Substituent Effects on the Stability and Antioxidant Activity of Spirodiazaselenuranes
Abstract
:1. Introduction
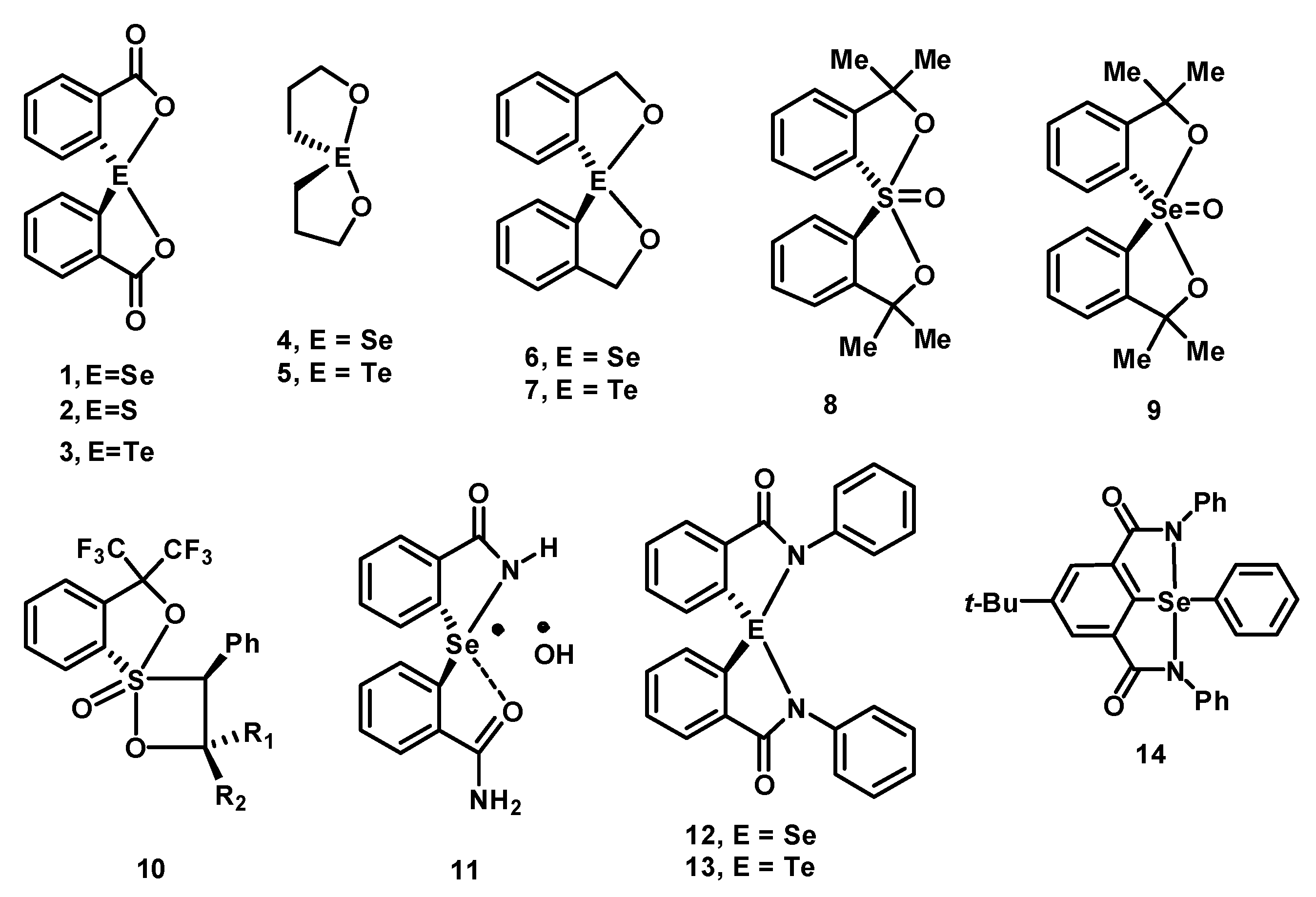
2. Results and Discussion
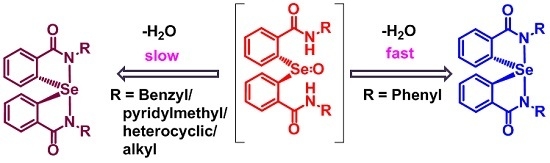
2.1. Synthesis of Spirodiazaselenuranes
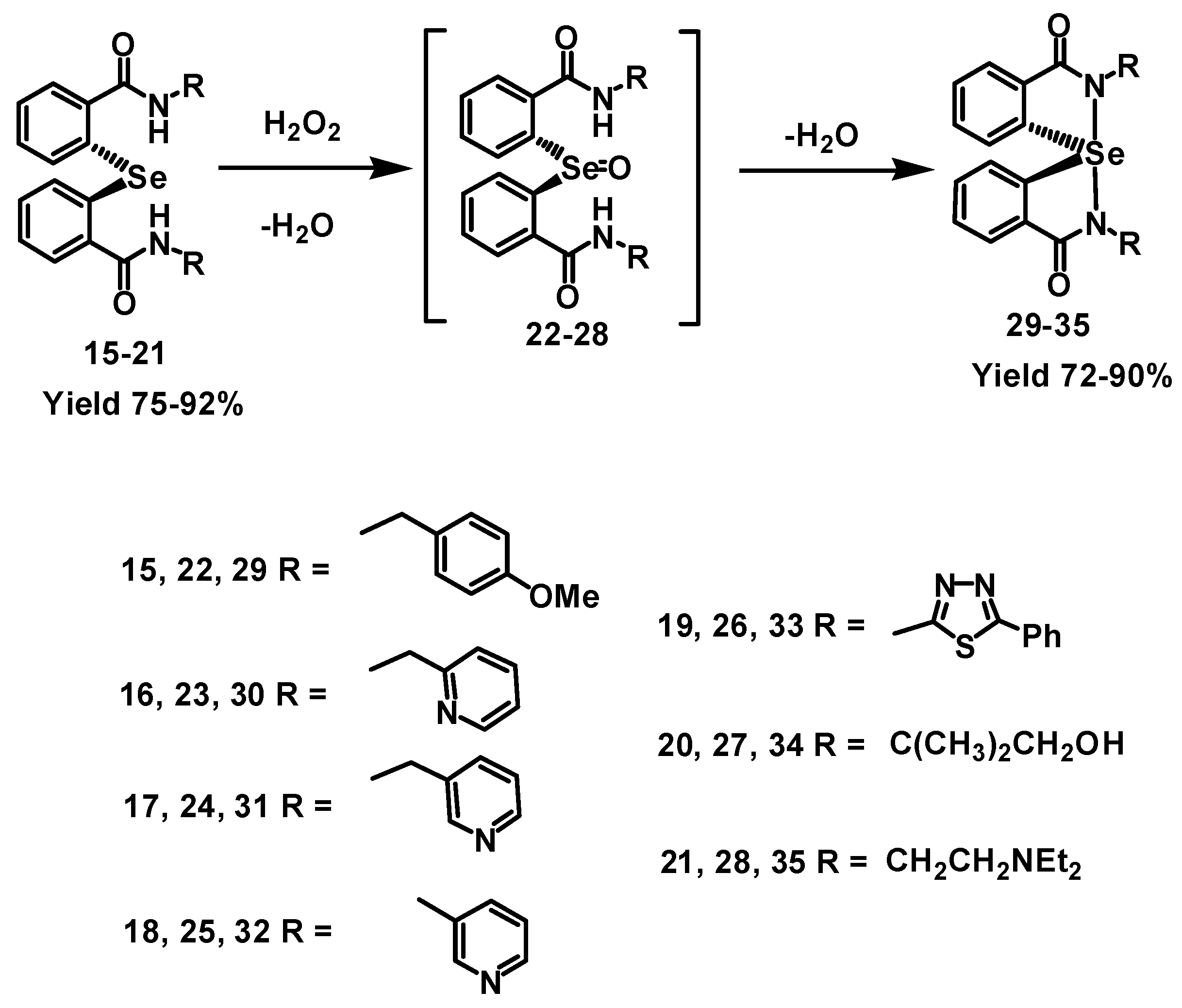
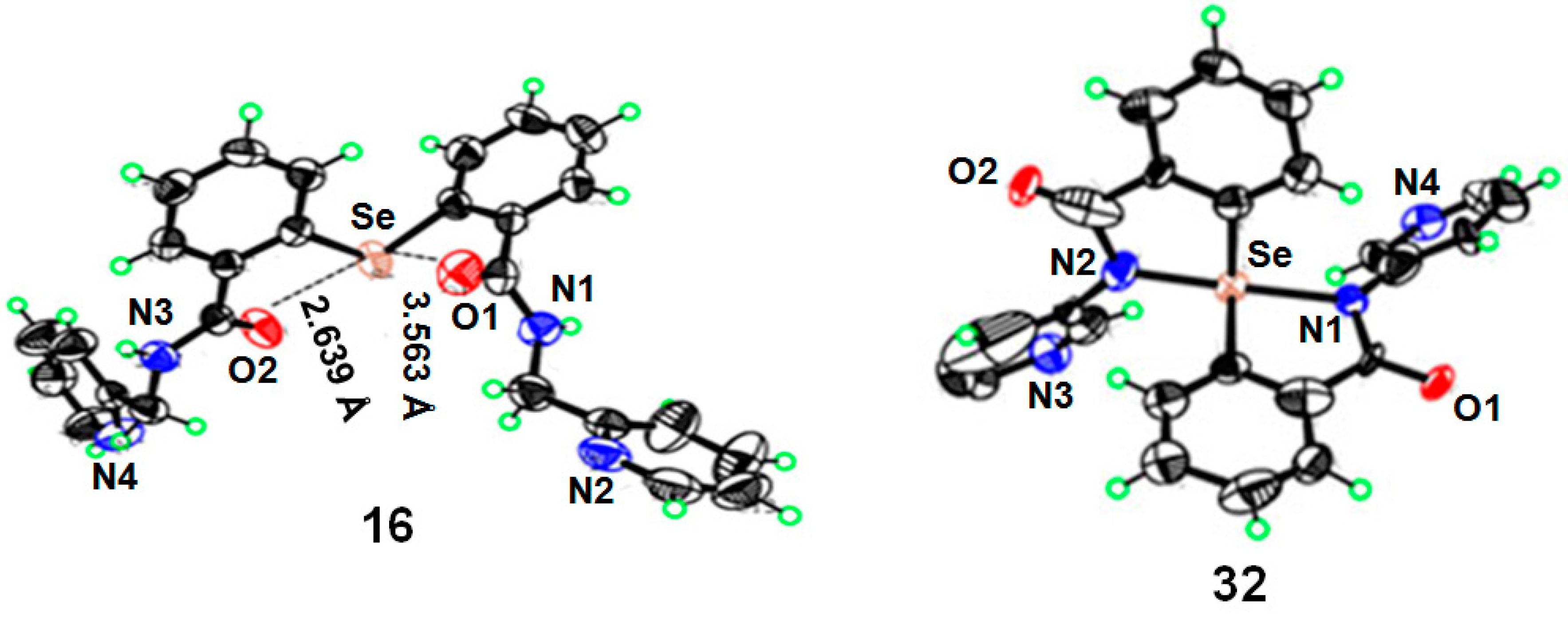
2.2. Mechanism of Cyclization
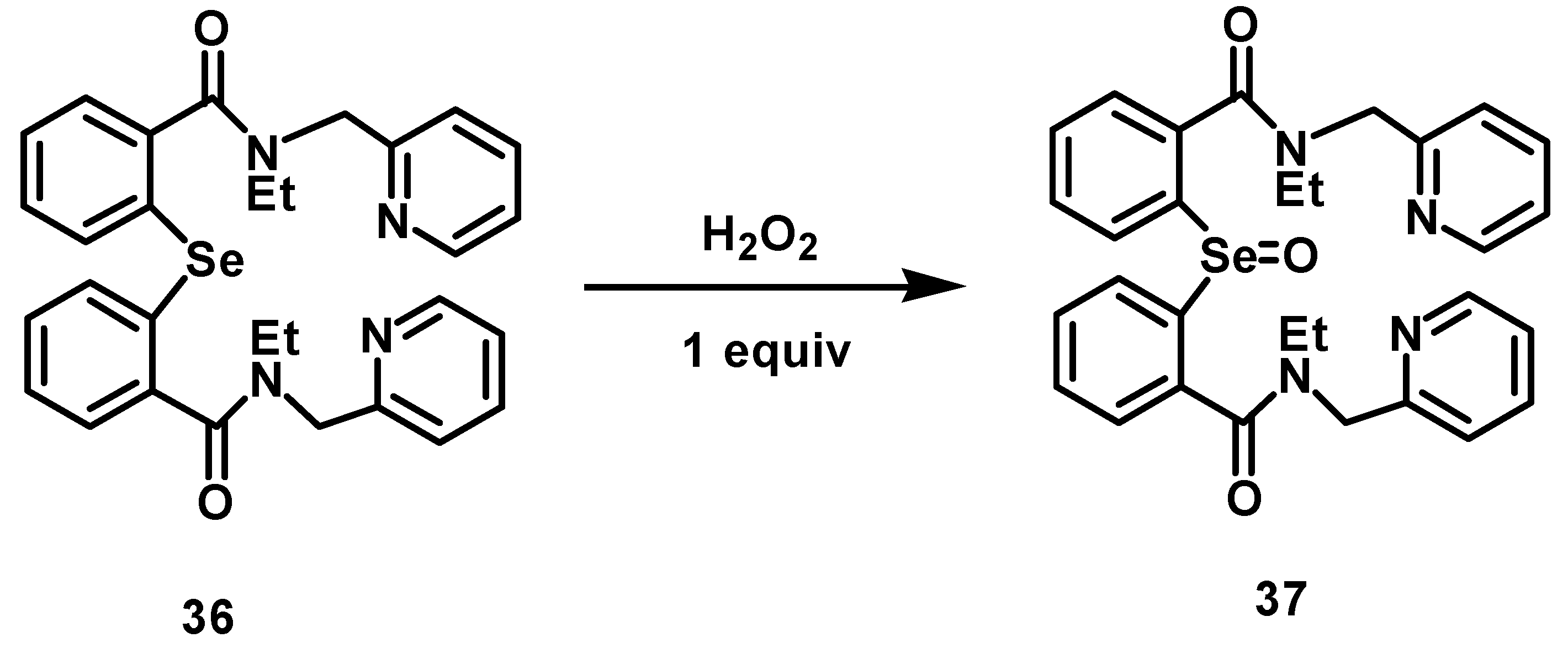
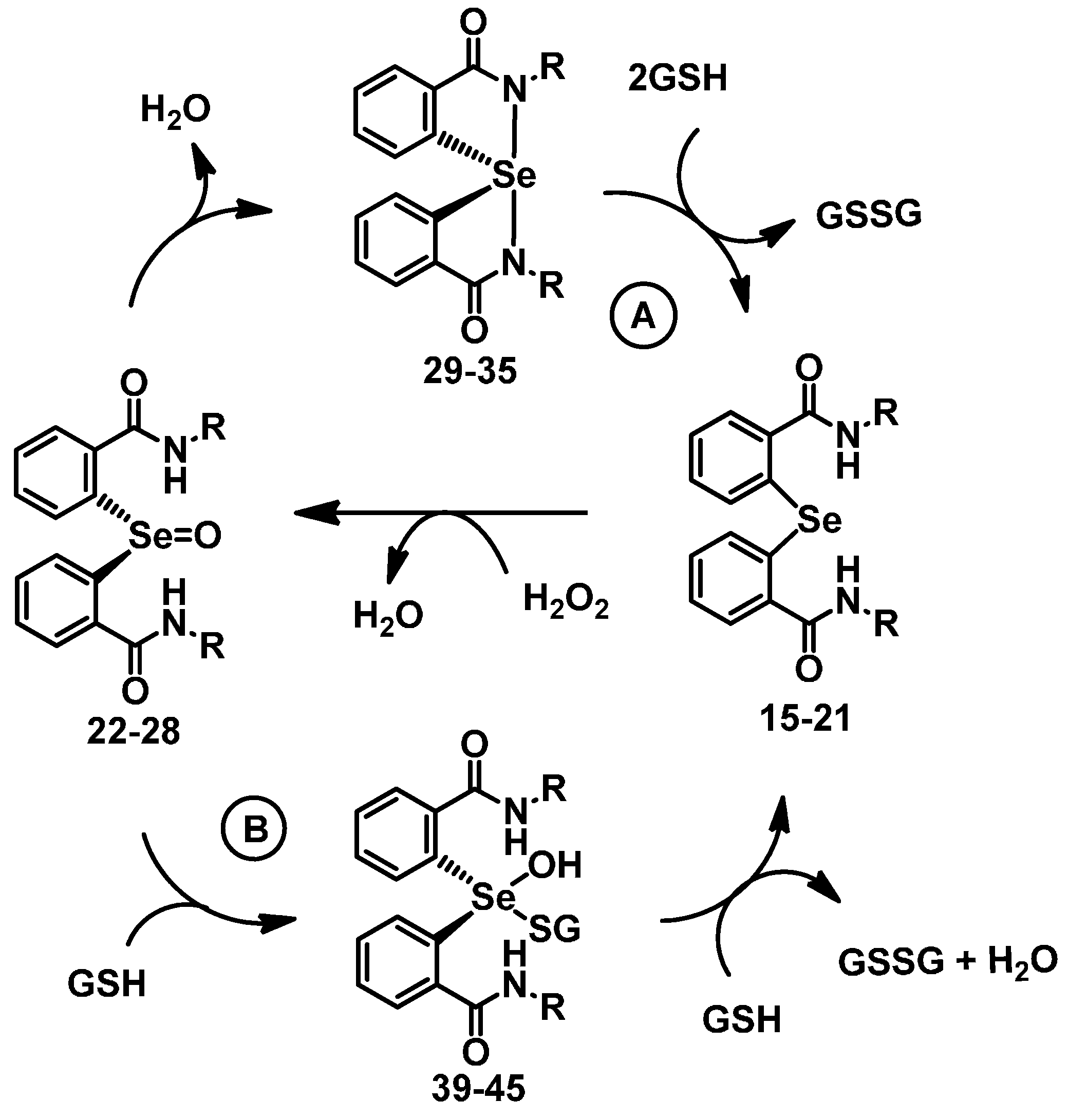
2.3. Glutathione Peroxidase (GPx)-Like Activity
| Compd | v0 (μM·min−1) [a] | Compd | v0 (μM·min−1) [a] |
|---|---|---|---|
| Ebselen | 98.0 ± 3.13 | 12 | 35.6 ± 1.25 |
| 15 | 37.3 ± 1.77 | 299 | 51.8 ± 2.91 |
| 16 | 65.7 ± 2.06 | 30 | 77.5 ± 3.99 |
| 17 | 74.1 ± 2.48 | 31 | 79.4 ± 2.87 |
| 18 | 52.8 ± 1.81 | 32 | 58.7 ± 0.95 |
| 19 | 42.3 ± 2.14 | 33 | 50.2 ± 1.80 |
| 20 | 54.8 ± 0.76 | 34 | 56.2 ± 1.53 |
| 21 | 50.7 ± 0.79 | 35 | 32.8 ± 0.65 |
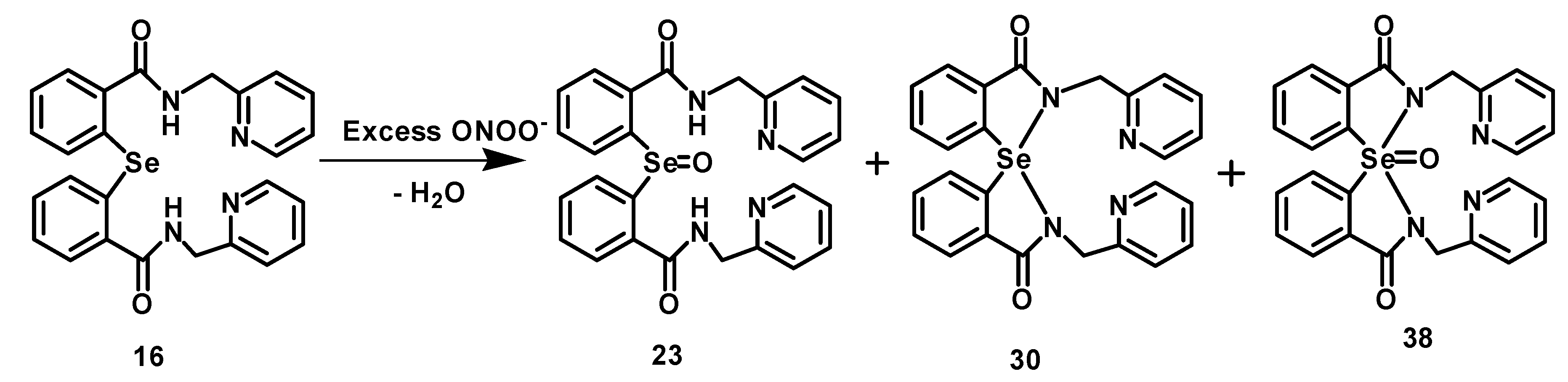
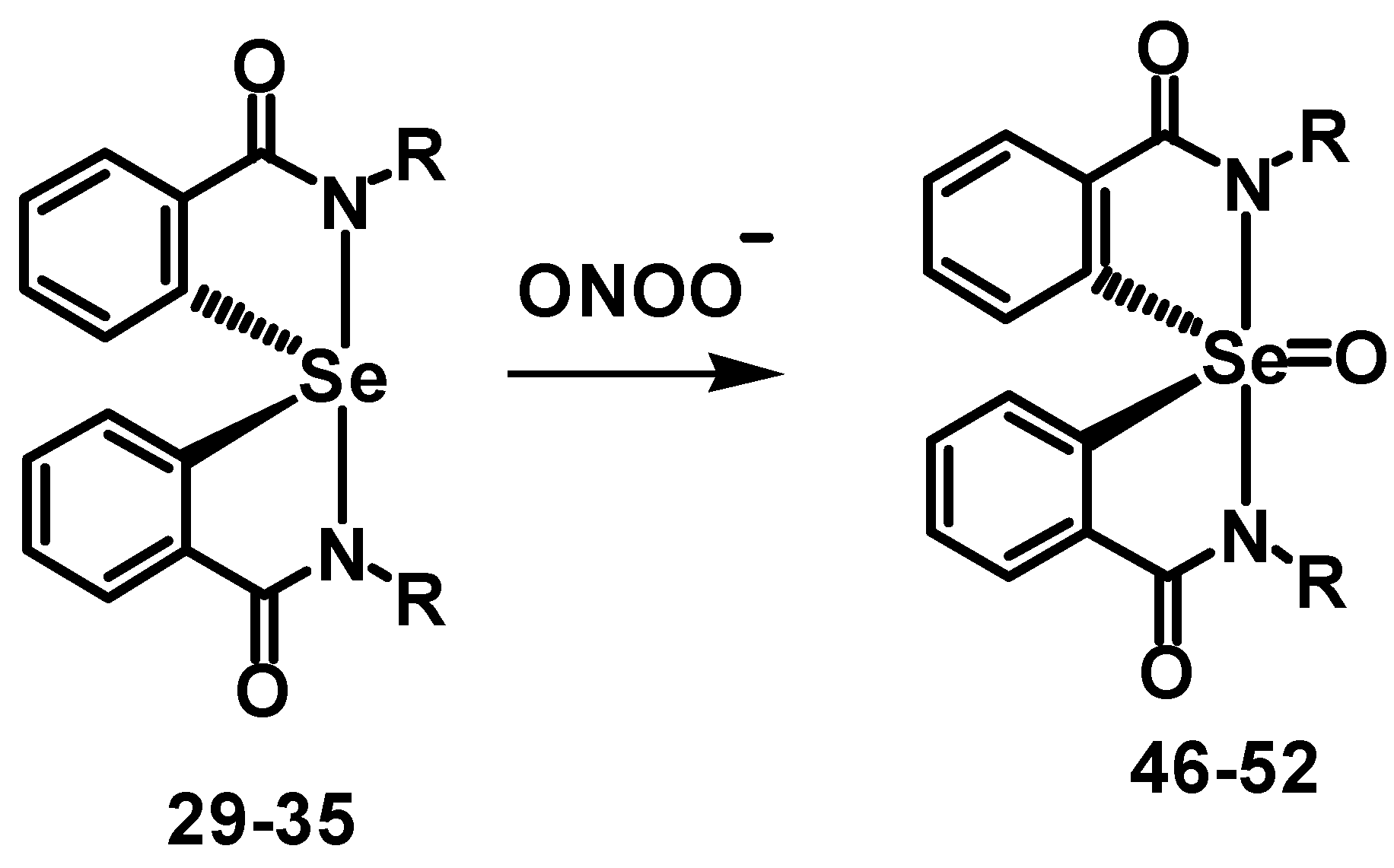
2.4. Inhibition of Peroxynitrite-Mediated Nitration and Oxidation

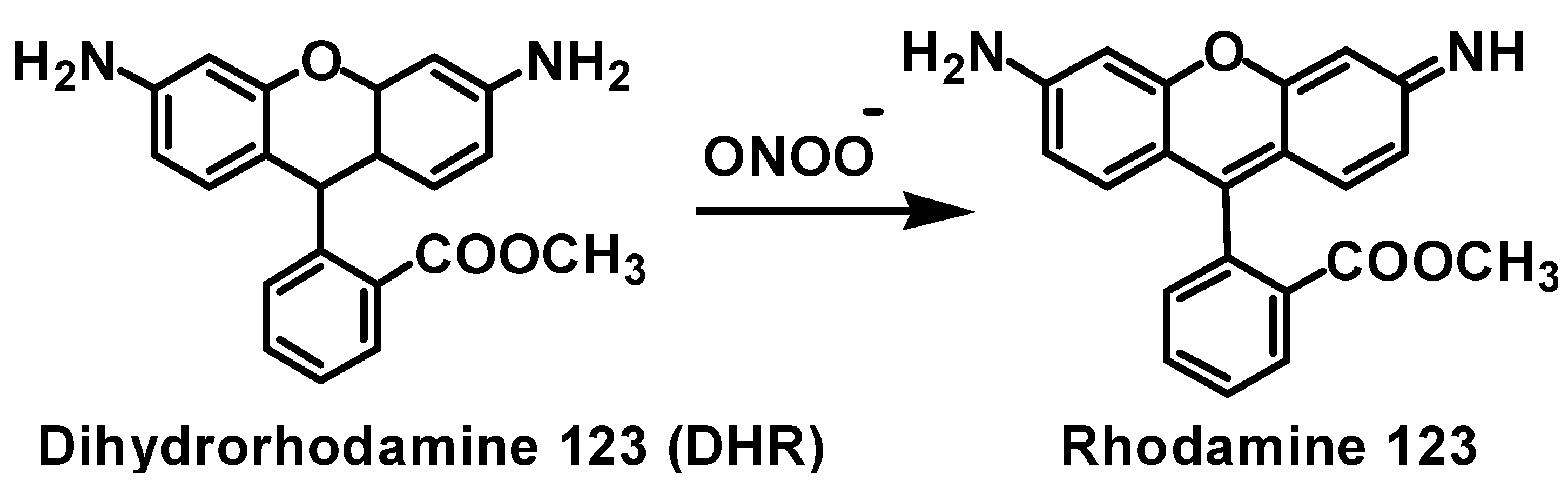
| Compd | IC50 µM [a] | Compd | IC50 µM [a] |
|---|---|---|---|
| ebselen | 0.92 ± 0.04 | 12 | 72.56 ± 1.28 |
| 15 | 8.83 ± 0.39 | 29 | 12.83 ± 0.49 |
| 16 | 5.55 ± 0.39 | 30 | 20.84 ± 0.70 |
| 17 | 4.23 ± 0.27 | 31 | 34.81 ± 0.15 |
| 18 | 2.50 ± 0.18 | 32 | 33.59 ± 0.30 |
| 19 | 18.33 ± 0.23 | 33 | 29.79 ± 0.16 |
| 20 | 16.37 ± 0.07 | 34 | 23.83 ± 0.96 |
| 21 | 21.30 ± 0.17 | 35 | 39.59 ± 0.26 |
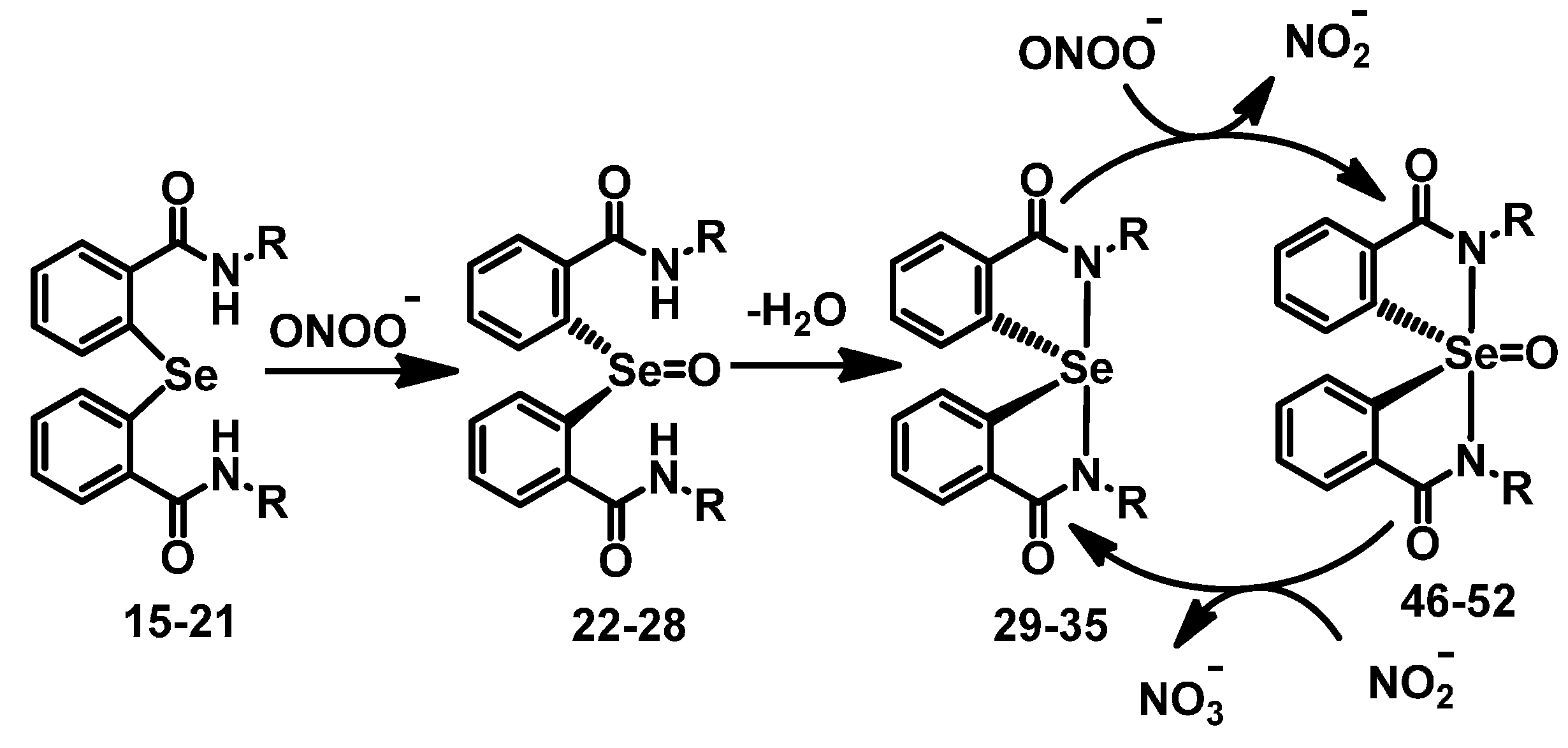
3. Experimental Section
3.1. General Procedures
3.2. GSH–GSSG Coupled Assay
