Synthesis and Herbicidal Activity of New Hydrazide and Hydrazonoyl Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
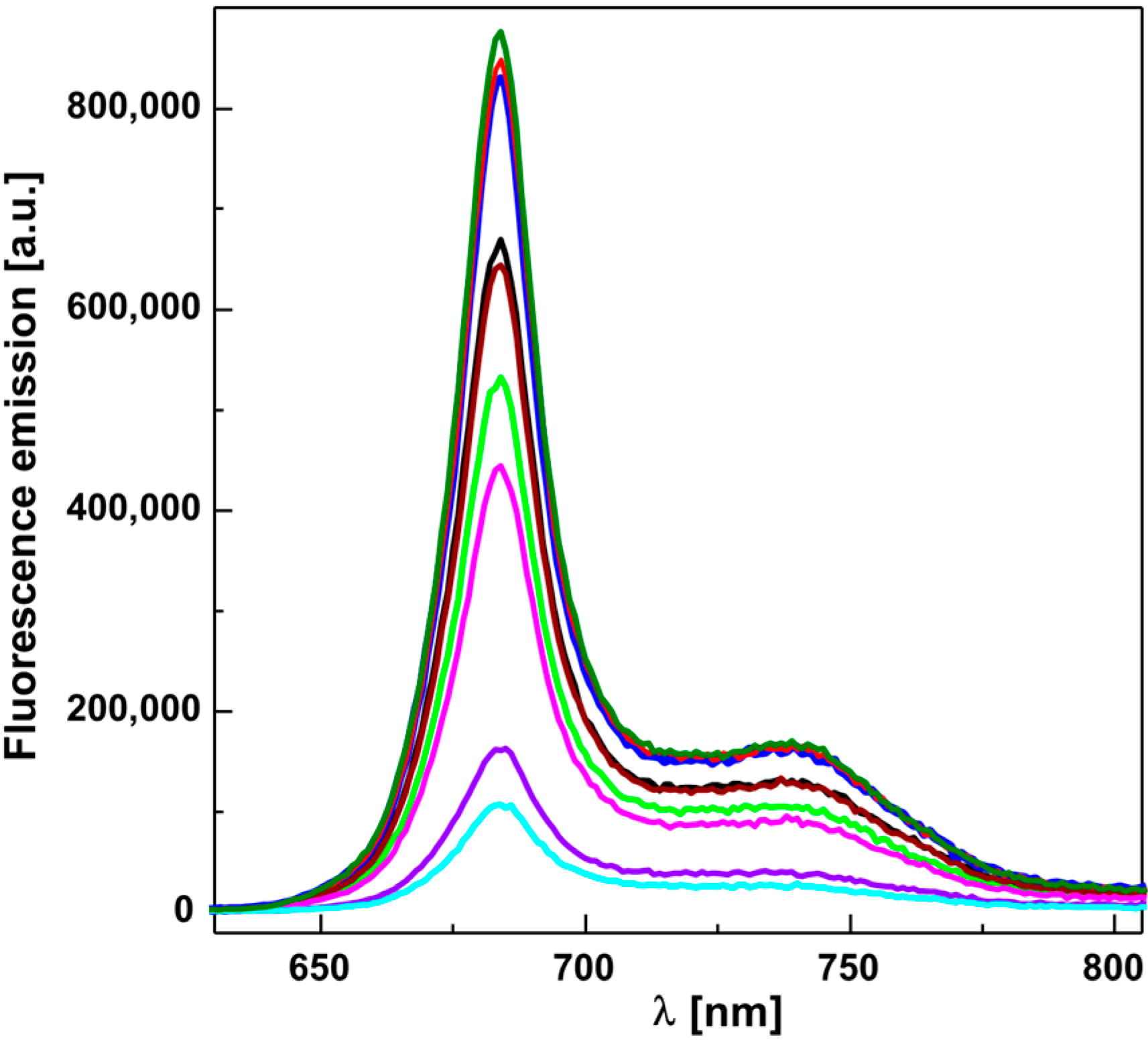
2.2. Inhibition of Photosynthetic Electron Transport (PET) in Spinach Chloroplasts
| Compound | PET Inhibition in Chloroplasts | Growth Inhibition of Chlorella vulgaris |
|---|---|---|
| 2a | no inhibition | no inhibition |
| 2b | 18.0 | 25.87 |
| 2c | no inhibition | 8.01 |
| 3b | 146.0 | 17.58 |
| 3c | 2213 | 10.32 |
| 3d | 61.4 | 13.32 |
| 3e | 2.34 | 12.30 |
| 4f | no inhibition | no inhibition |
| DCMU | 1.9 * | 7.3 ** |
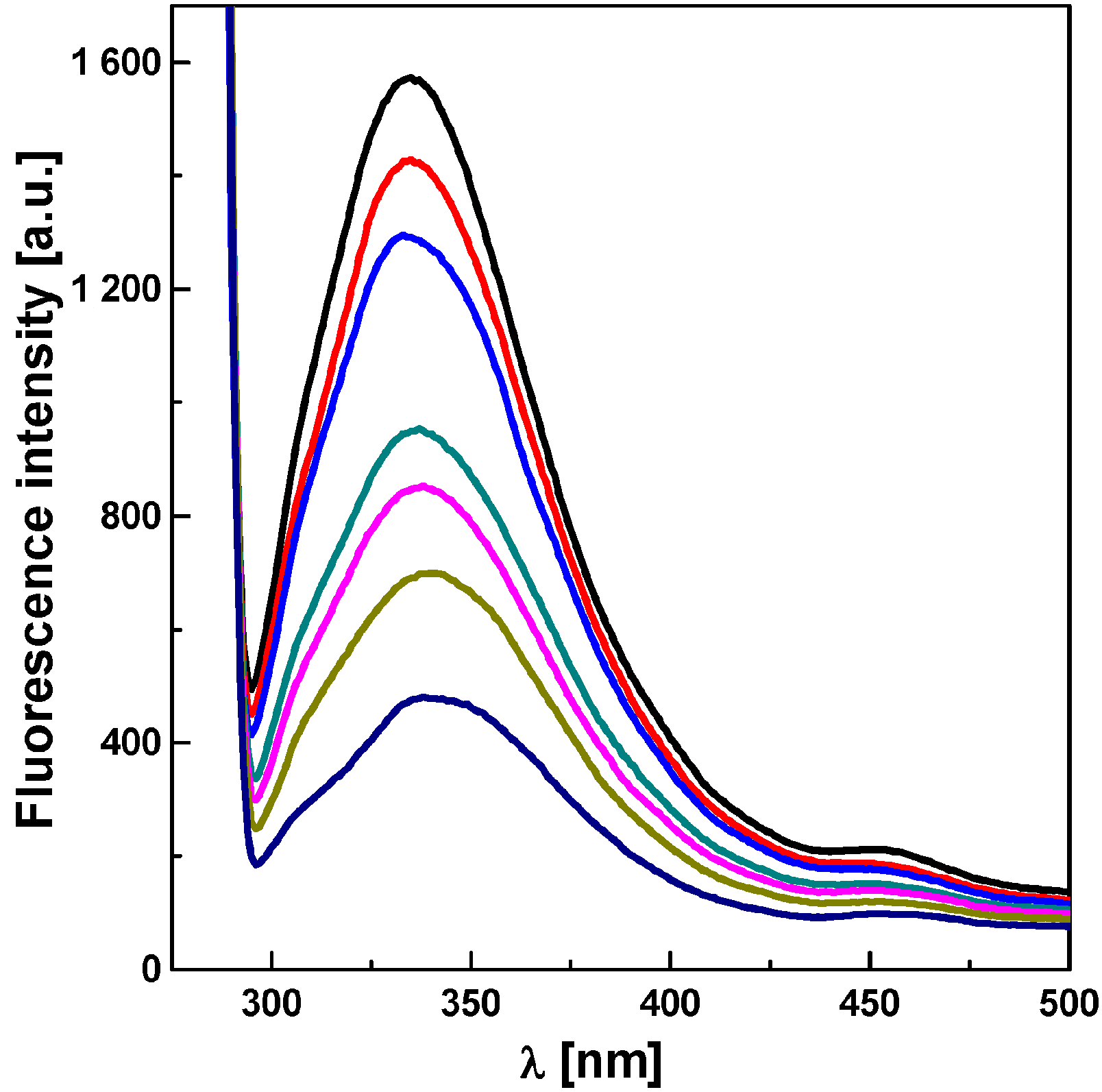
2.3. Inhibition of Algae Growth
3. Experimental Section
3.1. General Information
3.2. Synthesis
3.2.1. General Procedure for Synthesis of N′-[2,6-dinitro-4-(trifluoromethyl)phenyl]hydrazides 2a, 2b, 2c
N′-[2,6-Dinitro-4-(trifluoromethyl)phenyl]-4-methoxybenzohydrazide (2a)

4-(tert-Butyl)-N′-[2,6-dinitro-4-(trifluoromethyl)phenyl]benzohydrazide (2b)

N′-[2,6-Dinitro-4-(trifluoromethyl)phenyl]acethydrazide (2c)

3.2.2. General Procedure for Synthesis of N′-[2,6-dinitro-4-(trifluoromethyl)phenyl]hydrazonoyl Chlorides 3b, 3c, 3d, 3e
4-(tert-Butyl)-N′-[2,6-dinitro-4-(trifluoromethyl)phenyl]benzohydrazonoyl chloride (3b)

N′-[2,6-Dinitro-4-(trifluoromethyl)phenyl]acethydrazonoyl chloride (3c)

N′-[2,6-Dinitro-4-(trifluoromethyl)phenyl]-4-fluorobenzohydrazonoyl chloride (3d)

N′-[2,6-Dinitro-4-(trifluoromethyl)phenyl]thiophene-2-carbohydrazonoyl chloride (3e)

N′-[2,6-Dinitro-4-(trifluoromethyl)phenyl]-2-naphtohydrazonoyl cyanide (4f)

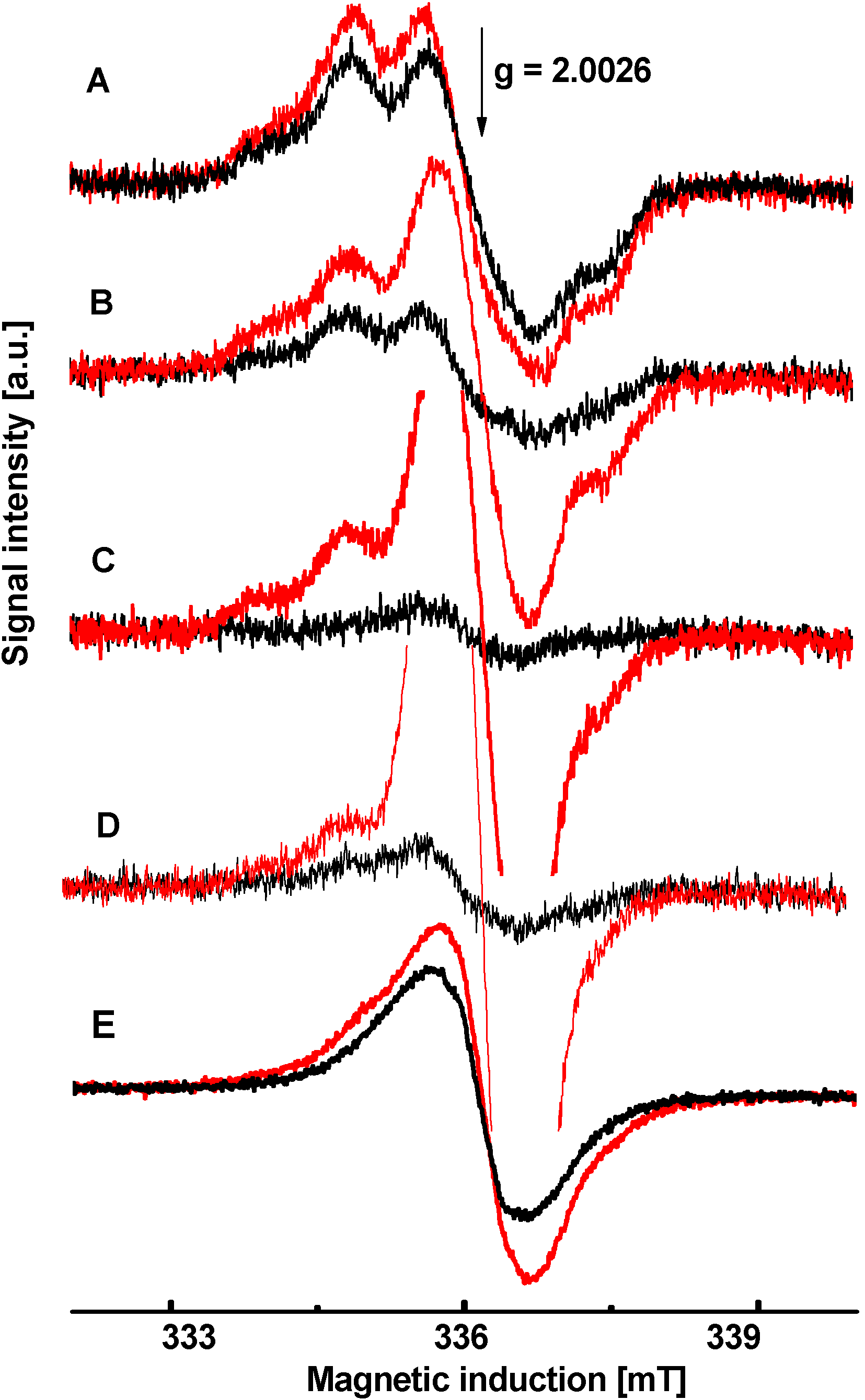
3.3. PET Study
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Eldehna, W.M.; Fares, M.; Abdel-Aziz, M.M.; Abdel-Aziz, H.A. Design, synthesis and antitubercular activity of certain nicotinic acid hydrazides. Molecules 2015, 20, 8800–8815. [Google Scholar] [CrossRef] [PubMed]
