Enzymatic Transesterification of Kraft Lignin with Long Acyl Chains in Ionic Liquids
Abstract
:1. Introduction
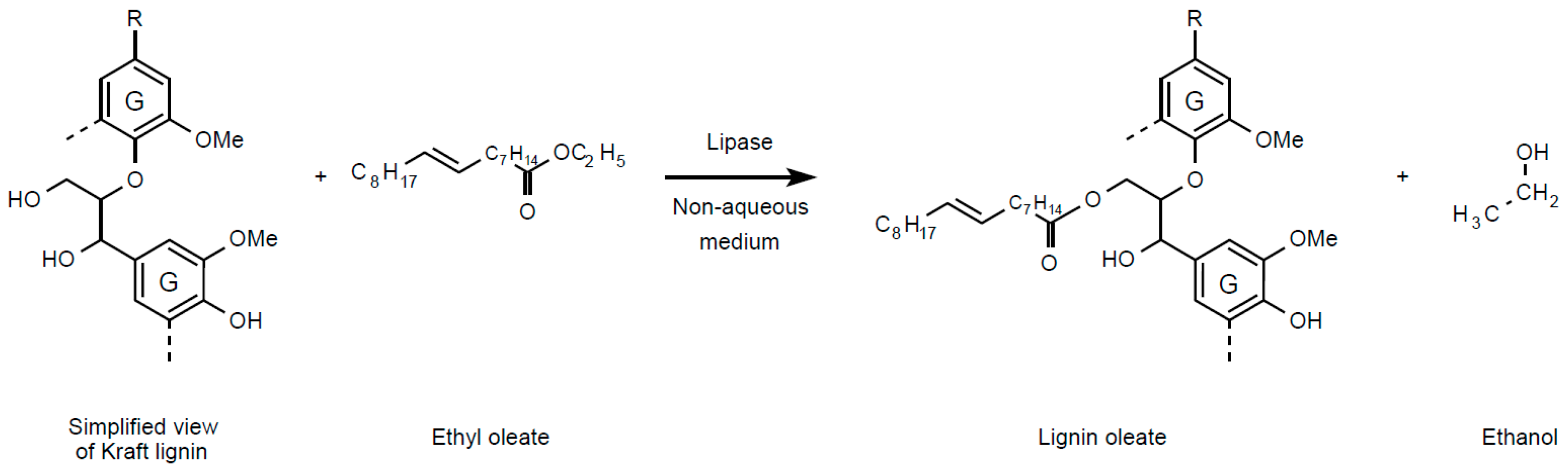
2. Results and Discussion
2.1. Lignin Solubility Study
| Solvents | Solubility of Lignin % (w/v) | Kamlet-Taft β Parameter Value Reference | |
|---|---|---|---|
| Dioxane | 6.7 ± 0.7 | 0.37 | [46] |
| [C4C1im][MeSO4] | 29.3 ± 1.3 | 0.60 | [43] |
| [C4C1im][OTf] | 14.7 ± 0.6 | 0.50 | [44] |
| [C4C1im][PF6] | 0.03 ± 0.01 | 0.21 | [42] |
2.2. Enzymatic Transesterification in Single ILs or Dioxane
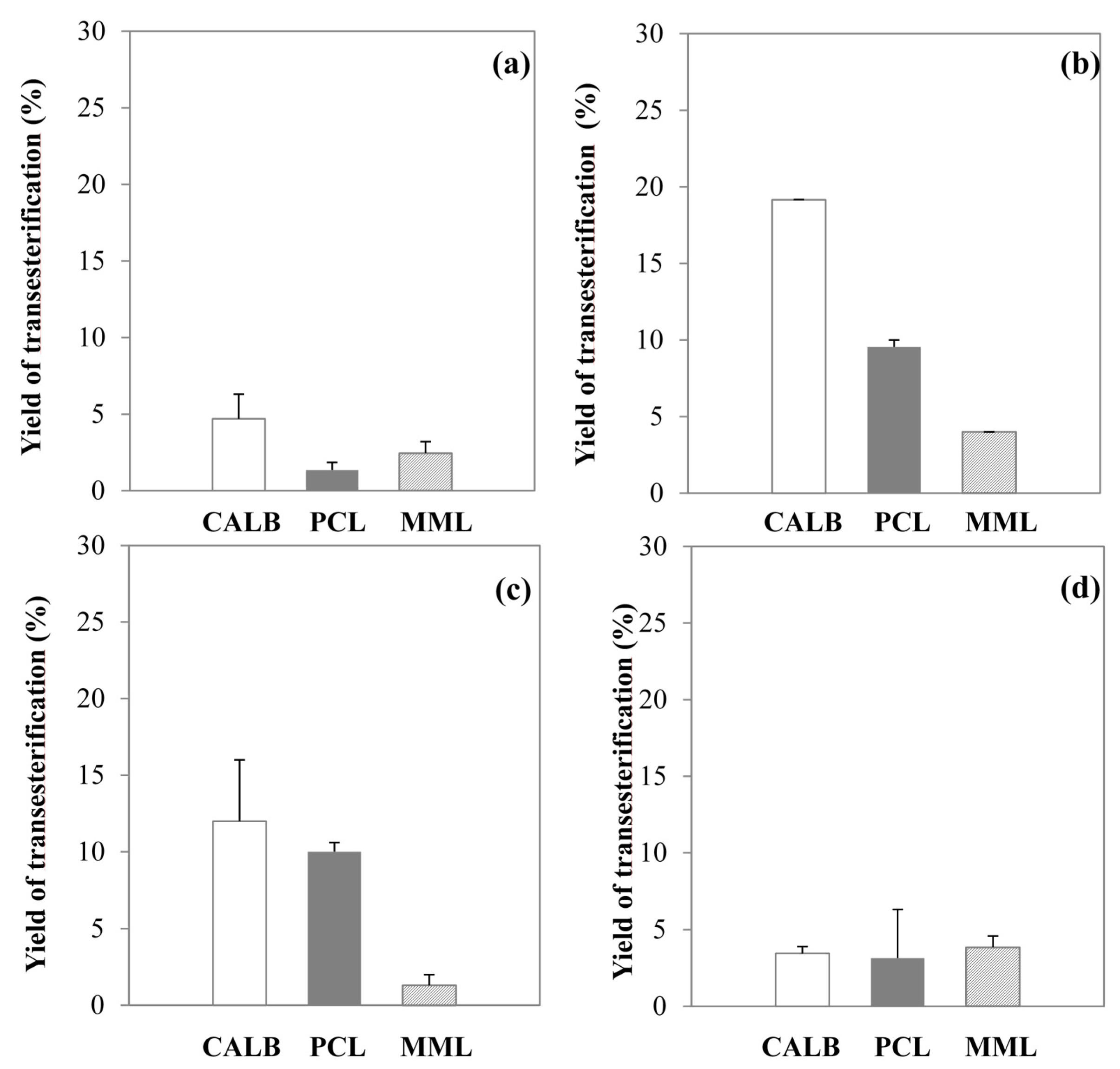
2.3. Enzymatic Transesterification in Binary Hydrophilic—Hydrophobic IL Systems
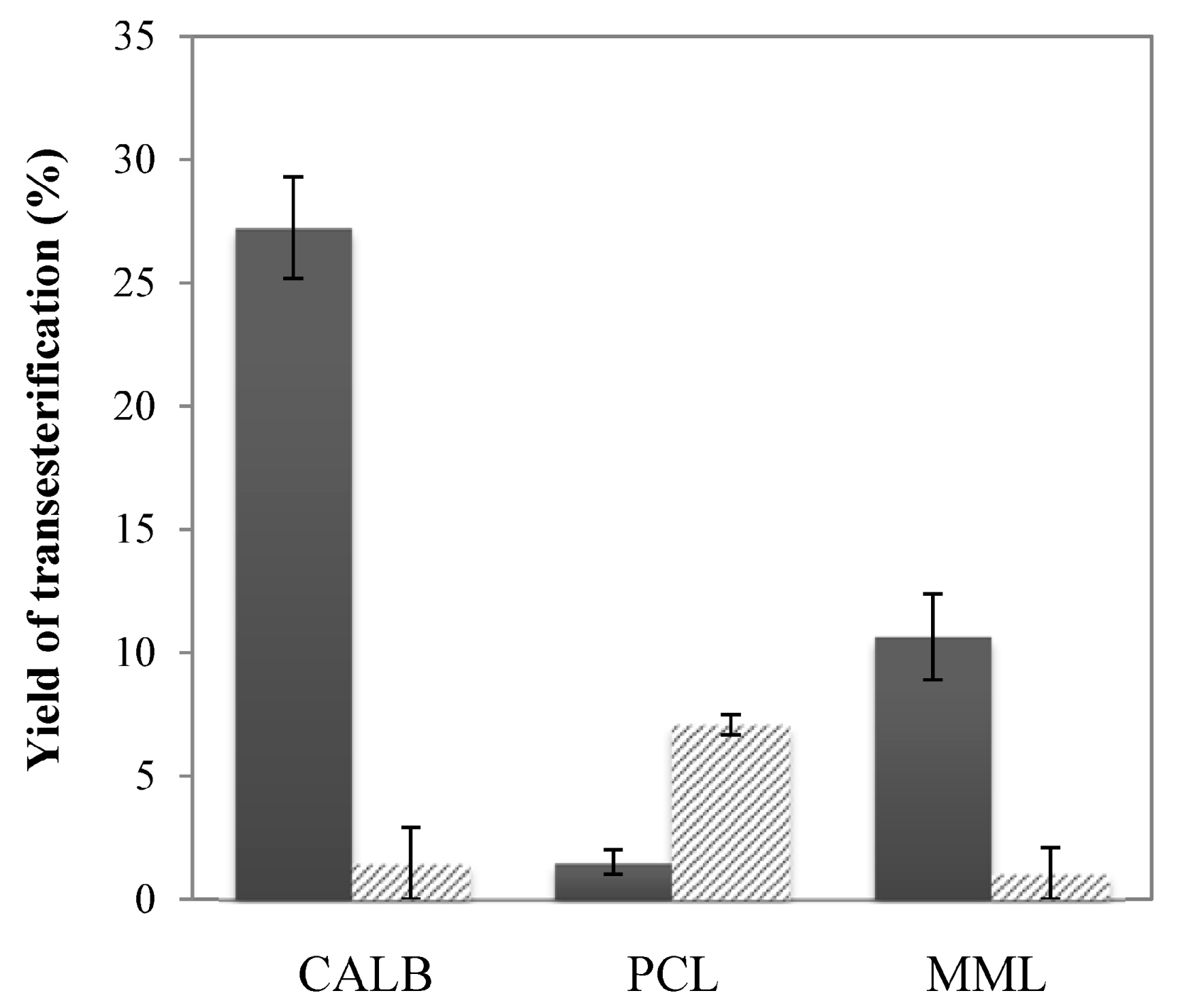
2.4. Enzymatic Transesterification in Hydrophilic IL with Hydrophobic IL-Coated Lipases
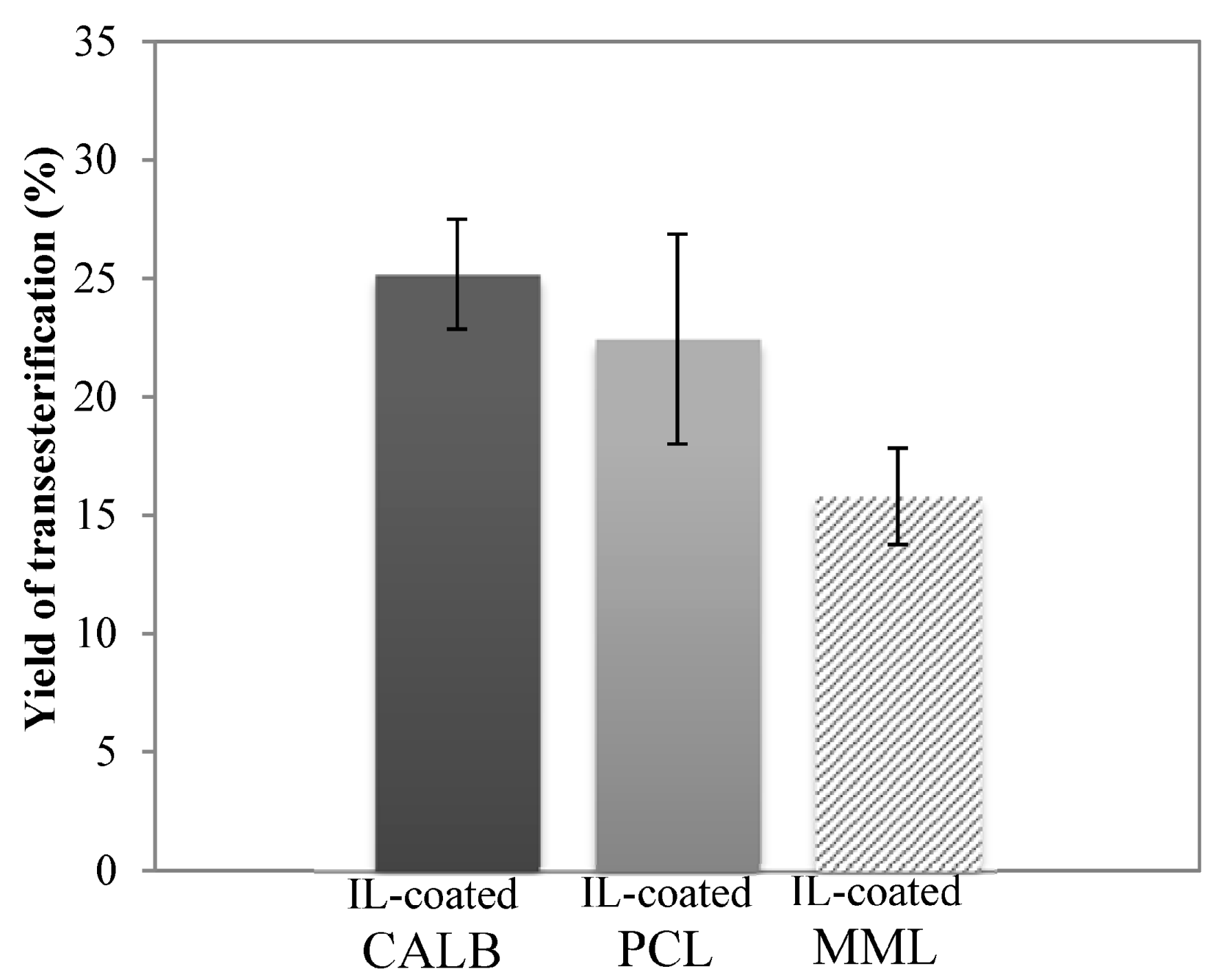
2.5. Structural Characterization of Modified Lignin
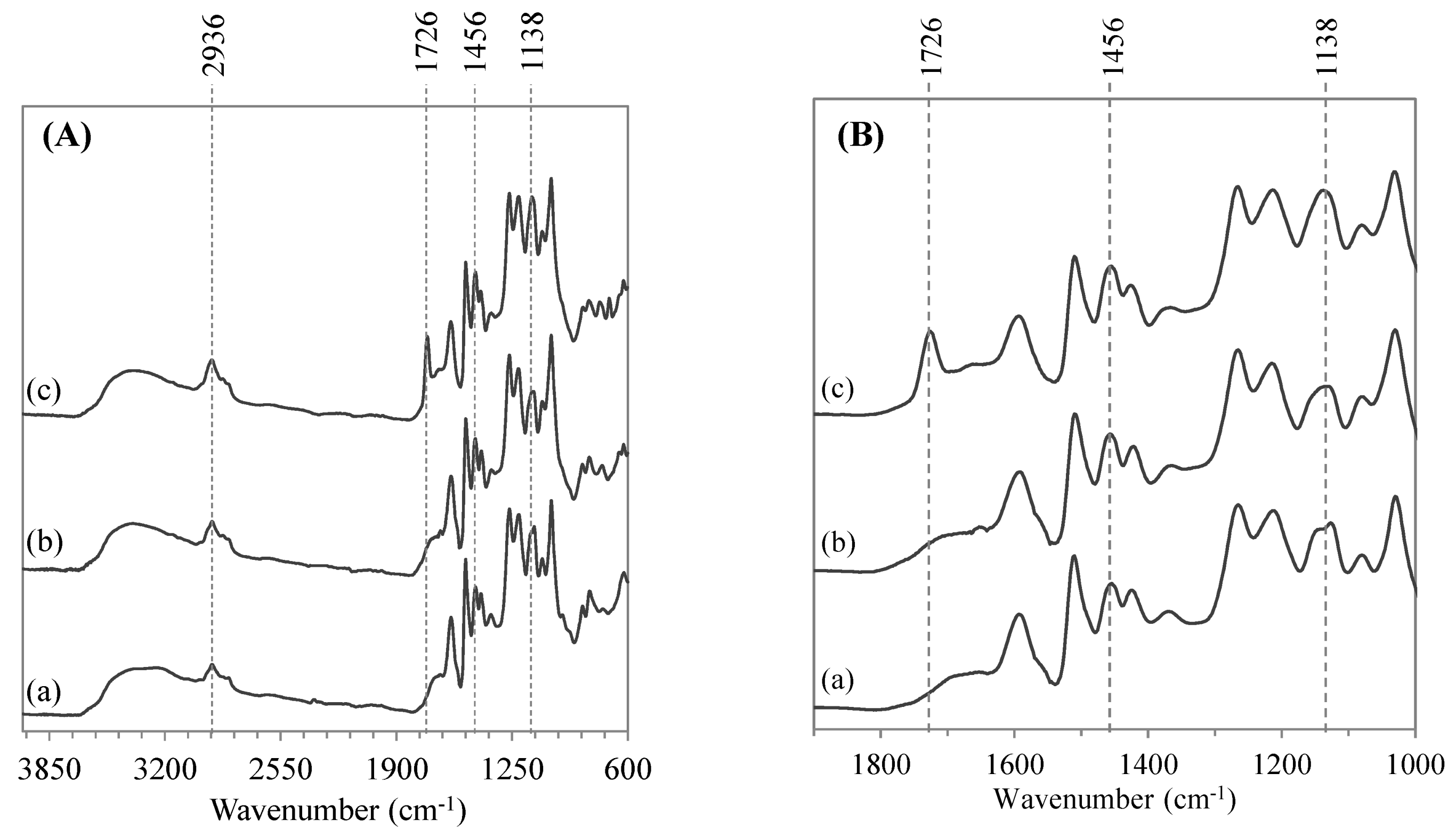
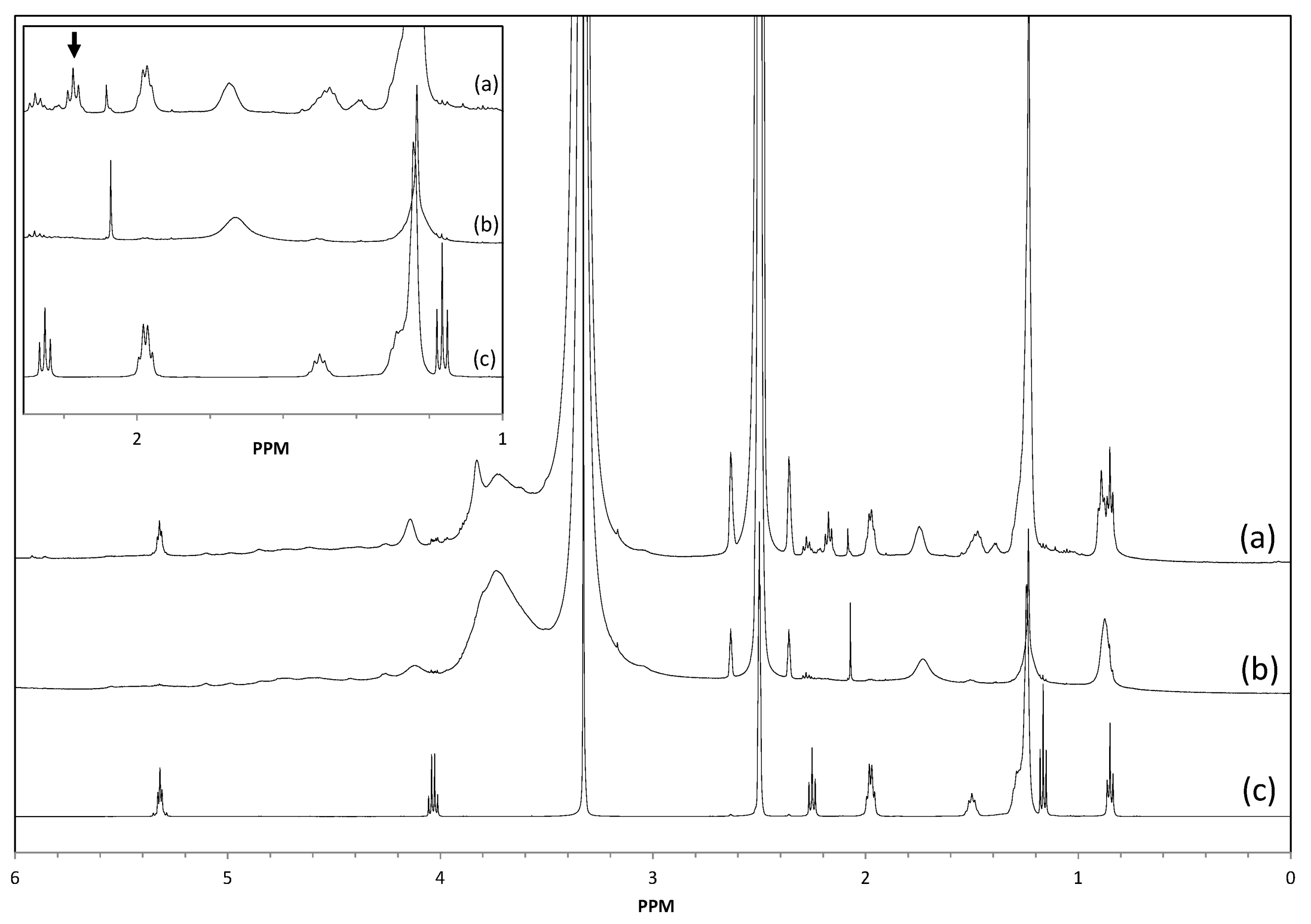
2.6. Thermal Properties of Modified Lignin
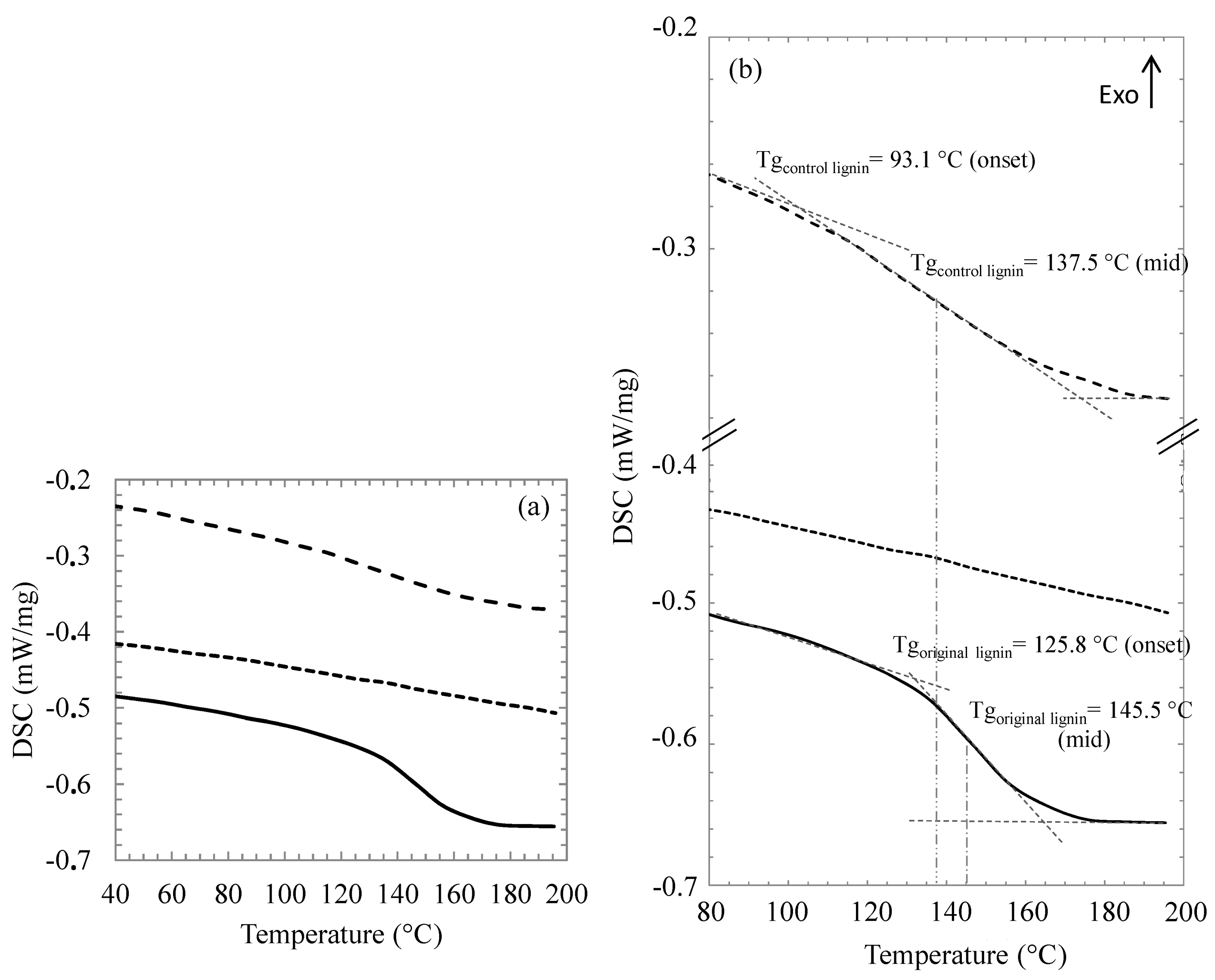
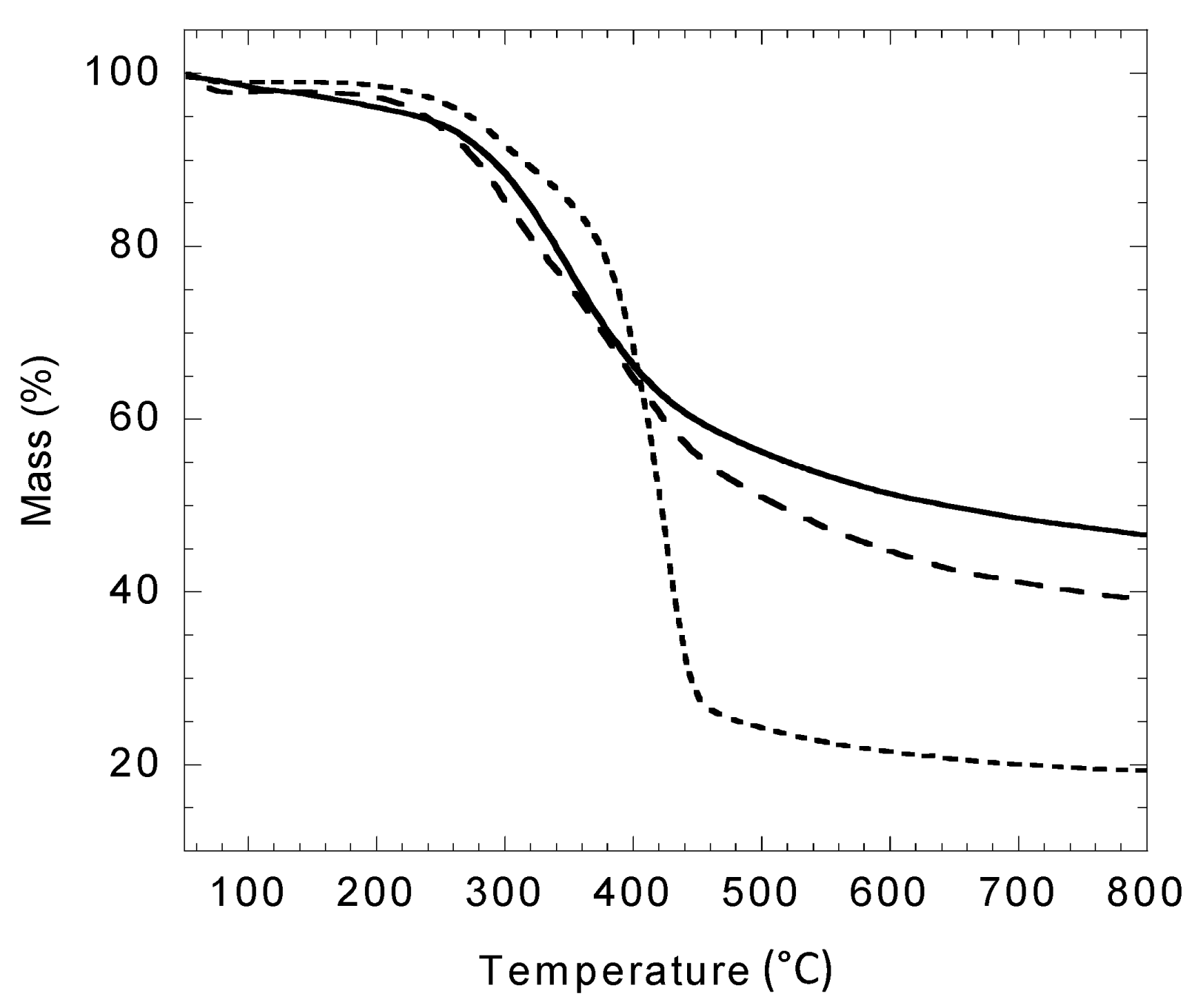
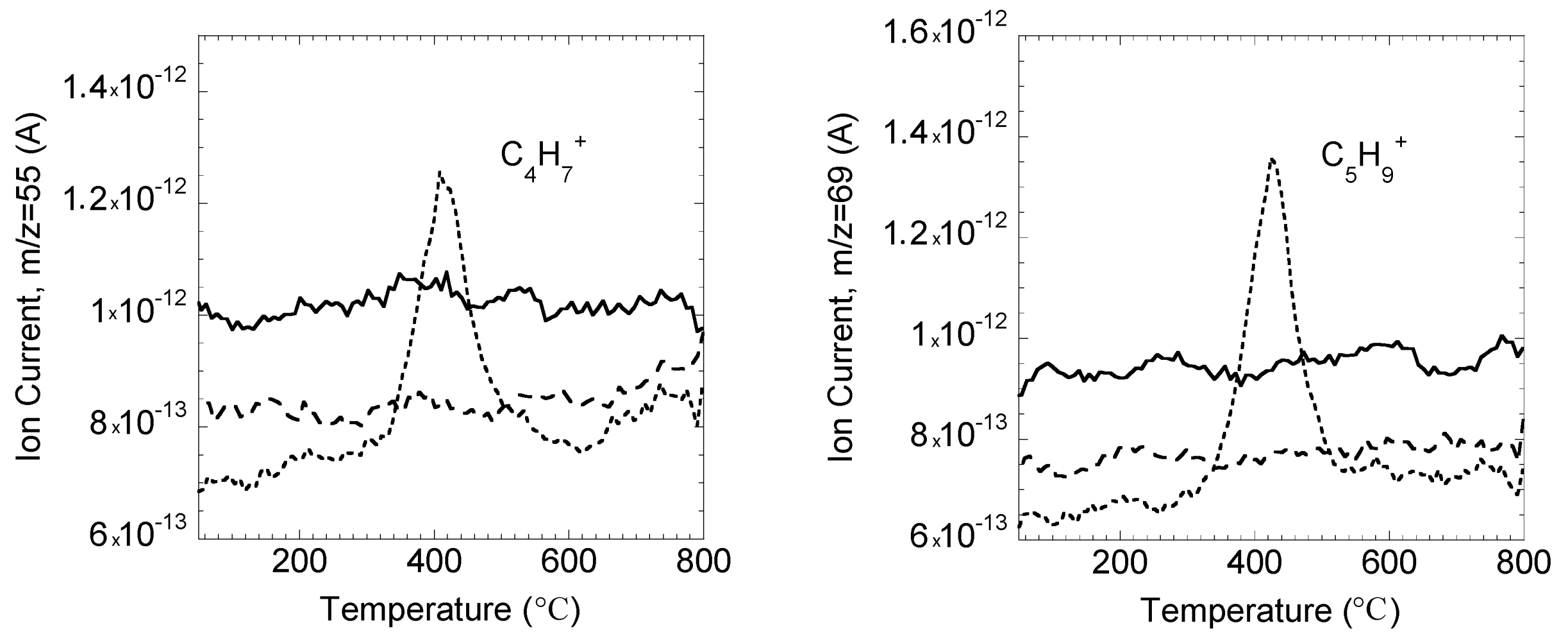
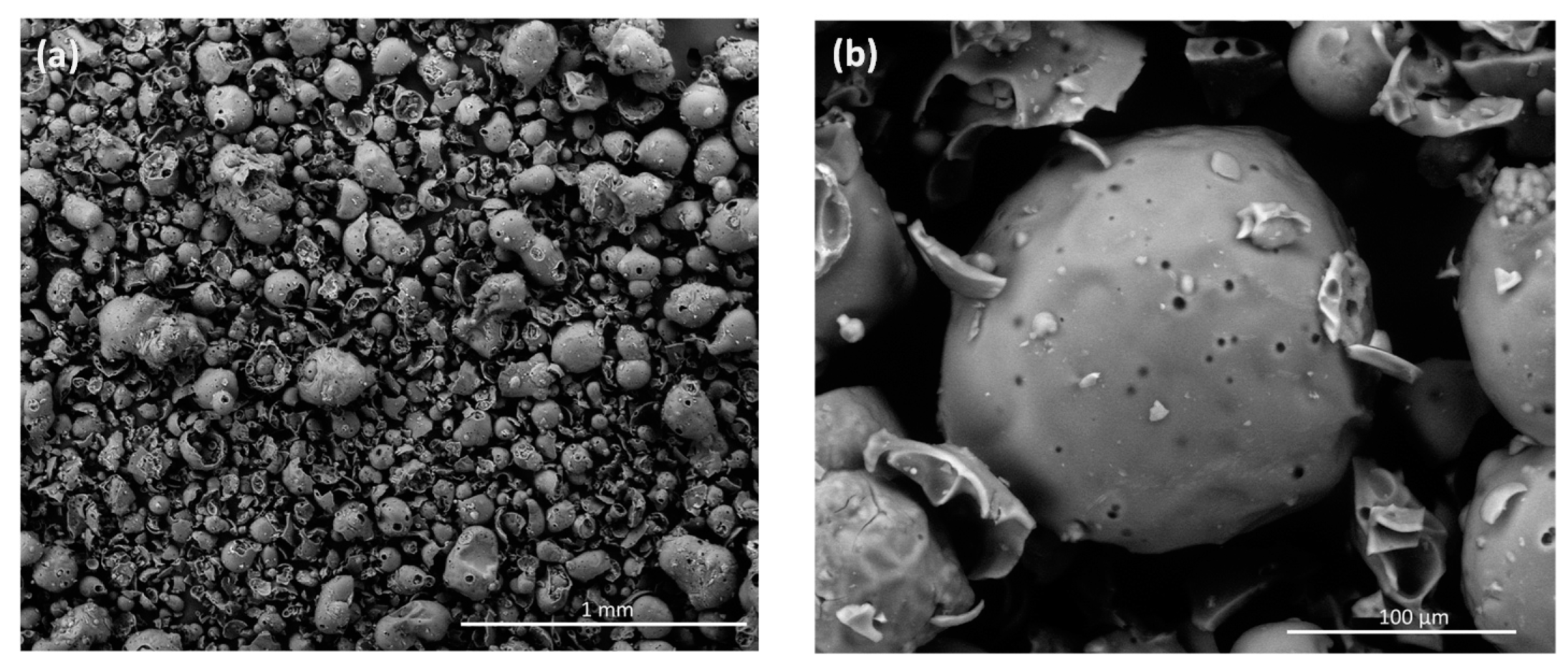
2.7. Textural Properties of Modified Lignin

3. Experimental Section
3.1. Materials
3.2. Solubility of Lignin
3.3. Enzymatic Esterification of Kraft Lignin
3.4. Lignin Recovery after Enzymatic Esterification
3.5. Quantitative Analysis by HPLC
3.6. Structural Characterization
3.7. Thermal Analyses
3.8. Scanning Electron Microscopy
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Doherty, W.O.S.; Mousavioun, P.; Fellows, C.M. Value-adding to cellulosic ethanol: Lignin polymers. Ind. Crops Prod. 2011, 33, 259–276. [Google Scholar] [CrossRef] [Green Version]
- Octave, S.; Thomas, D. Biorefinery: Toward an industrial metabolism. Biochimie 2009, 91, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Flores, F.G.; Dobado, J.A. Lignin as Renewable Raw Material. ChemSusChem 2010, 3, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Erdtman, H. Lignins: Occurrence, Formation, Structure and Reactions, K.V. Sarkanen and C.H. Ludwig, Eds., John Wiley & Sons, Inc., New York, 1971. 916. pp. $35.00. J. Polym. Sci. B Polym. Lett. 1972, 10, 228–230. [Google Scholar]
