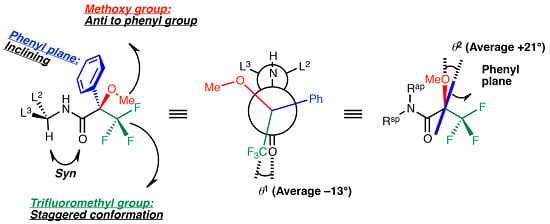
Characteristic Conformation of Mosher’s Amide Elucidated Using the Cambridge Structural Database
Abstract
:1. Introduction

2. Results and Discussion
2.1. Crystal Structures of MTPA Amides Deposited in the CSD
| No. | CCDC Number (b) | Reference | Chirality of MTPA (a) | Amine Moiety | R sp | R ap | O1–C1–C2–C3 (θ1) | C1–C2–C3–F3 | C1'–N–C1–O1 | X1′′–N–C1–O1 (c) | H1′–C1′–N–C1 | O2–C2–C5–C10 (θ2) | C1–C2–O2–C4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (d) | 199868 | [27] | R | Primary amine | Secondary alkyl group | H | −13.1(2) | −172.0(1) | 7.1(2) | −172.9 | −47.4 | 23.3(2) | 54.2(2) |
| 2 (d) | 199868 | [27] | R | Primary amine | Secondary alkyl group | H | −7.3(2) | −174.6(1) | 6.3(2) | −173.7 | −51.5 | 29.4(2) | 50.4(2) |
| 3 (d) | 222942 | [28] | R | Primary amine | Secondary alkyl group | H | 30.3(3) (e) | 175.6(2) | −4.2(5) | 169(4) | 47.0 | 11.2(7) | −44.4(4) |
| 4 (d) | 603055 | [29] | S | Primary amine | Secondary alkyl group | H | 35.0(4) (e) | 165.9(3) | 0.5(5) | −179.5 | 15.2 | −43.7(4) | −72.0(3) |
| 5 | 651954 | [30] | R | Primary amine | Primary alkyl group | H | −26(2) | −168(1) | −6(2) | 173 | – | 24(2) | 72(1) |
| 6 | 651954 | [30] | R | Primary amine | Primary alkyl group | H | −25(2) | −166(1) | 8(2) | −172 | – | 22(2) | 60(2) |
| 7 (d) | 678252 | [31] | R | Primary amine | Secondary alkyl group | H | −22.6(4) | −171.7(3) | 9.5(5) | −170.6 | 29.6 | 19.7(4) | 58.4(3) |