- Gilani, S.J.; Khan, S.A.; Siddiqui, N.; Verma, S.P.; Mullick, P.; Alam, O.J. Synthesis and in vitro antimicrobial activity of novel N-(6-chlorobenzo[d]thiazol-2-yl) hydrazine arboxamide derivatives of benzothiazole class. J. Enzym. Inhib. Med. Chem. 2011, 26, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Cacic, M.; Trkovnik, M.; Cacic, F.; Has-Schon, E. Synthesis and antimicrobial activity of some derivatives of (7-hydroxy-2-oxo-2H-chromen-4-yl)-acetic acid hydrazide. Molecules 2006, 11, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Kostecka, M. Synthesis of a new group of aliphatic hydrazide derivatives and the correlations between their molecular structure and biological activity. Molecules 2012, 17, 3560–3573. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Gangwal, R.; Sangamwar, A.T.; Jain, R. Synthesis, biological evaluation and 3D-QSAR study of hydrazide, semicarbazide and thiosemicarbazide derivatives of 4-(adamantan-1-yl)quinoline as anti-tuberculosis agents. Eur. J. Med. Chem. 2014, 85, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Judge, V.; Narasimhan, B.; Ahuja, M.; Sriram, D.; Yogeeswari, P.; de Clercq, E.; Pannecouque, C.; Balzarini, J. Synthesis, antimycobacterial, antiviral, antimicrobial activity and QSAR studies of N(2)-acyl isonicotinic acid hydrazide derivatives. Med. Chem. 2013, 9, 53–76. [Google Scholar] [CrossRef] [PubMed]
- Refat, H.M.; Fadda, A.A. Synthesis and antimicrobial activity of some novel hydrazide, benzochromenone, dihydropyridine, pyrrole, thiazole and thiophene derivatives. Eur. J. Med. Chem. 2013, 70, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Mohareb, R.M.; Hana, H.Y. Synthesis of progesterone heterocyclic derivatives of potential antimicrobial activity. Acta Pharm. 2008, 58, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Su, H.; Jiang, J.; Yang, X.; Nishida, Y. Design, synthesis and bioactivity of N-glycosyl-N′(5-substituted phenyl-2-furoyl) hydrazide derivatives. Int. J. Mol. Sci. 2014, 15, 6741–6756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozçelik, A.B.; Gökçe, M.; Orhan, I.; Kaynak, F.; Sahin, M.F. Synthesis