjugates. Molecules 2014, 19, 7646–7668. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.Y.; Xie, S.Q.; Du, Y.W.; Ma, Y.F.; Zhao, J.; Gao, W.Y.; Wang, C.J. Synthesis, cytotoxicity and apoptosis of naphthalimide polyamine conjugates as antitumor agents. Eur. J. Med. Chem. 2009, 44, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.Y.; Zhao, Z.H.; Zang, F.L.; Wang, Y.Q.; Wang, C.J. Spectroscopic study on the interaction between naphthalimide-polyamine conjugates and DNA. J. Photochem. Photobiol. B 2014, 138, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.Y.; Zang, F.L.; Luo, W.; Zhao, Z.H.; Wang, Y.Q.; Xu, X.J.; Wang, C.J. Spectroscopic study on the interaction between mononaphthalimide spermidine (MINS) and bovine serum albumin (BSA). J. Photochem. Photobiol. B 2015, 142, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, Z.; Liang, X.H.; Fan, J.; Han, Q. Study on photophysical and aggregation induced emission recognition of 1,8-naphthalimide probe for casein by spectroscopic method. Spectrochim. Acta A 2013, 108, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.S.; Kumbhar, A.A.; Heptullah, H.; Khan, A.A.; Gobre, V.V.; Gejji, S.P.; Puranik, V.G. Synthesis, electronic structure, DNA and protein binding, DNA cleavage, and anticancer activity of fluorophore-labeled copper(II) complexes. Inorg. Chem. 2011, 50, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.J.; Liu, Y.R.; Zhao, M.; Dong, J.X.; Qu, S.S. Spectroscopic studies on the interaction between methylene blue and bovine serum albumin. J. Photochem. Photobiol. A 2006, 179, 324–329. [Google Scholar] [CrossRef]
- Gelamo, E.L.; Silva, C.H.; Imasato, H.; Tabak, M. Interaction of bovine (BSA) and human (HSA) serum albumins with ionic surfactants: Spectroscopy and modeling. Biochim. Biophys. Acta 2002, 1594, 84–89. [Google Scholar] [CrossRef]
- De, S.; Girigoswami, A.; Das, S. Fluorescence probing of albumin–surfactant interaction. J. Coll. Int. Sci. 2005, 285, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Matei, I.; Ionescu, S.; Hillebrand, M. Interaction of fisetin with human serum albumin by fluorescence, circular dichroism spectroscopy and DFT calculations: Binding parameters and conformational changes. J. Lumin. 2011, 131, 1629–1635. [Google Scholar] [CrossRef]
- Hu, Y.J.; Liu, Y.; Zhang, L.X.; Zhao, R.M.; Qu, S.S. Study of interaction between colchicines and bovine serum albumin by fluorescence quenching method. J. Mol. Struct. 2005, 750, 174–178. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, L.N.; Zeng, B.R.; Kang, Q.L.; Dai, L.Z. Study on the binding of chloroamphenicol with bovine serum albumin by fluorescence and UV-vis spectroscopy. Spectrochim. Acta A 2010, 77, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.J.; Chen, Q.Y. The interaction between ionic liquids modified magnetic nanoparticles and bovine serum albumin and the cytotoxicity to HepG-2 cells. Spectrochim. Acta A 2014, 120, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, Q.W.; Wen, Y.Q. Spectroscopic studies on the interaction between nicotinamide and bovine serum albumin. Spectrochim. Acta A 2008, 71, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Lan, J.F.; Guan, M.; Sheng, F.L.; Zhang, H.X. Spectroscopic investigation of interaction between mangiferin and bovine serum albumin. Spectrochim. Acta A 2009, 73, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Nanda, R.K.; Sarkar, N.; Banerjee, R. Probing the interaction of ellagic acid with human serum albumin: A fluorescence spectroscopic study. J. Photochem. Photobiol. A 2007, 192, 152–158. [Google Scholar] [CrossRef]
- Memarpoor-Yazdi, M.; Mahaki, H. Probing the interaction of human serum albumin with vitamin B2 (riboflavin) and L-Arginine (L-Arg) using multi-spectroscopic, molecular modeling and zeta potential techniques. J. Lumin. 2013, 136, 150–159. [Google Scholar] [CrossRef]