| 8 (d) | 678252 | [31] | R | Primary amine | Secondary alkyl group | H | −14.4(5) | −173.2(3) | 5.2(6) | −174.8 | 28.3 | 22.5(4) | 54.2(4) |
| 9 (d,f) | 703912 | [32] | R | Primary amine | Secondary alkyl group | H | −67.7(6) (e) | −174.6(5) | 3.7(9) | 170(5) | −22.5 | −58.9(7) | −162.7(5) |
| 10 | 734247 | [33] | R | Primary amine | Primary alkyl group | H | −29.1(5) | −168.3(3) | −2.9(6) | 177.0 | – | 24.2(5) | 62.9(4) |
| 11 | 739753 | [34] | R | Primary amine | Primary alkyl group | H | −36.0(2) (e) | −164.7(2) | 7.1(3) | −172.9 | – | 49.8(2) | 64.5(2) |
| 12 (d) | 1218697 | [35] | R | Primary amine | Secondary alkyl group | H | −9.8(3) | −176.9(2) | −3.0(4) | 171(2) | 1.3 | 12.0(3) | 55.5(3) |
| 13 (d) | 1229820 | [36] | R | Primary amine | Secondary alkyl group | H | −31(1) (e) | −169.2(8) | 15(2) | 136 | −7 | 21 | 64 |
| 14 (d) | 1277744 | [37] | R | Primary amine | Secondary alkyl group | H | −28.4(3) | −170.3(2) | 3.9(4) | −176.0 | −14.4 | 26.9(3) | 65.2(3) |
| 15 (g) | 140352 | [38] | R | Secondary amine | Secondary alkyl group | Primary Alkyl group | −4.7(5) | −175.4(3) | 5.3(6) | −174.7(4) | 20.4(6) | 27.5(5) | 53.4(4) |
| 16 (g) | 167289 | [39] | S | Secondary amine | Secondary alkyl group | Primary alkyl group | 5.2(2) | 177.7(1) | −0.5(2) | −177.4(2) | 10(2) | −11.7(2) | −45.0(2) |