and antimicrobial, acetylcholinesterase and butyrylcholinesterase inhibitory activities of novel ester and hydrazide derivatives of 3(2H)-pyridazinone. Arzneimittelforschung 2010, 60, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Bartzatt, R.; Cirillo, S.L.; Cirillo, J.D. Small molecule hydrazide agents to inhibit growth and proliferation of mycobacterium tuberculosis. Med. Chem. 2012, 8, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Paprocka, R.; Modzelewska-Banachiewicz, B.; Wiese, M.; Eljaszewicz, A.; Michałkiewicz, J. Synthesis and anti-inflammatory activity of hydrazide derivatives of 2-methylidene-1,4-dicarboxybutanoic acid. Acta Pol. Pharm. 2012, 69, 1390–1394. [Google Scholar] [PubMed]
- Rollas, S.; Küçükgüzel, S.G. Biological activities of hydrazone derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Narasimhan, B. Hydrazides/hydrazones as antimicrobial and anticancer agents in the new millennium. Mini Rev. Med. Chem. 2013, 13, 971–987. [Google Scholar] [CrossRef] [PubMed]
- Narang, R.; Narasimhan, B.; Sharma, S. A review on biological activities and chemical synthesis of hydrazide derivatives. Curr. Med. Chem. 2012, 19, 569–612. [Google Scholar] [CrossRef] [PubMed]
- Parodi, S.; de Flora, S.; Cavanna, M.; Pino, A.; Robbiano, L.; Bennicelli, C.; Brambilla, G. DNA-damaging activity in vivo and bacterial mutagenicity of sixteen hydrazine derivatives as related quantitatively to their carcinogenicity. Cancer Res. 1981, 41, 1469–1482. [Google Scholar] [PubMed]
- Kak, S.N.; Kaul, B.L. Mutagenic activity of hydrazine and its combinations with maleic hydrazide and X-rays in barley. Cytobios 1975, 12, 123–128. [Google Scholar] [PubMed]
- Levi, B.Z.; Kuhn, J.C.; Ulitzur, S. Determination of the activity of 16 hydrazine derivatives in the bioluminescence test for genotoxic agents. Mutat. Res. 1986, 173, 233–237. [Google Scholar] [CrossRef]
- Mazza, M.; Montanari, L.; Pavanetto, F. Phytotoxicity of hydrazones of aromatic aldehydes. Farmaco Sci. 1976, 31, 334–344. [Google Scholar] [PubMed]