3.3. Peroxynitrite-Mediated Oxidation Assay
3.4. Inhibition of PN-Mediated Nitration of BSA
3.5. Electrophoretic Analysis
3.6. X-ray Crystallography
3.7. General Synthesis of Selenides
3.8. General Synthesis of Spirodiazaselenuranes
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Martin, J.C.; Perozzi, E.F. Isolable Oxysulfuranes in Organic Chemistry. Science 1976, 191, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Drabowicz, J.; Lyzwa, P.; Mikolajczyk, M. High-Coordinated Sulfur Compounds; Patai, S., Rappoport, Z., Eds.; Wiley: New York, NY, USA, 1993; pp. 799–956. [Google Scholar]
- Drabowicz, J. HyperValent Sulfuranes as Transient and Isolable Structures: Occurrence, Synthesis and ReactiVity; Akiba, K.Y., Ed.; Wiley: New York, NY, USA, 1999; pp. 211–240. [Google Scholar]
- Furukawa, N.; Sato, S. New aspects of hypervalent organosulfur compounds. Top. Curr. Chem. 1999, 205, 89–129. [Google Scholar]
- Lesser, R.; Weiss, R. Über Selenoxanthon und Selenoxanthon-carbonsäure. Über selenhaltige aromatische Verbindungen V. Ber. Dtsch. Chem. Ges. 1914, 47, 2510–2525. [Google Scholar] [CrossRef]
- Kapovits, I.; Kálmán, A. Formation and Structure of a Four-co-ordinate Organo-sulphur(IV) Compound. J. Chem. Soc. D 1971, 649–650. [Google Scholar] [CrossRef]
- Takaguchi, Y.; Furukawa, N. First synthesis and structural determination of 1,1′-spirobis(3H-2, 1-benzoxatellurole)-3,3′-dione ([10-Te-4(C202)]). Heteroat. Chem. 1995, 6, 481–484. [Google Scholar] [CrossRef]