- Chakar, F.S.; Ragauskas, A.J. Review of current and future softwood kraft lignin process chemistry. Ind. Crops Prod. 2004, 20, 131–141. [Google Scholar] [CrossRef]
- Gandini, A. The irruption of polymers from renewable resources on the scene of macromolecular science and technology. Green Chem. 2011, 13, 1061–1083. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Maldhure, A.; Chaudhari, A.; Ekhe, J. Thermal and structural studies of polypropylene blended with esterified industrial waste lignin. J. Therm. Anal. Calorim. 2011, 103, 625–632. [Google Scholar] [CrossRef]
- Saito, T.; Brown, R.H.; Hunt, M.A.; Pickel, D.L.; Pickel, J.M.; Messman, J.M.; Baker, F.S.; Keller, M.; Naskar, A.K. Turning renewable resources into value-added polymer: Development of lignin-based thermoplastic. Green Chem. 2012, 14, 3295–3303. [Google Scholar] [CrossRef]
- Thielemans, W.; Wool, R.P. Butyrated kraft lignin as compatibilizing agent for natural fiber reinforced thermoset composites. Compos. Part A Appl. Sci. Manuf. 2004, 35, 327–338. [Google Scholar] [CrossRef]
- Mariotti, N.; Hu, L.; Schorr, D.; Stevanovic, T.; Rodrigue, D.; Wang, X.M.; Diouf, P.N.; Grenier, D. New Bio-composites Containing Industrial Lignins. In Proceedings of the 55th International Convention of Society of Wood Science and Technology, Beijing, China, 27–31 August 2012.
- Nadji, H.; Bedard, Y.; Benaboura, A.; Rodrigue, D.; Stevanovic, T.; Riedl, B. Value-Added Derivatives of Soda Lignin from Alfa Grass (Stipa tenacissima). I. Modification and Characterization. J. Appl. Polym. Sci. 2010, 115, 1546–1554. [Google Scholar] [CrossRef]
- Nadji, H.; Diouf, P.N.; Benaboura, A.; Bedard, Y.; Riedl, B.; Stevanovic, T. Comparative study of lignins isolated from Alfa grass (Stipa tenacissima L.). Bioresour. Technol. 2009, 100, 3585–3592. [Google Scholar] [CrossRef] [PubMed]
- Sailaja, R.R.N.; Deepthi, M.V. Mechanical and thermal properties of compatibilized composites of polyethylene and esterified lignin. Mater. Des. 2010, 31, 4369–4379. [Google Scholar] [CrossRef]