- Hendrix, J.A.; Hemmerle, H.; Urmann, M.; Shutske, G.M.; Strupczewski, J.T.; Bordeau, K.J.; Jurcak, J.G.; Nieduzak, T.R.; Jackson, S.A.; Angell, P.; et al. Novel Heterocyclic Substituted Carbonyl Derivatives and Their Use as Dopamine D3 Receptor Ligands. US Patent 20090247509 A1; filed 4 November 2004, and issued 6 April 2004,
- Saleh, M.A. Mutagenic and carcinogenic effects of pesticides. J. Environ. Sci. Health B 1980, 15, 907–927. [Google Scholar] [CrossRef] [PubMed]
- Swietlinska, Z.; Zuk, J. Cytotoxic effects of maleic hydrazide. Mutat. Res. 1978, 55, 15–30. [Google Scholar] [CrossRef]
- Heath, R.L. Hydrazine as an electron donor to the water-oxidation site in photosynthesis. Biochim. Biophys. Acta 1971, 245, 160–164. [Google Scholar] [CrossRef]
- Messinger, J.; Renger, G. Generation, oxidation by the oxidized form of the tyrosine of polypeptide D2, and possible electronic configuration of the redox states S0, S−1, and S−2 of the water oxidase in isolated spinach thylakoids. Biochemistry 1993, 32, 9379–9386. [Google Scholar] [CrossRef] [PubMed]
- Förster, V.; Junge, W. On the action of hydroxylamine, hydrazine and their derivatives on the water-oxidizing complex. Photosynth. Res. 1986, 9, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Messinger, J.; Renger, G. The reactivity of hydrazine with photosystem II strongly depends on the redox state of the water oxidizing system. FEBS Lett. 1990, 277, 141–146. [Google Scholar] [CrossRef]
- He, P. The differences between hydroxylamine and hydrazine in affecting oxygen evolution of photosynthetic water splitting system in tobacco. Acta Phytophysiol. Sin. 1996, 22, 165–170. [Google Scholar]
- Koske, T.J.; Svec, L.V. Some effects of maleic hyrazide on light reactions of photosynthesis in isolated chloroplasts from Phaseolus vulgaris plants. Can. J. Plant Sci. 1975, 55, 145–149. [Google Scholar] [CrossRef]