- Zhang, Z.; Takahashi, S.; Saito, S.; Koizumi, T. First synthesis and stereochemistry of enantiomerically pure spiroselenurane and spirotellurane using the 2-exo-hydroxy-10-bornyl group as a chiral ligand. Tetrahedron Asymmetry 1998, 9, 3303–3317. [Google Scholar] [CrossRef]
- Zhang, J.; Saito, S.; Koizumi, T. Stereochemical Research on the Hydrolysis of Optically Pure Spirosulfuranes: Efficient Synthesis of Chiral Sulfoxides with Completely Opposite Stereochemistry. J. Org. Chem. 1998, 63, 9375–9384. [Google Scholar] [CrossRef]
- Zhang, J.; Saito, S.; Koizumi, T. Acidic and Basic Hydrolysis of an Optically Pure Spiro-λ4-sulfurane: Completely Opposite Stereochemical Outcome. J. Am. Chem. Soc. 1998, 120, 1631–1632. [Google Scholar] [CrossRef]
- Kapovits, I.; Rábai, J.; Szabó, D.; Czakó, K.; Kucsman, A.; Argay, G.; Fülöp, V.; Kálmán, A.; Koritsánsky, T.; Párkányi, L. Diaryldiacyloxyspirosulfuranes. Part 3. Sulfuranes with Five-, Six- and Sevenmembered Spirorings: Syntheses and Molecular Structures. J. Chem. Soc. Perkin Trans. 1993, 2, 847–853. [Google Scholar] [CrossRef]
- Back, T.G.; Moussa, Z.; Parvez, M. The Exceptional Glutathione Peroxidase-Like Activity of Di(3-hydroxypropyl) Selenide and the Unexpected Role of a Novel Spirodioxaselenanonane Intermediate in the Catalytic Cycle. Angew. Chem. Int. Ed. 2004, 43, 1268–1270. [Google Scholar] [CrossRef] [PubMed]
- Flohé, L.; Günzler, E.A.; Schock, H.H. Glutathione Peroxidase: A Selenoenzyme. FEBS Lett. 1973, 32, 132–134. [Google Scholar] [CrossRef]
- Jacob, C.; Giles, G.I.; Giles, N.M.; Sies, H. Sulfur and Selenium: The Role of Oxidation State in Protein Structure and Function. Angew. Chem. Int. Ed. 2003, 42, 4742–4758. [Google Scholar] [CrossRef] [PubMed]