- Thielemans, W.; Wool, R.P. Lignin Esters for Use in Unsaturated Thermosets: Lignin Modification and Solubility Modeling. Biomacromolecules 2005, 6, 1895–1905. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Sun, X.F.; Sun, R. The chemical modification of lignins with succinic anhydride in aqueous systems. Polym. Degrad. Stab. 2001, 71, 223–231. [Google Scholar] [CrossRef]
- Gifford, A.P.; Westland, J.A.; Neogi, A.N.; Ragnan, K.D. Low Tg Lignin Mixed Esters. U.S. Patent 20,100,152,428 A1, 12 December 2008. [Google Scholar]
- Tamminem, T.; Ropponen, J.; Hult, E.L.; Poppius-Levlin, K. Functionalized Lignin and Method of Producing the Same. Finland Patent WO2013050661 A1, 11 April 2013. [Google Scholar]
- Favrelle, A.; Bonnet, V.; Avondo, C.; Aubry, F.; Djedaïni-Pilard, F.; Sarazin, C. Lipase-catalyzed synthesis and characterization of novel lipidyl-cyclodextrins in solvent free medium. J. Mol. Catal. B Enzym. 2010, 66, 224–227. [Google Scholar] [CrossRef]
- Husson, E.; Humeau, C.; Paris, C.; Vanderesse, R.; Framboisier, X.; Marc, I.; Chevalot, I. Enzymatic acylation of polar dipeptides: Influence of reaction media and molecular environment of functional groups. Process Biochem. 2009, 44, 428–434. [Google Scholar] [CrossRef]
- Klibanov, A.M. Answering the question: “Why did biocatalysis in organic media not take off in the 1930s?”. Trends Biotechnol. 2000, 18, 85–86. [Google Scholar] [CrossRef]
- Lozano, P.; de Diego, T.; Carrié, D.; Vaultier, M.; Iborra, J.L. Enzymatic ester synthesis in ionic liquids. J. Mol. Catal. B Enzym. 2003, 21, 9–13. [Google Scholar] [CrossRef]
- Mattiasson, B.; Aldercreutz, P. Tailoring the microenvironment of enzymes in water-poor systems. Trends Biotechnol. 1991, 9, 394–398. [Google Scholar] [CrossRef]
- Sen, S.; Puskas, J. Green Polymer Chemistry: Enzyme Catalysis for Polymer Functionalization. Molecules 2015, 20, 9358–9379. [Google Scholar] [CrossRef] [PubMed]