- Haveman, J.; Duysens, L.N.M.; van Der Geest, T.C.M.; van Gorkom, H.J. Hydrazobenzene oxidation by 2,6-dichlorophenol-indophenol in a photoreaction catalyzed by system I of photosynthesis. Hydrazine compounds as donors for photosystem II. Biochim. Biophys. Acta 1972, 283, 316–327. [Google Scholar] [CrossRef]
- Gasparova, R.; Zbojek, D.; Lacova, M.; Kral’ova, K.; Gatial, A.; Horvath, B.; Krutosikova, A. Reactions of substituted furo [3,2-b]pyrrole-5-carboxhydrazides and their biological activity. Cent. Eur. J. Chem. 2005, 3, 622–646. [Google Scholar]
- Meyer, S.T.; Webster, J.D.; Young, D.H. Synergistic Algicidal Compositions Including Hydrazone Derivatives and Copper. US Patent 08906829 B2; filed 18 August 2011, and issued 9 December 2014,
- Heytler, P.G.; Prichard, W.W. A new class of uncoupling agents-carbonyl cyanide phenylhydrazones. Biochem. Biophys. Res. Commun. 1962, 7, 272–275. [Google Scholar] [CrossRef]
- Benz, R.; McLaughlin, S.S. The molecular mechanism of action of the proton ionophore FCCP (carbonylcyanide p-trifluoromethoxyphenylhydrazone). Biophys. J. 1983, 41, 381–398. [Google Scholar] [CrossRef]
- Avila, C.M.; Lopes, A.B.; Gonçalves, A.S.; da Silva, L.L.; Romeiro, N.C.; Miranda, A.L.; Sant’Anna, C.M.; Barreiro, E.J.; Fraga, C.A. Structure-based design and biological profile of (E)-N-(4-Nitrobenzylidene)-2-naphthohydrazide, a novel small molecule inhibitor of IκB kinase-β. Eur. J. Med. Chem. 2011, 46, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Leal, C.M.; Pereira, S.L.; Kümmerle, A.E.; Leal, D.M.; Tesch, R.; de Sant’Anna, C.M.; Fraga, C.A.; Barreiro, E.J.; Sudo, R.T.; Zapata-Sudo, G. Antihypertensive profile of 2-thienyl-3,4-methylenedioxybenzoylhydrazone is mediated by activation of the A2A adenosine receptor. Eur. J. Med. Chem. 2012, 55, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Murty, M.S.R.; Ram, K.R.; Rao, R.V.; Yadav, J.S.; Rao, J.V.; Velatooru, L.R. Synthesis of new S-alkylated-3-mercapto-1,2,4-triazole derivatives bearing cyclic amine moiety as potent anticancer agents. Lett. Drug Des. Discov. 2012, 9, 276–281. [Google Scholar] [CrossRef]
