New Polymorphic Forms of Pemetrexed Diacid and Their Use for the Preparation of Pharmaceutically Pure Amorphous and Hemipentahydrate Forms of Pemetrexed Disodium
Abstract
:1. Introduction
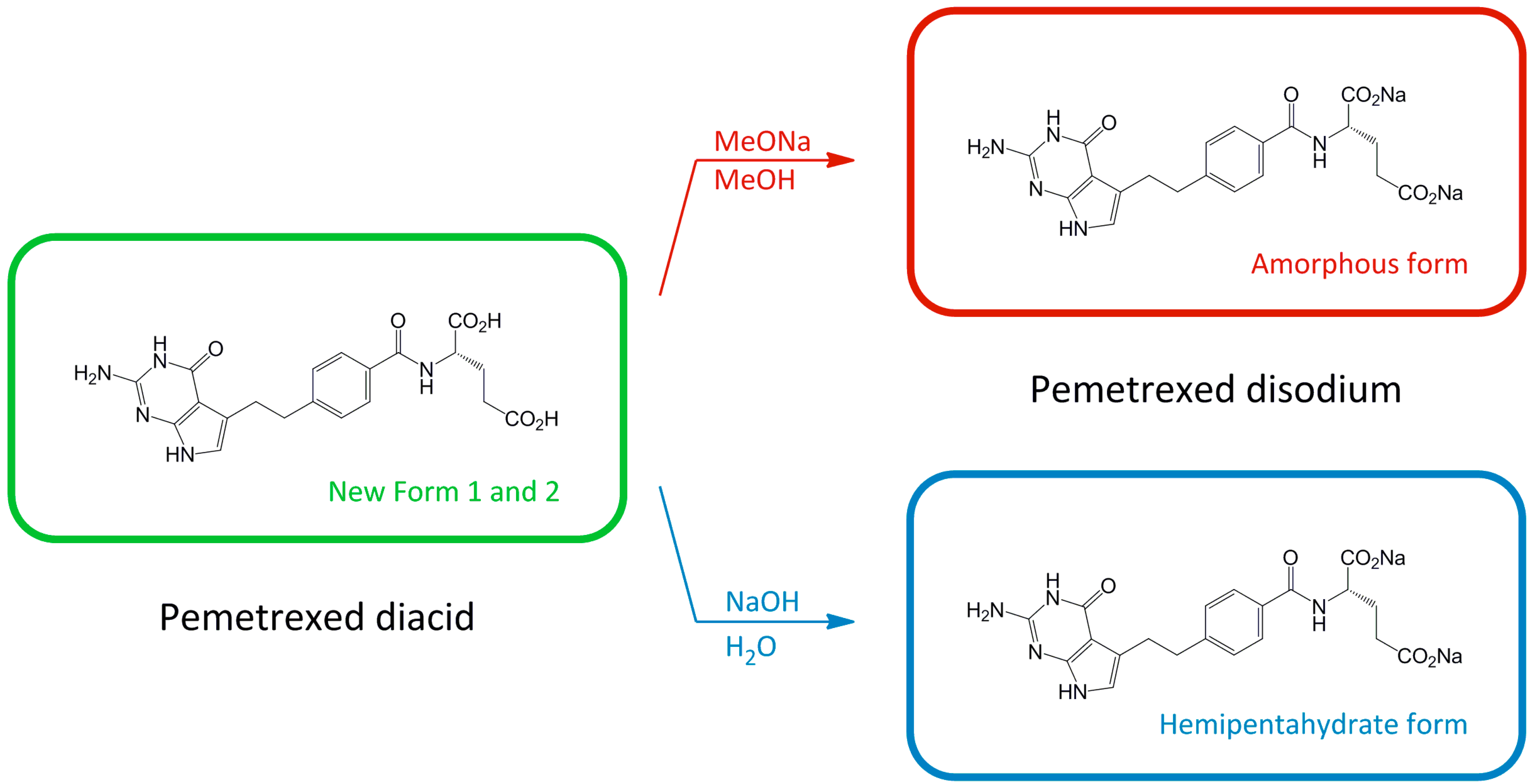
2. Results and Discussion
2.1. Characterization of Pemetrexed Diacid Forms 1 and 2
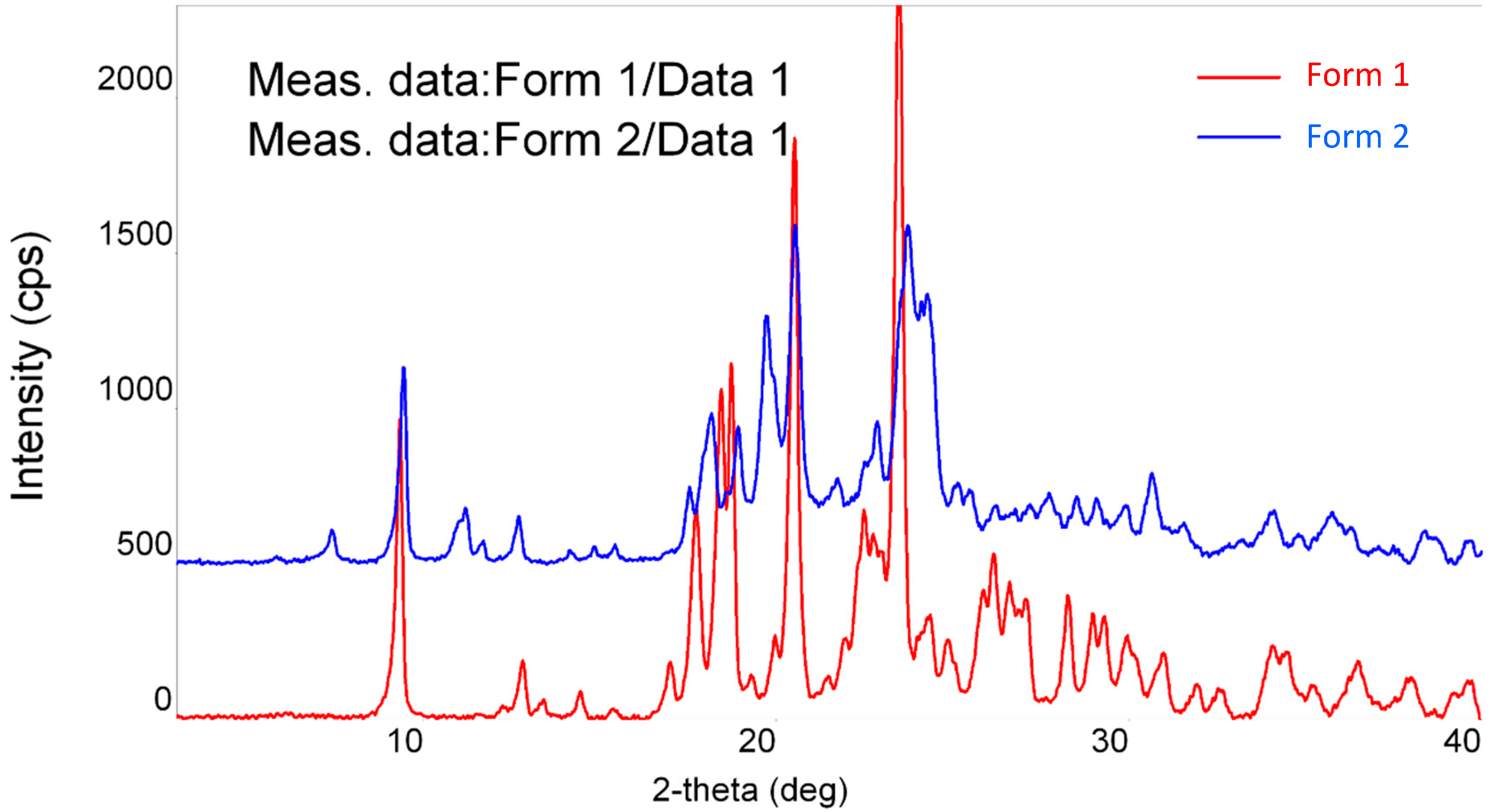
2.1.1. Powder X-ray Diffraction
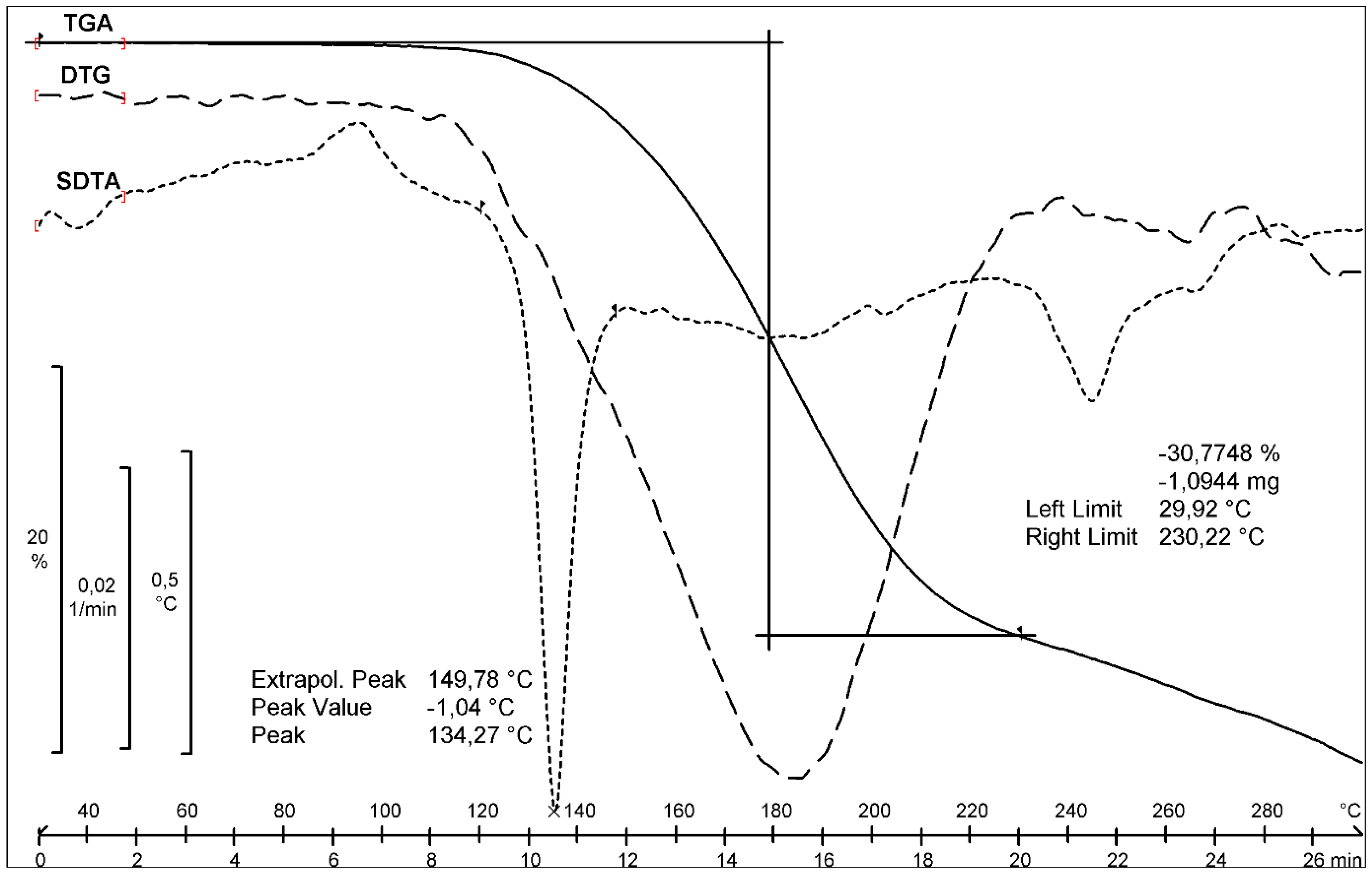
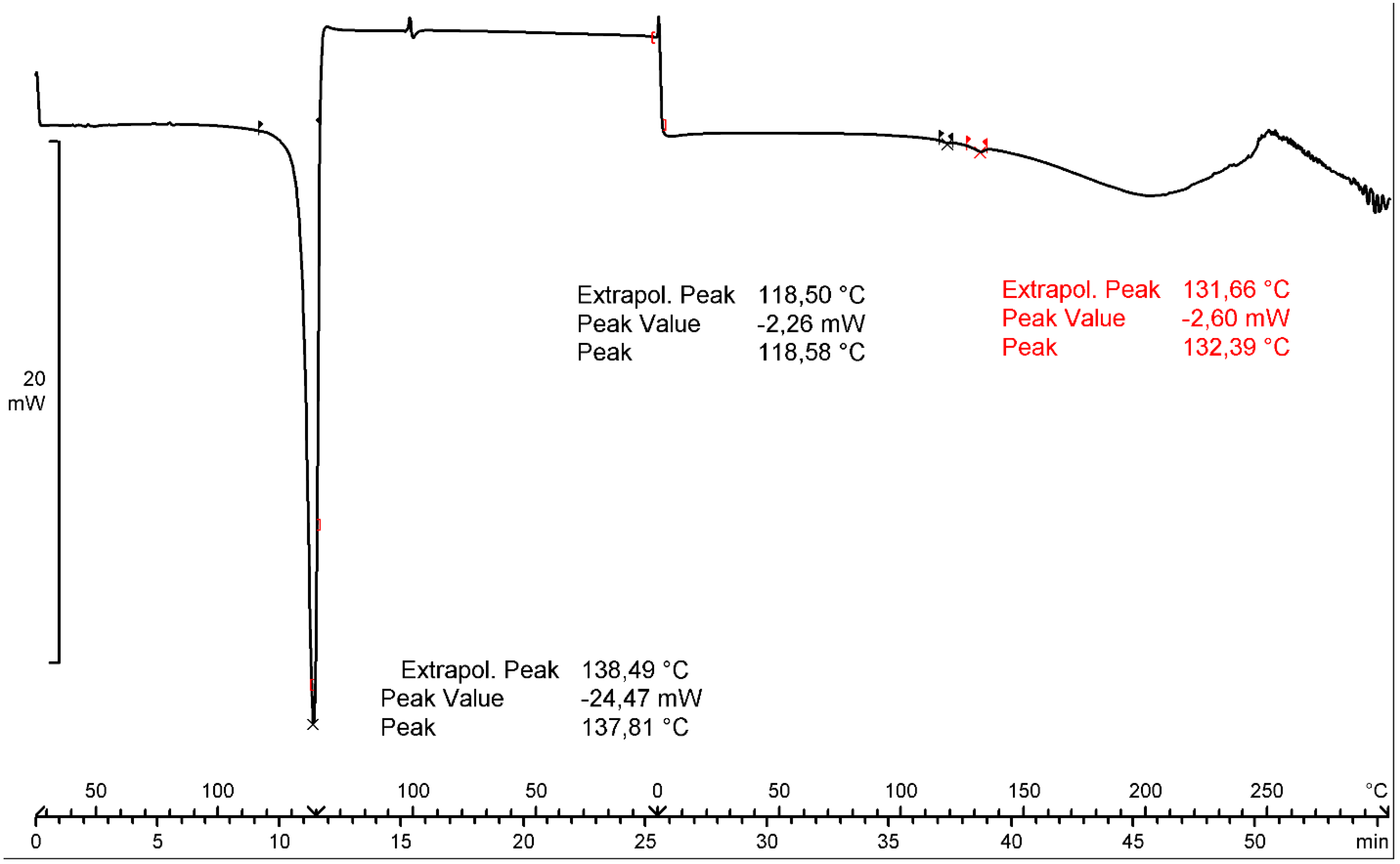
2.1.2. Thermal Analysis and Degradation Studies

| Sample | Impurities | Relative Retention Time | Area, [%] | Sum of Impurities, [%] |
|---|---|---|---|---|
| Profile | ≥0.05% | |||
| initial sample | Imp. 1 | 0.08 | 0.15 | 0.62 |
| Imp. 2 | 1.25 | 0.12 | ||
| heating 1 h/140 °C | Imp. 1 | 0.08 | 0.33 | 1.10 |
| Imp. 3 | 0.09 | 0.06 | ||
| Imp. 4 | 1.02 | 0.05 | ||
| Imp. 5 | 1.21 | 0.06 | ||
| Imp. 2 | 1.24 | 0.13 | ||