- Alissandratos, A.; Halling, P.J. Enzymatic acylation of starch. Bioresour. Technol. 2012, 115, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Bekhouche, M.; Blum, L.J.; Doumèche, B. Ionic Liquid-Inspired Cations Covalently Bound to Formate Dehydrogenase Improve its Stability and Activity in Ionic Liquids. ChemCatChem 2011, 3, 875–882. [Google Scholar] [CrossRef]
- Shill, K.; Padmanabhan, S.; Xin, Q.; Prausnitz, J.M.; Clark, D.S.; Blanch, H.W. Ionic liquid pretreatment of cellulosic biomass: Enzymatic hydrolysis and ionic liquid recycle. Biotechnol. Bioeng. 2011, 108, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Doherty, T.V.; Linhardt, R.J.; Dordick, J.S. Ionic liquid-mediated selective extraction of lignin from wood leading to enhanced enzymatic cellulose hydrolysis. Biotechnol. Bioeng. 2009, 102, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Pena-Pereira, F.; Namieśnik, J. Ionic Liquids and Deep Eutectic Mixtures: Sustainable Solvents for Extraction Processes. ChemSuschem 2014, 7, 1784–1800. [Google Scholar] [CrossRef] [PubMed]
- Anugwom, I.; Eta, V.; Virtanen, P.; Mäki-Arvela, P.; Hedenström, M.; Hummel, M.; Sixta, H.; Mikkola, J.-P. Switchable Ionic Liquids as Delignification Solvents for Lignocellulosic Materials. ChemSuschem 2014, 7, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Mäki-Arvela, P.; Anugwom, I.; Virtanen, P.; Sjöholm, R.; Mikkola, J.P. Dissolution of lignocellulosic materials and its constituents using ionic liquids—A review. Ind. Crops Prod. 2010, 32, 175–201. [Google Scholar] [CrossRef]
- Pu, Y.; Jiang, N.; Ragauskas, A.J. Ionic Liquid as a Green Solvent for Lignin. J. Wood Chem. Technol. 2007, 27, 23–33. [Google Scholar] [CrossRef]
- Husson, E.; Humeau, C.; Blanchard, F.; Framboisier, X.; Marc, I.; Chevalot, I. Chemo-selectivity of the N,O-enzymatic acylation in organic media and in ionic liquids. J. Mol. Catal. B Enzym. 2008, 55, 110–117. [Google Scholar] [CrossRef]
- Lozano, P. Enzymes in neoteric solvents: From one-phase to multiphase systems. Green Chem. 2010, 12, 555–569. [Google Scholar] [CrossRef]