- Grimster, N.P.; Connelly, S.; Baranczak, A.; Dong, J.; Krasnova, L. Aromatic sulfonyl fluorides covalently kinetically stabilize transthyretin to prevent amyloidogenesis while affording a fluorescent conjugate. J. Am. Chem. Soc. 2013, 135, 5656–5658. [Google Scholar] [CrossRef] [PubMed]
- Schwenker, G. The reaction of isonicotinic acid hydrazide with 2,4-dinitrochlorobenzene. Arch. Pharm. Ber. Dtsch. Pharm. Ges. 1958, 291, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Defusco, A.A.; Strauss, M.J. Displacement-cyclization reactions of mono-substituted hydrazines with chloronitrobenzenes and chloronitropyrimidines. New routes to 8-azapurine and benzopyrazole derivatives. J. Heterocycl. Chem. 1981, 18, 351–355. [Google Scholar] [CrossRef]
- Karrer, F.; Hall, R.G. Cyanohydrazone Derivatives. US Patent 6417386 B2; filed 13 February 2002, and issued 19 July 2002,
- Hemming, K.; Luheshi, A.B.N.; Redhouse, A.D.; Smaalley, R.K.; Thompson, J.R.; Kennewell, P.D.; Westwood, R. 1,3-Dipolar cycloadditions of 2-Ethoxy- and 2-(ethylthio)-1-azetines with nitrile oxides, nitrile ylides and nitrilimines: An unexpected 1,2,4-triazole formation. Tetrahedron 1993, 49, 4383–4408. [Google Scholar] [CrossRef]
- Huisgen, I.; Grashey, R.; Aufderhaar, E.; Kunz, R.; Siedel, M. 1.3-Dipolare Cycloadditionen, VI. Anlagerung der Nitrilimine an Azomethine und Isocyanate. Chem. Ber. 1964, 97, 1085–1095. [Google Scholar] [CrossRef]
- Polumbrik, O.M.; Ryabokon, I.G.; Matkovskii, L.N. Perfluorophenyl-containing verdazyl radicals. Chem. Heterocycl. Comp. 1980, 16, 882–885. [Google Scholar] [CrossRef]
- Doležal, M.; Miletín, M.; Kuneš, J.; Kráľová, K. Substituted Amides of Pyrazine-2-carboxylic acids: Synthesis and Biological Activity. Molecules 2002, 7, 363–373. [Google Scholar] [CrossRef]
- Gonec, T.; Kos, J.; Zadrazilova, I.; Pesko, M.; Govender, R.; Keltosova, S.; Chambel, B.; Pereira, D.; Kollar, P.; Imramovsky, A.; et al. Antibacterial and herbicidal activity of ring-aubstituted 2-hydroxynaphthalene-1-carboxanilides. Molecules 2013, 18, 9397–9419. [Google Scholar] [CrossRef] [PubMed]