- Back, T.G.; Kuzma, D.; Parvez, M. Aromatic Derivatives and Tellurium Analogues of Cyclic Seleninate Esters and Spirodioxyselenuranes That Act as Glutathione Peroxidase Mimetics. J. Org. Chem. 2005, 70, 9230–9236. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.K.; Patel, U.; Roy, D.; Sunoj, R.B.; Singh, H.B.; Wolmershäuser, G.; Butcher, R.J. O-Hydroxylmethylphenylchalcogens: Synthesis, Intramolecular Nonbonded Chalcogen···OH Interactions, and Glutathione Peroxidase-like Activity. J. Org. Chem. 2005, 70, 9237–9247. [Google Scholar] [CrossRef] [PubMed]
- Drabowicz, J.; Martin, J.C. Stereochemistry of Spirosulfuranes and Their Oxides: Static and Dynamic aspects. Pure Appl. Chem. 1996, 68, 951–956. [Google Scholar] [CrossRef]
- Drabowicz, J.; Luczak, J.; Micolajczyk, M.; Yamamoto, Y.; Matsukawa, S.; Akiba, K.-Y. First Optically Active Selenurane Oxide: Resolution of C2-Symmetric 3,3,3′,3′-Tetramethyl-1,1′-Spirobi [3h,2,1]-Benzoxaselenole Oxide. Chirality 2004, 16, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, T.; Ohno, F.; Okazaki, R.; Ikeda, H.; Inagaki, S. Experimental and Theoretical Evidence for Oxirane Formation Reaction of entacoordinate 1,2λ6-Oxathietanes with Retention of Configuration. J. Am. Chem. Soc. 1996, 118, 12455–12456. [Google Scholar] [CrossRef]
- Kuzma, D.; Parvez, M.; Back, T.G. Formation of a Spirodiazaselenurane and its Corresponding Azaselenonium Derivatives from the Oxidation of 2,2-selenobis(benzamide). Structure, Properties and Glutathione Peroxidase Activity. Org. Biomol. Chem. 2007, 5, 3213–3217. [Google Scholar]