- Garcia-Lorenzo, A.; Tojo, E.; Tojo, J.; Teijeira, M.; Rodriguez-Berrocal, F.J.; Gonzalez, M.P.; Martinez-Zorzano, V.S. Cytotoxicity of selected imidazolium-derived ionic liquids in the human Caco-2 cell line. Sub-structural toxicological interpretation through a QSAR study. Green Chem. 2008, 10, 508–516. [Google Scholar] [CrossRef]
- Egorova, K.S.; Ananikov, V.P. Toxicity of Ionic Liquids: Eco(cyto)activity as Complicated, but Unavoidable Parameter for Task-Specific Optimization. ChemSuschem 2014, 7, 336–360. [Google Scholar] [CrossRef] [PubMed]
- Schorr, D.; Diouf, P.N.; Stevanovic, T. Evaluation of industrial lignins for biocomposites production. Ind. Crops Prod. 2014, 52, 65–73. [Google Scholar] [CrossRef]
- Virsu, P.; Liljeblad, A.; Kanerva, A.; Kanerva, L.T. Preparation of the enantiomers of 1-phenylethan-1,2-diol. Regio- and enantioselectivity of acylase I and Candida antarctica lipases A and B. Tetrahedron Asymmetry 2001, 12, 2447–2455. [Google Scholar] [CrossRef]
- Nicolosi, G.; Spatafora, C.; Tringali, C. Chemo-enzymatic preparation of resveratrol derivatives. J. Mol. Catal. B Enzym. 2002, 16, 223–229. [Google Scholar] [CrossRef]
- Chebil, L.; Humeau, C.; Falcimaigne, A.; Engasser, J.-M.; Ghoul, M. Enzymatic acylation of flavonoids. Process Biochem. 2006, 41, 2237–2251. [Google Scholar] [CrossRef]
- Zhang, X.; Adachi, S.; Watanabe, Y.; Matsuno, R. Lipase-catalyzed synthesis of O-lauroyl l-serinamide and O-lauroyl l-threoninamide. Food Res. Int. 2005, 38, 297–300. [Google Scholar] [CrossRef]
- Ab Rani, M.A.; Brant, A.; Crowhurst, L.; Dolan, A.; Lui, M.; Hassan, N.H.; Hallett, J.P.; Hunt, P.A.; Niedermeyer, H.; Perez-Arlandis, J.M.; et al. Understanding the polarity of ionic liquids. Phys. Chem. Chem. Phys. 2011, 13, 16831–16840. [Google Scholar] [CrossRef] [PubMed]
- Doherty, T.V.; Mora-Pale, M.; Foley, S.E.; Linhardt, R.J.; Dordick, J.S. Ionic liquid solvent properties as predictors of lignocellulose pretreatment efficacy. Green Chem. 2010, 12, 1967–1975. [Google Scholar] [CrossRef]