| heating 1 h/150 °C | Imp. 1 | 0.08 | 0.29 | 1.24 |
| Imp. 3 | 0.09 | 0.05 | ||
| Imp. 5 | 1.21 | 0.09 | ||
| Imp. 2 | 1.23 | 0.06 | ||
| Imp. 6 | 1.25 | 0.15 |
2.2. Characterization of Pemetrexed Disodium Forms and Their Interconversion Relationship
2.2.1. Powder X-ray Diffraction
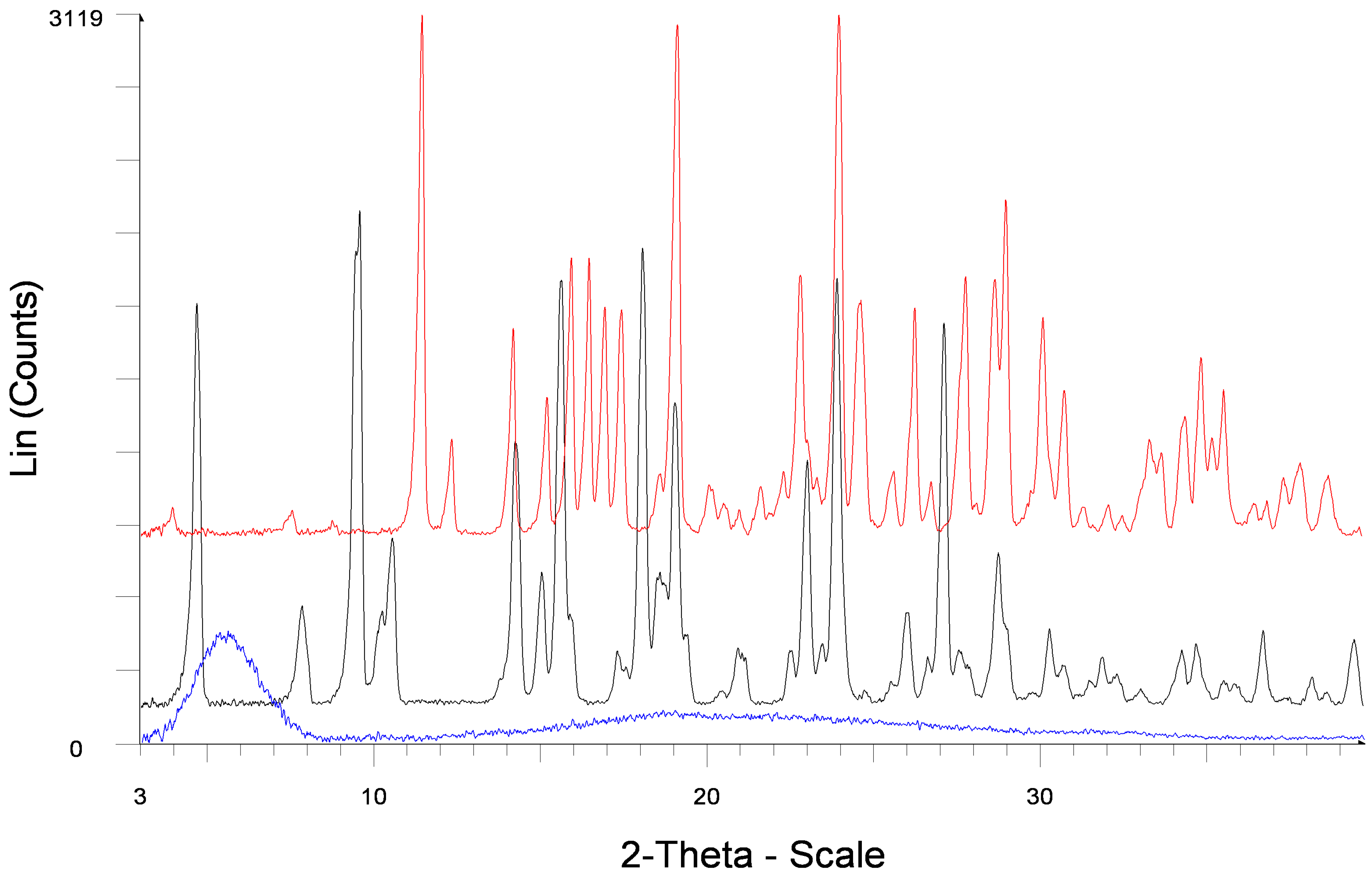
2.2.2. Thermal Analysis
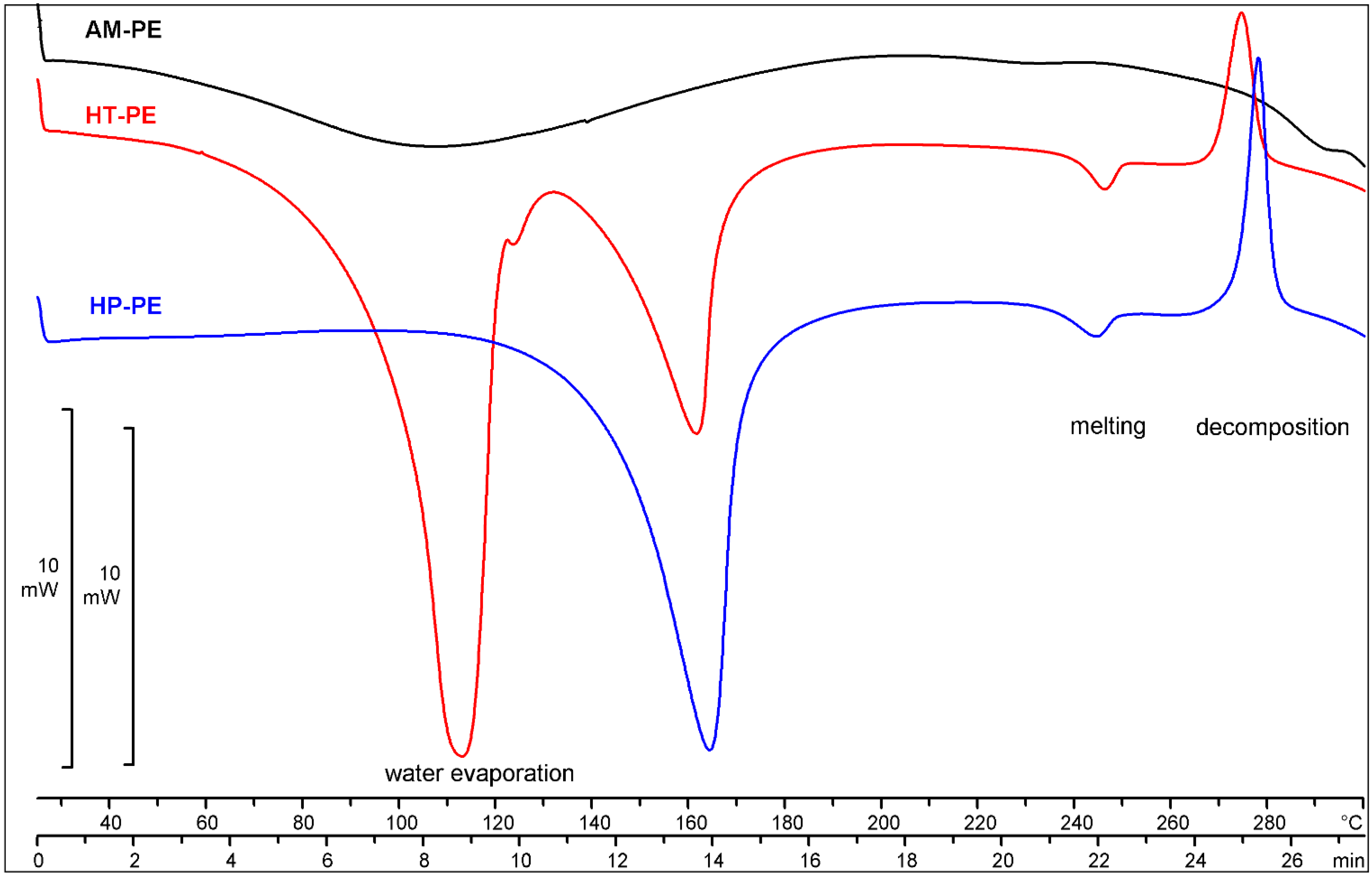
| Sample | Increase in Mass according Ph. Eur., [%] | Water Content, [%] | Mass Loss |
|---|---|---|---|
| TGA, [%] | |||
| AM-PE, initial | -- | 6.5 | 7.8 * |
| AM-PE, 24 h 25 °C 80% RH | 17.4 | 18.5 | 18.5 |
| HP-PE, inital | -- | 9.0 | 9.1 |
| HP-PE, 24 h 25 °C 80% RH | 21.1 | 21.3 | 21.3 |
| Sample | Impurities | Relative Retention Time | Area, [%] | Sum of Impurities, [%] |
|---|---|---|---|---|
| Profile | ≥0.05% | |||
| AM-PE, initial | unknown | 1.1 | 0.06 | 0.34 |
| AM-PE, 24 h 25 °C 80% RH | unknown | 1.1 | 0.05 | 0.39 |