| 17 (g) | 241708 | [40] | S | Secondary amine | Secondary alkyl group | Primary alkyl group | 6.9(2) | 176.0(1) | −1.7(2) | 169.5(1) | 48.1 | −15.4(2) | −45.1(2) |
| 18 (g) | 251663 | [41] | R | Secondary amine | Secondary alkyl group | Primary alkyl group | −12.4(2) | −172.3(1) | 1.8(2) | −168.2(1) | 24.3 | 24.5(2) | 56.7(2) |
| 19 | 288331 | [42] | S | Secondary amine | Secondary alkyl group | Secondary alkyl group | 7.7(3) | 176.5(2) | 8.7(3) | 174.0(2) | −1.3 | −14.0(3) | −50.1(3) |
| 20 | 296547 | [43] | R | Secondary amine | Primary alkyl group | Primary alkyl group | –13.4(8) | −171.7(5) | −0.9(9) | −170.9(6) | – | 12.6(7) | 47.4(7) |
| 21 (g) | 604432 | [44] | R | Secondary amine | Secondary alkyl group | Primary alkyl group | −4.1(5) | −176.3(3) | 8.7(5) | −170.3(3) | 17.1 | 15.3(4) | 51.7(4) |
| 22 (g) | 605818 | [45] | R | Secondary amine | Secondary alkyl group | Primary alkyl group | −15.6(2) | −174.1(1) | 5.8(2) | −164.3(1) | −58.3 | 6.9(2) | 52.1(1) |
| 23 | 638938 | [46] | R | Secondary amine | Primary alkyl group | Primary alkyl group | −6.2(5) | −173.8(3) | −1.8(5) | −173.7(3) | – | 27.7(4) | 51.2(4) |
| 24 (g) | 675390 | [47] | R | Secondary amine | Secondary alkyl group | Primary alkyl group | −14.6(2) | −174.7(1) | 10.1(3) | −164.8(2) | −56.5 | 9.2(2) | 50.4(2) |
| 25 (g) | 706349 | [48] | R | Secondary amine | Secondary alkyl group | Primary alkyl group | −13.7(4) | −172.3(3) | 5.9(5) | −168.6(3) | 19(2) | 19.3(4) | 59.4(4) |