- Jeličić, A.; García, N.; Löhmannsröben, H.-G.; Beuermann, S. Prediction of the Ionic Liquid Influence on Propagation Rate Coefficients in Methyl Methacrylate Radical Polymerizations Based on Kamlet-Taft Solvatochromic Parameters. Macromolecules 2009, 42, 8801–8808. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Abboud, J.L.M.; Taft, R.W. An Examination of Linear Solvation Energy Relationships. In Progress in Physical Organic Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 485–630. [Google Scholar]
- Hofmann, K.; Schreiter, K.; Seifert, A.; Ruffer, T.; Lang, H.; Spange, S. Solvatochromism and linear solvation energy relationship of diol- and proline-functionalized azo dyes using the Kamlet-Taft and Catalan solvent parameter sets. New J. Chem. 2008, 32, 2180–2188. [Google Scholar] [CrossRef]
- Torres, C.; Bernabé, M.; Otero, C. Part II. Two enzymatic procedures for the selective synthesis of malic acid monoesters. Enzym. Microb. Technol. 1999, 25, 753–761. [Google Scholar] [CrossRef]
- Torres, C.; Otero, C. Part I. Enzymatic synthesis of lactate and glycolate esters of fatty alcohols. Enzym. Microb. Technol. 1999, 25, 745–752. [Google Scholar] [CrossRef]
- Gremos, S.; Zarafeta, D.; Kekos, D.; Kolisis, F. Direct enzymatic acylation of cellulose pretreated in BMIMCl ionic liquid. Bioresour. Technol. 2011, 102, 1378–1382. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Ray, M.J.; To, T.Q.; Leak, D.J.; Murphy, R.J.; Welton, T. Ionic liquid pretreatment of lignocellulosic biomass with ionic liquid-water mixtures. Green Chem. 2011, 13, 2489–2499. [Google Scholar] [CrossRef]
- Lee, S.H.; Ha, S.H.; Hiep, N.M.; Chang, W.-J.; Koo, Y.-M. Lipase-catalyzed synthesis of glucose fatty acid ester using ionic liquids mixtures. J. Biotechnol. 2008, 133, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Van Rantwijk, F.; Sheldon, R.A. Biocatalysis in Ionic Liquids. Chem. Rev. 2007, 107, 2757–2785. [Google Scholar] [CrossRef] [PubMed]
- Carta, G.; Gainer, J.L.; Gibson, M.E. Synthesis of esters using a nylon-immobilized lipase in batch and continuous reactors. Enzym. Microb. Technol. 1992, 14, 904–910. [Google Scholar] [CrossRef]