| HP-PE, initial | -- | -- | -- | 0.15 |
| HP-PE, 24 h 25 °C 80% RH | -- | -- | -- | 0.22 |
3. Experimental Section
3.1. Materials
3.1.1. Preparation of Pemetrexed Diacid Form 1
3.1.2. Preparation of Pemetrexed Diacid Form 2
3.1.3. Crystallization of Pemetrexed Diacid Forms 1 and 2
3.1.4. Preparation of Amorphous Pemetrexed Disodium
3.1.5. Preparation of Hemipentahydrate Pemetrexed Disodium
3.2. Methods
3.2.1. Powder X-ray Diffraction
3.2.2. Differential Scanning Calorimetry
3.2.3. Thermogravimetry
3.2.4. Water Determination
3.2.5. High Performance Liquid Chromatography
3.2.6. Gas Chromatography Methods (a Detailed Description is Provided in the Supporting Information)
3.2.7. Hygroscopicity Test
4. Conclusions
Supplementary Materials
Acknowledgments
Associated Content
Author Contributions
Conflicts of Interest
References and Notes
- Fukuoka, E.; Makita, M.; Nakamura, Y. Glassy State of Pharmaceuticals. 5. Relaxation During Cooling and Heating of Glass by Differential Scanning Calorimetry. Chem. Pharm. Bull. 1991, 39, 2087–2090. [Google Scholar] [CrossRef]
- Sidoryk, K.; Malińska, M.; Bańkowski, K.; Kubiszewski, M.; Łaszcz, M.; Bodziachowska-Panfil, M.; Kossykowska, M.; Giller, T.; Kutner, A.; Woźniak, K. Physicochemical characteristics of sunitinib malate and its process-related impurities. J. Pharm. Sci. 2013, 102, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Byrn, S.R.; Pfeiffer, R.R.; Stowell, J.G. Solid-State Chemistry of Drugs, 2nd ed.; SSCI, Inc.: West Lafayette, Indiana, 1999; pp. 259–366. [Google Scholar]
- Hilfiker, R. (Ed.) Polymorphism in the Pharmaceutical Industry, 1st ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006.
- ICH Topic Q 6 A. Specifications: Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products: Chemical Substances; European Medicines Agency: London, UK, 2000.