- Otevrel, J.; Bobal, P.; Zadrazilova, I.; Govender, R.; Pesko, M.; Keltosova, S.; Koleckarova, P.; Marsalek, P.; Imramovsky, A.; Coffey, A.; et al. Antimycobacterial and photosynthetic electron transport inhibiting activity of ring-substituted 4-arylamino-7-chloroquinolinium chlorides. Molecules 2013, 18, 10648–10670. [Google Scholar] [CrossRef] [PubMed]
- Gonec, T.; Kos, J.; Zadrazilova, I.; Pesko, P.; Keltosova, K.; Tengler, J.; Bobal, P.; Kollar, P.; Cizek, A.; Kralova, K.; et al. Antimycobacterial and herbicidal activity of ring-substituted 1-hydroxynaphthalene-2-carboxanilides. Bioorg. Med. Chem. 2013, 21, 6531–6541. [Google Scholar] [CrossRef] [PubMed]
- Jandourek, O.; Dolezal, M.; Paterova, P.; Kubicek, V.; Pesko, M.; Kunes, J.; Coffey, A.; Guo, J.; Kralova, K. N-Substituted 5-amino-6-methylpyrazine-2,3-dicarbonitriles: Microwave-assisted synthesis and biological properties. Molecules 2014, 19, 651–671. [Google Scholar] [CrossRef] [PubMed]
- Fedtke, C. Biochemistry and Physiology of Herbicide Action; Springer Verlag: Berlin-Heidelberg, Germany; New York, NY, USA, 1982. [Google Scholar]
- Govindjee, E. Sixty-three years since Kautsky: Chlorophyll a fluorescence. Aust. J. Plant Physiol. 1995, 22, 131–160. [Google Scholar] [CrossRef]
- Debus, R.J.; Barry, B.A.; Babcock, G.T.; McIntosh, L. Site-directed mutagenesis identifies a tyrosine radical involved in the photosynthetic oxygen-evolving system. Proc. Natl. Acad. Sci. USA 1988, 85, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Debus, R.J.; Barry, B.A.; Sithole, I.; Babcock, G.T.; McIntosh, L. Directed mutagenesis indicates that the donor to P+680 in photosystem II is tyrosine-161 of the D1 polypeptide. Biochemistry 1988, 27, 9071–9074. [Google Scholar] [CrossRef] [PubMed]
- Hoff, A.J. Application of ESR in photosynthesis. Phys. Rep. 1979, 54, 75–200. [Google Scholar] [CrossRef]