- Sarma, B.K.; Manna, D.; Minoura, M.; Mugesh, G. Synthesis, Structure, Spirocyclization Mechanism, and Glutathione Peroxidase-like Antioxidant Activity of Stable Spirodiazaselenurane and Spirodiazatellurane. J. Am. Chem. Soc. 2010, 132, 5364–5374. [Google Scholar] [CrossRef] [PubMed]
- Lamani, D.S.; Bhowmick, D.; Mugesh, G. Spirodiazaselenuranes: Synthesis, Structure and Antioxidant Activity. Org. Biomol. Chem. 2012, 10, 7933–7943. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, K.; Singh, H.B.; Goel, N.; Singh, U.P.; Butcher, R.J. Synthesis and Structural Characterization of Pincer Type Bicyclic Diacyloxy- and Diazaselenuranes. Dalton Trans. 2011, 40, 9858–9867. [Google Scholar] [CrossRef] [PubMed]
- Dahlen, B.; Lindgren, B. Formation and Crystal Structure of 3,3′-Spiroby(3-selenaphthalide). Acta Chem. Scand. 1973, 6, 2218–2220. [Google Scholar] [CrossRef]
- Bhabak, K.P.; Mugesh, G. Amide-Based Glutathione Peroxidase Mimics: Effect of Secondary and Tertiary Amide Substituents on Antioxidant Activity. Chem. Asian J. 2009, 4, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Szabó, C.; Ischiropoulos, H.; Radi, R. Peroxynitrite: Biochemistry, Pathophysiology and Development of Therapeutics. Nat. Rev. Drug Discov. 2007, 6, 662–680. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.S.; Beckman, T.W.; Chen, J.; Marshall, P.A.; Freeman, B.A. Apparent Hydroxyl Radical Production by Peroxynitrite: Implications for Endothelial Injury from Nitric Oxide and Superoxide. Proc. Natl. Acad. Sci. USA 1990, 87, 1620–1624. [Google Scholar] [CrossRef] [PubMed]
- Radi, R.; Peluffo, G.; Alvarez, M.N.; Naviliat, M.; Cayota, A. Unraveling Peroxynitrite Formation in Biological Systems. Free Radic. Biol. Med. 2001, 30, 463–488. [Google Scholar] [CrossRef]