- Hess, R.; Bornscheuer, U.; Capewell, A.; Scheper, T. Lipase-catalyzed synthesis of monostearoylglycerol in organic solvents from 1,2-O-isopropylidene glycerol. Enzym. Microb. Technol. 1995, 17, 725–728. [Google Scholar] [CrossRef]
- Dong, F.-X.; Zhang, L.; Tong, X.-Z.; Chen, H.-B.; Wang, X.-L.; Wang, Y.-Z. Ionic liquid coated lipase: Green synthesis of high molecular weight poly(1,4-dioxan-2-one). J. Mol. Catal. B Enzym. 2012, 77, 46–52. [Google Scholar] [CrossRef]
- Mutschler, J.; Rausis, T.; Bourgeois, J.-M.; Bastian, C.; Zufferey, D.; Mohrenz, I.V.; Fischer, F. Ionic liquid-coated immobilized lipase for the synthesis of methylglucose fatty acid esters. Green Chem. 2009, 11, 1793–1800. [Google Scholar] [CrossRef]
- Faix, O. Fourier transformed infrared spectroscopy. In Methods in Lignin Chemistry; Lin, S.Y., Dence, C.W., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 1992; p. 93. [Google Scholar]
- Glasser, W.G.; Jain, R.K. Lignin Derivatives. I. Alkanoates. Holzforschung 1993, 47, 225–233. [Google Scholar] [CrossRef]
- Cui, C.; Sadeghifar, H.; Sen, S.; Argyropoulos, D.S. Toward Thermoplastic Lignin Polymers; Part II: Thermal and Polymer Characteristics of Kraft Lignin and Derivatives. BioResources 2013, 8, 864–886. [Google Scholar] [CrossRef]
- Fox, S.C.; McDonald, A.G. Chemical and thermal characterization of three industrial lignins and their corresponding lignin esters. BioResources 2010, 5, 990–1009. [Google Scholar]
- Ghosh, I.; Jain, R.K.; Glasser, W.G. Blends of Biodegradable Thermoplastics with Lignin Esters. In Lignin: Historical, Biological, and Materials Perspectives; Glasser, W.G., Northey, R.A., Schultz, T.P., Eds.; ACS Symposium Series; 1999; Volume 742, pp. 331–350. Available online: http://pubs.acs.org/doi/abs/10.1021/bk-2000-0742.ch017 (accessed on 7 September 2015).
- Glasser, W.G.; Dave, V.; Frazier, C.E. Molecular weight distribution of (semi-) commercial lignin derivatives. J. Appl. Polym. Sci. 1993, 13, 545–559. [Google Scholar] [CrossRef]
- Ghosh, I.; Jain, R.K.; Glasser, W.G. Multiphase materials with lignin. XV. Blends of cellulose acetate butyrate with lignin esters. J. Appl. Polym. Sci. 1999, 74, 448–457. [Google Scholar] [CrossRef]