| 26 (h) | 707825 | [49] | S | Secondary amine | Me | Secondary alkyl group | 8.6(3) | 173.1(2) | 0.1(3) | 173.6(2) | – | −12.7(3) | −50.0(3) |
| 27 (h) | 707825 | [49] | S | Secondary amine | Me | Secondary alkyl group | 4.0(3) | 176.7(2) | 0.3(3) | −174.7(2) | – | −14.1(3) | −51.0(2) |
| 28 (h) | 707825 | [49] | S | Secondary amine | Me | Secondary alkyl group | 1.1(3) | 178.0(2) | 1.3(3) | −176.7(2) | – | –18.4(3) | −48.3(3) |
| 29 (h) | 707825 | [49] | S | Secondary amine | Me | Secondary alkyl group | 3.6(3) | 177.1(2) | 1.2(3) | –179.7(2) | – | −9.4(3) | −46.0(3) |
| 30 | 766837 | [50] | S | Secondary amine | Primary alkyl group | Primary alkyl group | 9.4(2) | 175.1(1) | 2.0(2) | 177.8(1) | – | −14.7(2) | −44.7(1) |
| 31 (g) | 830079 | [51] | R | Secondary amine | Tertiary alkyl group | Primary alkyl group | −18(1) | −173.1(6) | −1(1) | −178.3(7) | – | 20(1) | 55.8(9) |
| 32 | 1104875 | [52] | R | Secondary amine | Primary alkyl group | Primary alkyl group | −2.2(2) | −176.5(2) | −5.2(3) | −176.7(2) | – | 27.1(2) | 45.2(2) |
| 33 | 1105464 | [53] | R | Secondary amine | Primary alkyl group | Primary alkyl group | −8.2(8) | 178.4(5) | −0.7(9) | −172.4(6) | – | 12.0(8) | 46.6(8) |
| 34 | 1105464 | [53] | R | Secondary amine | Primary alkyl group | Primary alkyl group | –9.0(8) | –175.6(5) | –6.1(9) | –168.0(6) | – | 13.7(8) | 45.8(7) |
| 35 (g) | 1267150 | [54] | S | Secondary amine | Secondary alkyl group | Primary alkyl group | 7.1(6) | 176.2(4) | −3.0(7) | −179.1(4) | 19.5 | −15.3(6) | −42.6(5) |