- Salgo, M.G.; Bermudez, E.; Squadrito, G.L.; Battista, J.R.; Pryor, W.A. DNA Damage and Oxidation of Thiols Peroxynitrite Causes in Rat Thymocytes. Arch. Biochem. Biophys. 1995, 322, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Burney, S.; Niles, J.C.; Dedon, P.C.; Tannenbaum, S.R. DNA Damage in Deoxynucleosides and Oligonucleotides Treated with Peroxynitrite. Chem. Res. Toxicol. 1999, 12, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Rubbo, H.; Radi, R.; Trujillo, M.; Telleri, R.; Kalyanaraman, B.; Barnes, S.; Kirk, M.; Freeman, B.A. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J. Biol. Chem. 1994, 269, 26066–26075. [Google Scholar] [PubMed]
- Violi, F.; Marino, R.; Milite, M.T.; Loffredo, L. Nitric Oxide and its Role in Lipid Peroxidation. Diabetes/Metab. Res. Rev. 1999, 15, 283–288. [Google Scholar] [CrossRef]
- MacMillan-Crow, L.A.; Crow, J.P.; Kerby, J.D.; Beckman, J.S.; Thompson, J.A. Nitration and Inactivation of Manganese Superoxide Dismutase in Chronic Rejection of Human Renal Allografts. Proc. Natl. Acad. Sci. USA 1996, 93, 11853–11858. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.S.; Lin, H.; Crowley, J.R.; Vuletich, J.L.; Osawa, Y.; Hollenberg, P.F. Peroxynitrite-Mediated Nitration of Tyrosine and Inactivation of the Catalytic Activity of Cytochrome P450 2B1. Chem. Res. Toxicol. 1998, 11, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Knapp, L.T.; Kanterewicz, B.I.; Hayes, E.L.; Klann, E. Peroxynitrite-Induced Tyrosine Nitration and Inhibition of Protein Kinase C. Biochem. Biophys. Res. Commun. 