- FDA. Guidance for Industry, ANDAs: Pharmaceutical Solid Polymorphism. Chemistry, Manufacturing and Controls Information; U.S. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER): Rockville, MD, USA, 2007.
- Antineoplastics and immunosuppressants. In Martindale—The Complete Drug Reference, 13th ed.; Sweetman, S.C. (Ed.) The Pharmaceutical Press: London, UK, 2002; p. 564.
- O’Neil, M.J. (Ed.) The Merck Index—An Encyclopedia of Chemicals, Drugs and Biologicals, 14th ed.; Merck Research Laboratories: Whitehouse Station, NJ, USA, 2006; p. 7079.
- Novak, K.M. (Ed.) Drug Facts and Comparisons, 59th ed.; Wolters Kluwer Health: St. Louis, MO, USA, 2005; p. 2283.
- Cohen, M.H.; Justice, R.; Pazdur, R. Approval summary: Pemetrexed in the initial treatment of advanced/metastatic non-small cell lung cancer. Oncologist 2009, 14, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Pemetrexed disodium heptahydrate. In European Pharmacopoeia; EDQM, Council of Europe: Strasbourg, France, 2013; Volume 8, p. 2637.
- Chelius, E.C.; Reutzel-Edens, S.M.; Van den Berghe Snorek, S. A Novel Crystalline Form of N-[4-[2-(2-amino-4,7-dihydro-4-oxo-3H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]-l-glutamic Acid and Process Therefor. Int. Patent Appl. WO 01/62760 A2, 30 August 2001. [Google Scholar]
- Palle, R.V.; Nariyam, S.M.; Patel, V.B.; Vinjamuri, R.R.S.; Devarakonda, S.N.; Yarraguntla, S.R.; Mudapaka, V.K.; Nalivela, V. Solid Forms of Pemetrexed. Int. Patent Appl. WO 2008/124485 A2, 16 October 2008. [Google Scholar]
- Li, J.; Cai, Z.; Wang, M. Amorphous Polymorph for Pemetrexed Disodium and Preparation Method Thereof. CN Pat. Appl. No. 101575338, 11 November 2009. [Google Scholar]
- Patel, N.S.; Kilaru, S.; Thennati, R. Stable Amorphous Form of Pemextred Disodium. Europ. Pat. Appl. No. 2072518 A1, 24 June 2009. [Google Scholar]
- Patel, N.S.; Kilaru, S.; Thennati, R. Stable Amorphous Form of Pemetrexed Disodium. US Pat. Appl. No. 2009/181990 A1, 16 July 2009. [Google Scholar]
- Kadaboina, R.; Nariyam, S.M.; Ramakrishnan, S.; Peddireddy, S.; Baig, M.A.; Duggirala, N. Amorphous Pemetrexed Disodium. Int. Patent Appl. WO 2010/028105, 11 March 2010. [Google Scholar]
- Palle, R.V.; Nariyam, S.M.; Patel, V.B.; Vinjamuri, R.R.S.; Devarakonda, S.N.; Yarraguntla, S.R.; Mudapaka, V.K.; Nalivela, V. Solid Forms of Pemetrexed. US Pat. Appl. No. 2010/063072 A1, 11 March 2010. [Google Scholar]
- European Medicines Agency. Protelos: EPAR—Scientific Discussion. 2004. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_Scientific_Discussion/human/000564/WC500025606.pdf (accessed on 20 September 2004).