- Pavlíková, S.; Šeršeň, F.; Jesenák, K.; Gaplovska, K.; Čík, G. Efficacy of modified natural zeolites in the protection against the damaging effect of 4-chlorophenol on algal growth. Fresenius Environ. Bull. 2010, 19, 3055–3058. [Google Scholar]
- Šeršeň, F.; Balgavý, P.; Devínsky, F. Electron spin resonance study of chloroplast photosynthetic activity in the presence of amphiphilic amines. Gen. Physiol. Biophys. 1990, 9, 625–633. [Google Scholar] [PubMed]
- Šeršeň, F.; Kráľová, K.; Macho, V. New findings about the inhibitory action of phenylcarbamates and phenylthiocarbamates on photosynthetic apparatus. Pestic. Biochem. Physiol. 2000, 68, 113–118. [Google Scholar] [CrossRef]
- Šeršeň, F.; Kráľová, K.; Peško, M.; Cigáň, M. Effect of Pb2+ ions on photosynthetic apparatus. Gen. Physiol. Biophys. 2014, 33, 131–136. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šeršeň, F.; Gregáň, F.; Peško, M.; Dvoranová, D.; Kráľová, K.; Matkovičová, Z.; Gregáň, J.; Donovalová, J. Synthesis and Herbicidal Activity of New Hydrazide and Hydrazonoyl Derivatives. Molecules 2015, 20, 14139-14154. https://doi.org/10.3390/molecules200814139
Šeršeň F, Gregáň F, Peško M, Dvoranová D, Kráľová K, Matkovičová Z, Gregáň J, Donovalová J. Synthesis and Herbicidal Activity of New Hydrazide and Hydrazonoyl Derivatives. Molecules. 2015; 20(8):14139-14154. https://doi.org/10.3390/molecules200814139
Chicago/Turabian StyleŠeršeň, František, Fridrich Gregáň, Matúš Peško, Dana Dvoranová, Katarína Kráľová, Zuzana Matkovičová, Juraj Gregáň, and Jana Donovalová. 2015. "Synthesis and Herbicidal Activity of New Hydrazide and Hydrazonoyl Derivatives" Molecules 20, no. 8: 14139-14154. https://doi.org/10.3390/molecules200814139
APA StyleŠeršeň, F., Gregáň, F., Peško, M., Dvoranová, D., Kráľová, K., Matkovičová, Z., Gregáň, J., & Donovalová, J. (2015). Synthesis and Herbicidal Activity of New Hydrazide and Hydrazonoyl Derivatives. Molecules, 20(8), 14139-14154. https://doi.org/10.3390/molecules200814139