| 36 (g) | 1267151 | [54] | R | Secondary amine | Secondary alkyl group | Primary alkyl group | −14.0(7) | −170.3(4) | 8.4(7) | −163.3(5) | 24.4 | 29.7(7) | 59.7(6) |
| 37 (g) | 1280861 | [55] | R | Secondary amine | Secondary alkyl group | Primary alkyl group | −15.6(6) | −170.9(4) | 11.4(7) | −174.1(4) | −15.1 | 28.5(7) | 50.1(6) |
| 38 (g) | 1280861 | [55] | R | Secondary amine | Secondary alkyl group | Primary alkyl group | −5.5(6) | −177.6(4) | 7.8(7) | −177.4(4) | −17.0 | 25.0(6) | 41.3(6) |
| 39 | 1294281 | [56] | R | Secondary amine | Primary alkyl group | Primary alkyl group | −6(1) | −175.0(7) | 1(1) | −172.0(7) | – | 26(1) | 44.0(9) |
| 40 | 1294281 | [56] | R | Secondary amine | Primary alkyl group | Primary alkyl group | −15(1) | −172.9(7) | 1(1) | −163.7(8) | – | 14(1) | 47(1) |
| 41 (i) | 113953 | [57] | R | Aniline derivative | ortho-Substituted phenyl group | H | −17(1) | −174.9(6) | −11(1) | 178.6 | – | 8(1) | 54.6(8) |
| 42 (i) | 113953 | [57] | R | Aniline derivative | ortho-Substituted phenyl group | H | −28(1) | −168.0(7) | −1(1) | −170.3 | – | 47.7(9) | 65.9(8) |
| 43 (f,i) | 113953 | [57] | R | Aniline derivative | ortho-Substituted phenyl group | H | −58.4(9) (e) | −175.1(6) | −1(1) | 176.4 | – | −56.4(9) | −153.6(6) |
| 44 (i) | 113953 | [57] | R | Aniline derivative | ortho-Substituted phenyl group | H | −15(1) | −170.8(6) | 6(1) | 175.4 | – | 36.1(9) | 57.7(8) |