- Christie, W. Gas chromatography-mass spectrometry methods for structural analysis of fatty acids. Lipids 1998, 33, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Fierro, V.; Torné-Fernández, V.; Celzard, A. Kraft lignin as a precursor for microporous activated carbons prepared by impregnation with ortho-phosphoric acid: Synthesis and textural characterisation. Microporous Mesoporous Mater. 2006, 92, 243–250. [Google Scholar] [CrossRef]
- Pua, F.-L.; Fang, Z.; Zakaria, S.; Guo, F.; Chia, C.-H. Direct production of biodiesel from high-acid value Jatropha oil with solid acid catalyst derived from lignin. Biotechnol. Biofuels 2011, 4. [Google Scholar] [CrossRef] [PubMed]
- Prado, R.; Erdocia, X.; Serrano, L.; Labidi, J. Lignin purification with green solvents. Cellul. Chem. Technol. 2012, 46, 221–225. [Google Scholar]
- Singh, R.; Singh, S.; Trimukhe, K.D.; Pandare, K.V.; Bastawade, K.B.; Gokhale, D.V.; Varma, A.J. Lignin–carbohydrate complexes from sugarcane bagasse: Preparation, purification, and characterization. Carbohydr. Polym. 2005, 62, 57–66. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hulin, L.; Husson, E.; Bonnet, J.-P.; Stevanovic, T.; Sarazin, C. Enzymatic Transesterification of Kraft Lignin with Long Acyl Chains in Ionic Liquids. Molecules 2015, 20, 16334-16353. https://doi.org/10.3390/molecules200916334
Hulin L, Husson E, Bonnet J-P, Stevanovic T, Sarazin C. Enzymatic Transesterification of Kraft Lignin with Long Acyl Chains in Ionic Liquids. Molecules. 2015; 20(9):16334-16353. https://doi.org/10.3390/molecules200916334
Chicago/Turabian StyleHulin, Lise, Eric Husson, Jean-Pierre Bonnet, Tatjana Stevanovic, and Catherine Sarazin. 2015. "Enzymatic Transesterification of Kraft Lignin with Long Acyl Chains in Ionic Liquids" Molecules 20, no. 9: 16334-16353. https://doi.org/10.3390/molecules200916334
APA StyleHulin, L., Husson, E., Bonnet, J.-P., Stevanovic, T., & Sarazin, C. (2015). Enzymatic Transesterification of Kraft Lignin with Long Acyl Chains in Ionic Liquids. Molecules, 20(9), 16334-16353. https://doi.org/10.3390/molecules200916334