- Good Manufacturing Practice in Pharmaceutical Industry. Available online: http://www.pharmacistspharmajournal.org/2011/06/good-manufacturing-practice-in.html (accessed on 16 July 2011).
- Michalak, O.; Jatczak, K.; Pucko, W.; Witkowska, A.; Bujak, I.; Łaszcz, M.; Trzcińska, K.; Kościuch, M.; Zagrodzka, J.; Kutner, A. The Preparation Process Development of an Amorphous Form of Pharmaceutically Pure Pemetrexed Disodium. PL Pat. Appl. No. 403942, 17 May 2013. [Google Scholar]
- Michalak, O.; Jatczak, K.; Pucko, W.; Witkowska, A.; Łaszcz, M.; Bujak, I.; Groman, A.; Cybulski, M. Process for the Preparation of High Purity Amorphous Pemetrexed Disodium and Crystalline Forms of N-[4-[2-(2-Amino-4,7-dihydro-4-oxo-3H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]-l-glutamic Acid. Int. Patent Appl. WO 2014/185797 A1, 20 November 2014. [Google Scholar]
- Busolli, J.; Diulgheroff, N.; Nemethne, R.C.; Pirkes, M.; Pontiroli, A.; Villa, M.; Aronhime, J. Crystalline Forms of Pemetrexed Diacid and Processes for the Preparation Thereof. Int. Patent Appl. WO 2008/021405 A1, 21 February 2008. [Google Scholar]
- Luo, J.; Lin, M.; Zhu, Z.; Luo, J.; Ye, W.; Qin, Y.; Deng, J. New Crystalline Forms of Pemetrexed Diacid, and Preparations Thereof. Int. Patent Appl. WO 2010/031357 A1, 25 March 2010. [Google Scholar]
- Characters section in monographs. In European Pharmacopoeia, 8th ed.; EDQM, Council of Europe: Strasbourg, France, 2013; Chapter 5.11.
- Sample Availability: Samples of the compounds AM-PE and HP-PE are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalak, O.; Łaszcz, M.; Jatczak, K.; Witkowska, A.; Bujak, I.; Groman, A.; Cybulski, M. New Polymorphic Forms of Pemetrexed Diacid and Their Use for the Preparation of Pharmaceutically Pure Amorphous and Hemipentahydrate Forms of Pemetrexed Disodium. Molecules 2015, 20, 13814-13829. https://doi.org/10.3390/molecules200813814
Michalak O, Łaszcz M, Jatczak K, Witkowska A, Bujak I, Groman A, Cybulski M. New Polymorphic Forms of Pemetrexed Diacid and Their Use for the Preparation of Pharmaceutically Pure Amorphous and Hemipentahydrate Forms of Pemetrexed Disodium. Molecules. 2015; 20(8):13814-13829. https://doi.org/10.3390/molecules200813814
Chicago/Turabian StyleMichalak, Olga, Marta Łaszcz, Kamil Jatczak, Anna Witkowska, Iwona Bujak, Aleksandra Groman, and Marcin Cybulski. 2015. "New Polymorphic Forms of Pemetrexed Diacid and Their Use for the Preparation of Pharmaceutically Pure Amorphous and Hemipentahydrate Forms of Pemetrexed Disodium" Molecules 20, no. 8: 13814-13829. https://doi.org/10.3390/molecules200813814
APA StyleMichalak, O., Łaszcz, M., Jatczak, K., Witkowska, A., Bujak, I., Groman, A., & Cybulski, M. (2015). New Polymorphic Forms of Pemetrexed Diacid and Their Use for the Preparation of Pharmaceutically Pure Amorphous and Hemipentahydrate Forms of Pemetrexed Disodium. Molecules, 20(8), 13814-13829. https://doi.org/10.3390/molecules200813814