2001, 286, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Giles, G.I.; Fry, F.H.; Tasker, K.M.; Holme, A.L.; Peers, C.; Green, K.N.; Klotz, L.O.; Sies, H.; Jacob, C. Evaluation of Sulfur, Selenium and Tellurium Catalysts with Antioxidant Potential. Org. Biomol. Chem. 2003, 1, 4317–4322. [Google Scholar] [CrossRef] [PubMed]
- Masumoto, H.; Sies, H. The Reaction of Ebselen with Peroxynitrite. Chem. Res. Toxicol. 1996, 9, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Masumoto, H. Ebselen as a Glutathione Peroxidase Mimic and as a Scavenger of Peroxynitrite. Adv. Pharmacol. 1997, 38, 229–246. [Google Scholar] [PubMed]
- Bian, K.; Gao, Z.; Weisbrodt, N.; Murad, F. The nature of heme/iron-induced protein tyrosine nitration. Proc. Natl. Acad. Sci. USA 2003, 100, 5712–5717. [Google Scholar] [CrossRef] [PubMed]
- Bhabak, K.P.; Mugesh, G. Antithyroid Drugs and their Analogues Protect Against Peroxynitrite-Mediated Protein Tyrosine Nitration-A Mechanistic Study. Chem. Eur. J. 2010, 16, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Komarov, P.; Sies, H.; de Groot, H. Inhibition of superoxide and nitric oxide release and protection from reoxygenation injury by ebselen in rat kupffer cells. Hepatology 1992, 15, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Bhabak, K.P.; Vernekar, A.A.; Jakka, S.R.; Roy, G.; Mugesh, G. Mechanistic Investigations on the Efficient Catalytic Decomposition of Peroxynitrite by Ebselen Analogues. Org. Biomol. Chem. 2011, 9, 5193–5200. [Google Scholar] [CrossRef] [PubMed]