- Sirajuddin, M.; Ali, S.; Badshah, A. Drug-DNA interactions and their study by UV-Visible, fluorescence spectroscopies and cyclic voltametry. J. Photochem. Photobiol. B 2013, 124, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Li, N.Q.; Jiang, Y.Y. Electrochemical studies of porphyrin interacting with DNA and determination of DNA. Anal. Chim. Acta 1997, 344, 97–104. [Google Scholar] [CrossRef]
- Hu, Y.J.; Liu, Y.; Xiao, X.H. Investigation of the interaction between Berberine and human serum albumin. Biomacromolecules 2009, 10, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.D.; Subramanian, S. Thermodynamics of protein association reactions: Forces contributing to stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Zhao, G.J.; Li, G.Y.; Han, K.L. Fluorescence quenching phenomena facilitated by excited-state hydrogen bond strengthening for fluorenone derivatives in alcohols. J. Photochem. Photobiol. A 2010, 209, 181–184. [Google Scholar] [CrossRef]
- Horne, D.S. Casein micelle structure and stability. In Milk Proteins-From Expression to Food; Thompson, A., Boland, M., Singh, H., Eds.; Academic Press: New York, NY, USA, 2008; pp. 133–162. [Google Scholar]
- Zhang, Y.Z.; Chen, X.X.; Dai, J.; Zhang, X.P.; Liu, Y.X.; Liu, Y. Spectroscopic studies on the interaction of lanthanum(III) 2-oxo-propionic acid salicyloyl hydrazone complex with bovine serum albumin. Luminescence 2008, 23, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Xiang, X.; Mei, P.; Dai, J.; Zhang, L.L.; Liu, Y. Spectroscopic studies on the interaction of congo red with bovine serum albumin. Spectrochim. Acta A 2009, 72, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.L.; Wu, F.Y.; Liu, A.H. Study of the interaction between 2,5-di-[2-(4-hydroxy-phenyl)ethylene]-terephthalonitril and bovine serum albumin by fluorescence spectroscopy. Spectrochim. Acta A 2011, 79, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Tang, B.P.; Zhang, H.M.; Zhou, Q.H.; Zhang, G.C. Studies on the interaction between imidacloprid and human serum albumin: Spectroscopic approach. J. Photochem. Photobiol. B 2009, 94, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Sandhya, B.; Hegde, A.H.; Kalanur, S.S.; Katrahalli, U.; Seetharamap, J. Interaction of triprolidine hydrochloride with serum albumins: Thermodynamic and binding characteristics, and influence of site probes. J. Pharm. Biomed. Anal. 2011, 54, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Muller-Renaud, S.; Dupont, D.; Dulieu, P. Development of a biosensor immunoassay for the quantification of alphas1-casein in milk. J. Dairy. Res. 2005, 72, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.M.; Yue, Y.Y.; Yang, Q.; Yan, N.; Chen, X.G. Complexes between C.I. Acid Orange 6 and human serum albumin, a multi-spectroscopic approach to investigate the binding behavior. J. Lumin. 2011, 131, 1222–1228. [Google Scholar] [CrossRef]
- Mahaki, H.; Memarpoor-Yazdi, M.; Chamani, J.; Saberi, M.R. Interaction between ropinirole hydrochloride and aspirin with human serum albumin as binary and ternary systems by multi-spectroscopic, molecular modeling and zeta potential. J. Lumin. 2013, 134, 758–771. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Ran, X.; Fang, M.; Shi, J.; Qin, H.; Goh, J.M.; Song, J. Resurrecting abandoned proteins with pure water: CD and NMR studies of protein fragments solubilized in salt-free water. Biophys. J. 2006, 91, 4201–4209. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Liu, R. Phenotypic characterization of the binding of tetracycline to human serum albumin. Biomacromolecules 2011, 12, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, P.F.J.; Bonvin, A.M.J.J.; Bochicchio, B.; Pepe, A.; Alix, A.J.P.; Tamburro, A.M. Kinetics and thermodynamics of type VIII beta-turn formation: A CD, NMR, and microsecond explicit molecular dynamics study of the GDNP tetrapeptide. Biophys. J. 2006, 90, 2745–2759. [Google Scholar] [CrossRef] [PubMed]