| 45 (i) | 1310848 | [58] | R | Aniline derivative | ortho-Substituted phenyl group | H | −2(2) | −172(1) | −5(3) | – | – | 35(2) | 51(2) |
| 46 (f,i) | 1310848 | [58] | R | Aniline derivative | ortho-Substituted phenyl group | H | −60(2) (e) | 179(2) | 3(3) | – | – | −34(2) | −172(2) |
| 47 (i) | 1310848 | [58] | R | Aniline derivative | ortho-Substituted phenyl group | H | −41(2) (e) | −169(2) | 0(3) | – | – | 13(2) | 71(2) |
| 48 (f,i) | 1310848 | [58] | R | Aniline derivative | ortho-Substituted phenyl group | H | −51(2) (e) | −165(2) | −1(3) | – | – | 85(2) | −154(2) |
| 49 | 655554 | [59] | R | Benzotriazole | ortho-Substituted phenyl group | N | −9.3(1) | −175.21(7) | −2.8(1) | 178.81(8) | – | 25.7(1) | 46.6(1) |
| 50 (f) | 1142231 | [60] | S | Diethyl 1-aminoalkylphos-phonate derivative | 1-(Diethoxy-phophoryl)alkyl group | H | 57(3) (e) | 169(2) | 9(4) | – | −14 | 77(2) | 148(2) |
| 51 | 1142231 | [60] | S | Diethyl 1-aminoalkylphos-phonate derivative | 1-(Diethoxy-phophoryl)alkyl group | H | 24(3) | 172(2) | 4(3) | – | −12 | −35(2) | −65(2) |
| 52 | 1236701 | [61] | R | Diethyl 1-aminoalkylphos-phonate derivative | 1-(Diethoxy-phophoryl)alkyl group | H | −23.1(5) | −171.5(3) | −3.1(6) | 176.6 | 19.2 | 7.3(5) | 59.3(4) |
| 53 | 1236702 | [61] | R | Diethyl 1-aminoalkylphos-phonate derivative | 1-(Diethoxy-phophoryl)alkyl group | H | −19.4(6) | −174.3(4) | −3.2(7) | 177.2 | 18.8 | 5.8(6) | 55.8(5) |