- Uppu, R.M.; Pryor, W.A. Synthesis of Peroxynitrite in a Two-Phase System Using Isoamyl Nitrite and Hydrogen Peroxide. Anal. Biochem. 1996, 236, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXTL Version 5.10,Structure Determination Software Suite; Bruker AXS: Madison, WI, USA, 1998. [Google Scholar]
- Sheldrick, G.M. Phase annealing in SHELX-90: Direct methods for larger structures. Acta Crystallogr. Sect. A 1990, 46, 467–473. [Google Scholar] [CrossRef]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Gualardi, A. Completion and refinement of crystal structures with SIR92. J. Appl. Crystallogr. 1993, 26, 343–350. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS, A Program for Adsorption Correction with the siemens SMART Area Detection System; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. SHELX-97, Program for the Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Samples Availability: Samples of the compounds 29–35 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamani, D.S.; Bhowmick, D.; Mugesh, G. Substituent Effects on the Stability and Antioxidant Activity of Spirodiazaselenuranes. Molecules 2015, 20, 12959-12978. https://doi.org/10.3390/molecules200712959
Lamani DS, Bhowmick D, Mugesh G. Substituent Effects on the Stability and Antioxidant Activity of Spirodiazaselenuranes. Molecules. 2015; 20(7):12959-12978. https://doi.org/10.3390/molecules200712959
Chicago/Turabian StyleLamani, Devappa S., Debasish Bhowmick, and Govindasamy Mugesh. 2015. "Substituent Effects on the Stability and Antioxidant Activity of Spirodiazaselenuranes" Molecules 20, no. 7: 12959-12978. https://doi.org/10.3390/molecules200712959