- Ojha, H.; Mishra, K.; Hassan, M.I.; Chaudhury, N.K. Spectroscopic and isothermal titration calorimetry studies of binding interaction of ferulic acid with bovine serum albumin. Thermochim. Acta 2012, 548, 56–64. [Google Scholar] [CrossRef]
- Zhang, G.W.; Zhao, N.; Wang, L. Fluorescence spectrometric studies on the binding of puerarin to human serum albumin using warfarin, ibuprofen and digitoxin as site markers with the aid of chemometrics. J. Lumin. 2011, 131, 2716–2724. [Google Scholar] [CrossRef]
- Ojha, H.; Murari, B.M.; Anand, S.; Hassan, M.I.; Ahmad, F.; Chaudhury, N.K. Interaction of DNA minor groove binder Hoechst 33258 with bovine serum albumin. Chem. Pharm. Bull. 2009, 57, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Brandl, M.; Weiss, M.S.; Jabs, A.; Sühnel, J.; Hilgenfeld, R. C-H⋯π-interactions in proteins. J. Mol. Biol. 2001, 307, 357–377. [Google Scholar] [CrossRef] [PubMed]
- Otosu, T.; Nishimoto, E.; Yamashita, S. Multiple conformational state of human serum albuminaround single tryptophan residue at various pH revealed by time-resolved fluorescence spectroscopy. J. Biochem. 2010, 147, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Paliwal, S.; Sharma, M.; Singh, A.; Sharma, S.; Yadav, D. Pharmacophore based virtual screening, molecular docking and biological evaluation to identify novel PDE5 inhibitors with vasodilatory activity. Bioorg. Med. Chem. Lett. 2014, 24, 3137–3141. [Google Scholar] [CrossRef] [PubMed]
- The PyMOL Molecular Graphics System; DeLano Scientific: San Carlos, CA, USA, 2002.
- Sekula, B.; Zielinski, K.; Bujacz, A. Crystallographic studies of the complexes of bovine and equine serum albumin with 3,5-diiodosalicylic acid. Int. J. Biol. Macromol. 2013, 60, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Cornell, W.D.; Cieplak, P.; Bayly, C.I.; Gould, I.R.; Merz, K.M.; Ferguson, D.M.; Spellmeyer, D.C.; Fox, T.; Caldwell, J.W.; Kollman, P.A. A second generation force field for the simulation of proteins, nucleic acids and organic molecules. J. Am. Chem. Soc. 1995, 117, 5179–5197. [Google Scholar] [CrossRef]
- Hornak, V.; Abel, R.; Okur, A.; Strockbine, B.; Roitberg, A. Simmerling, C. Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins 2006, 65, 712–725. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Cieplak, P.; Kollman, P.A. How Well Does a Restrained Electrostatic Potential (RESP) Model perform in calcluating conformational energies of organic and biological molecules? J. Comput. Chem. 2000, 21, 1049–1074. [Google Scholar] [CrossRef]
- Halgren, T.A. Merck molecular force field: I–V. J. Comput. Chem. 1996, 17, 490–641. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Z.-Y.; Song, L.-N.; Zhao, Y.; Zang, F.-L.; Zhao, Z.-H.; Chen, N.-H.; Xu, X.-J.; Wang, C.-J. Spectroscopic Study on the Interaction between Naphthalimide-Polyamine Conjugates and Bovine Serum Albumin (BSA). Molecules 2015, 20, 16491-16523. https://doi.org/10.3390/molecules200916491
Tian Z-Y, Song L-N, Zhao Y, Zang F-L, Zhao Z-H, Chen N-H, Xu X-J, Wang C-J. Spectroscopic Study on the Interaction between Naphthalimide-Polyamine Conjugates and Bovine Serum Albumin (BSA). Molecules. 2015; 20(9):16491-16523. https://doi.org/10.3390/molecules200916491
Chicago/Turabian StyleTian, Zhi-Yong, Li-Na Song, Yuan Zhao, Feng-Lei Zang, Zhong-Hua Zhao, Nan-Hao Chen, Xue-Jun Xu, and Chao-Jie Wang. 2015. "Spectroscopic Study on the Interaction between Naphthalimide-Polyamine Conjugates and Bovine Serum Albumin (BSA)" Molecules 20, no. 9: 16491-16523. https://doi.org/10.3390/molecules200916491
APA StyleTian, Z.-Y., Song, L.-N., Zhao, Y., Zang, F.-L., Zhao, Z.-H., Chen, N.-H., Xu, X.-J., & Wang, C.-J. (2015). Spectroscopic Study on the Interaction between Naphthalimide-Polyamine Conjugates and Bovine Serum Albumin (BSA). Molecules, 20(9), 16491-16523. https://doi.org/10.3390/molecules200916491