| 54 | 1236703 | [61] | R | Diethyl 1-aminoalkylphos-phonate derivative | 1-(Diethoxy-phophoryl)alkyl group | H | −24(2) | −166(1) | 4(2) | −173 | 11 | 31(2) | 59(2) |
| 55 | 1236703 | [61] | R | Diethyl 1-aminoalkylphos-phonate derivative | 1-(Diethoxy-phophoryl)alkyl group | H | −24(2) | −166(1) | 9(2) | −171 | −14 | 35(2) | 57(1) |
| 56 | 1216345 | [62] | R | Oxazolidine-2-selone derivative | Selenoxo group | Secondary alkyl group | −15.9(4) | −172.9(2) | 24.5(4) | −147.8(3) | – | 18.5(4) | 56.6(3) |
| 57 | 630372 | [63] | R | Thiocarbamide derivative | N-Substituted thiocarbamoyl group | Secondary alkyl group | −16.7(3) | −174.1(2) | 11.2(4) | −152.6(2) | – | 24.8(3) | 57.7(3) |
| 58 | 143886 | [64] | R | p-Toluene-sulfonamide derivative | p-Toluene-sulfonyl group | Primary alkyl group | −5.4(6) | −177.4(3) | 7.4(5) (j) | −177.6(4) | – | 18.2(6) | 37.8(5) |

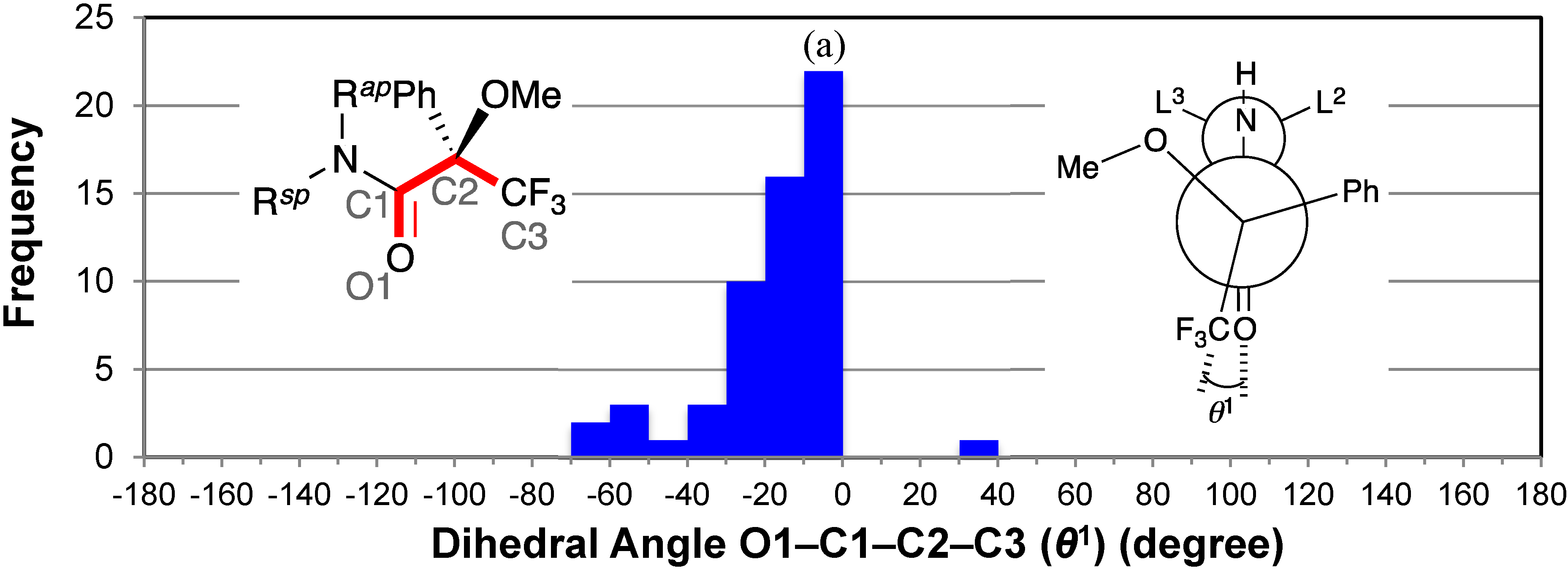
2.2. Dihedral Angles of Amide Carbonyl Group and Trifluoromethyl Group: O1–C1–C2–C3
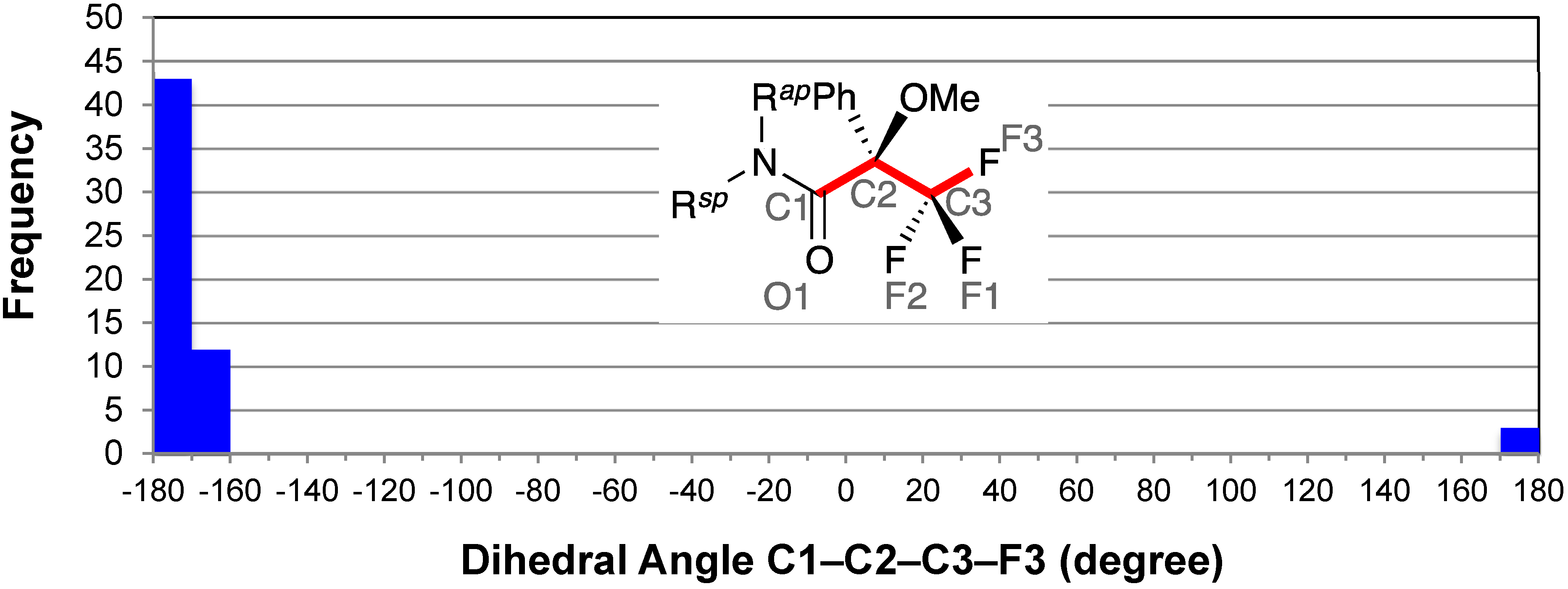
2.3. Staggered Conformation of Trifluoromethyl Group: C1–C2–C3–F3
2.4. Resonance Effects of Amide Bond: C1′–N–C1–O1
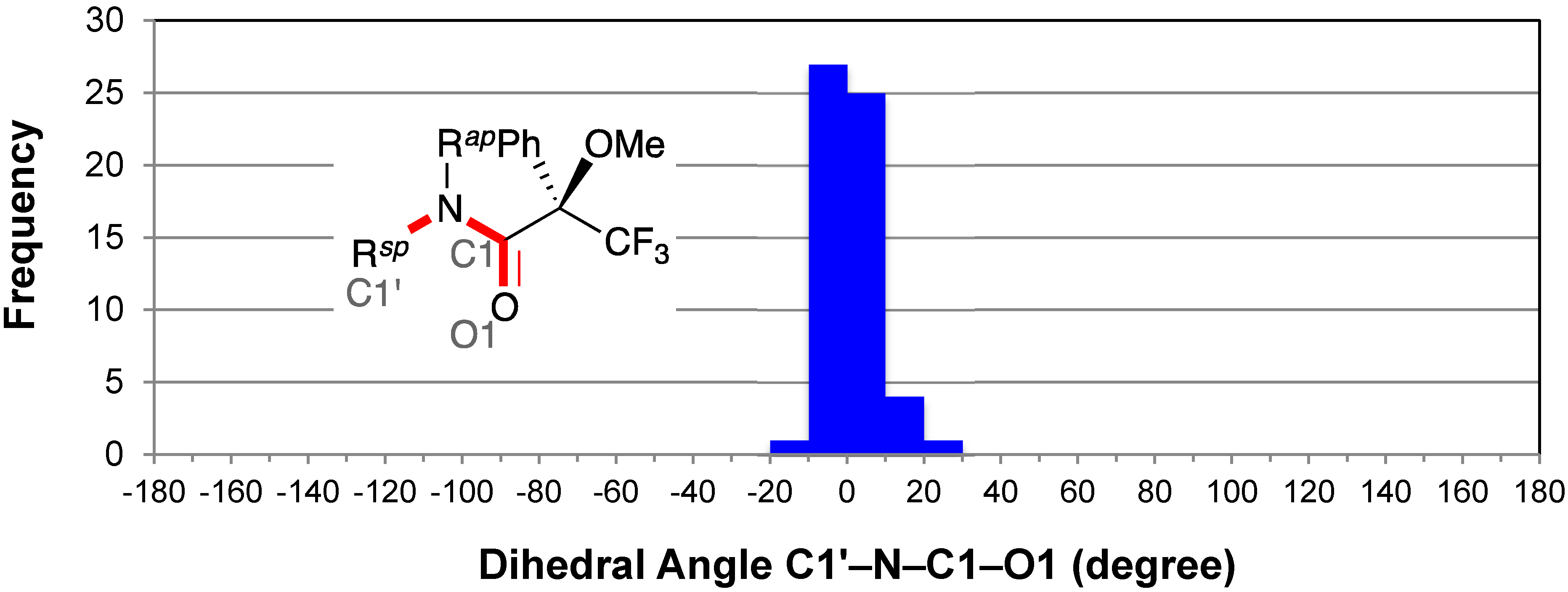
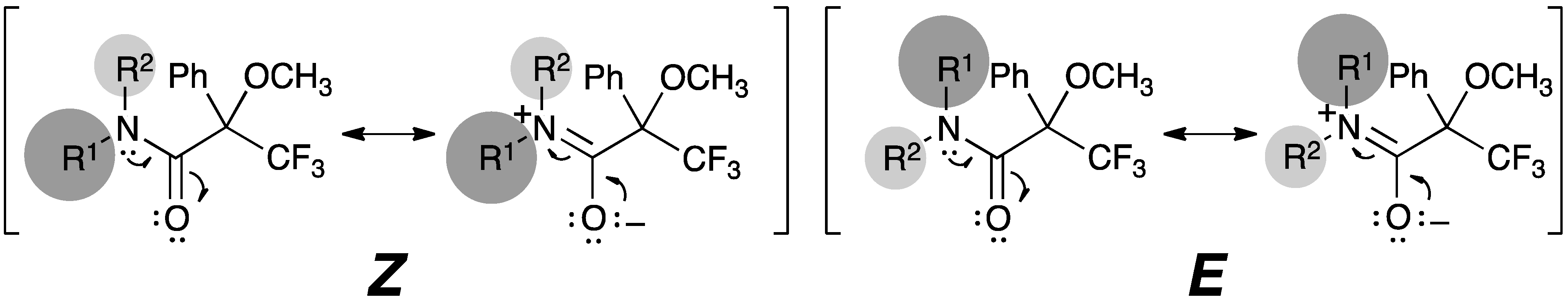
2.5. Resonance Effects of Amide Bond: X1′′–N–C1–O1
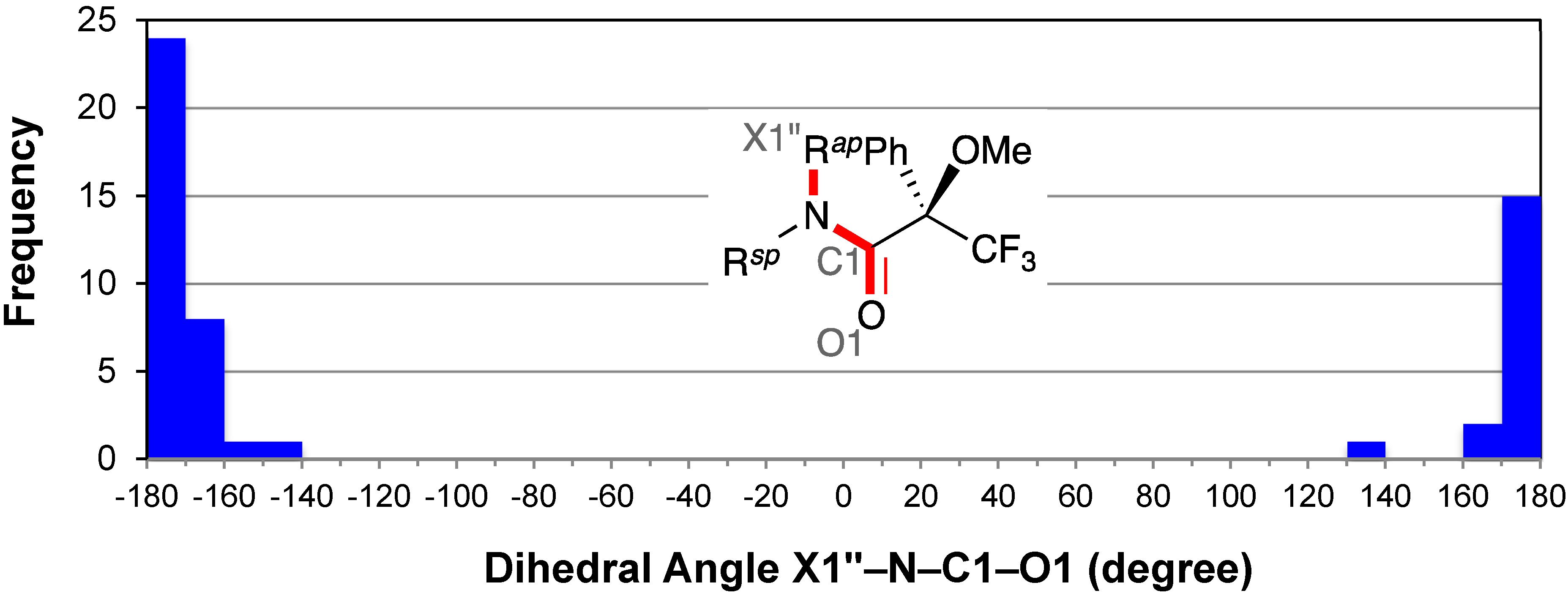
2.6. Conformation of the Amine Moiety: H1′–C1′–N–C1
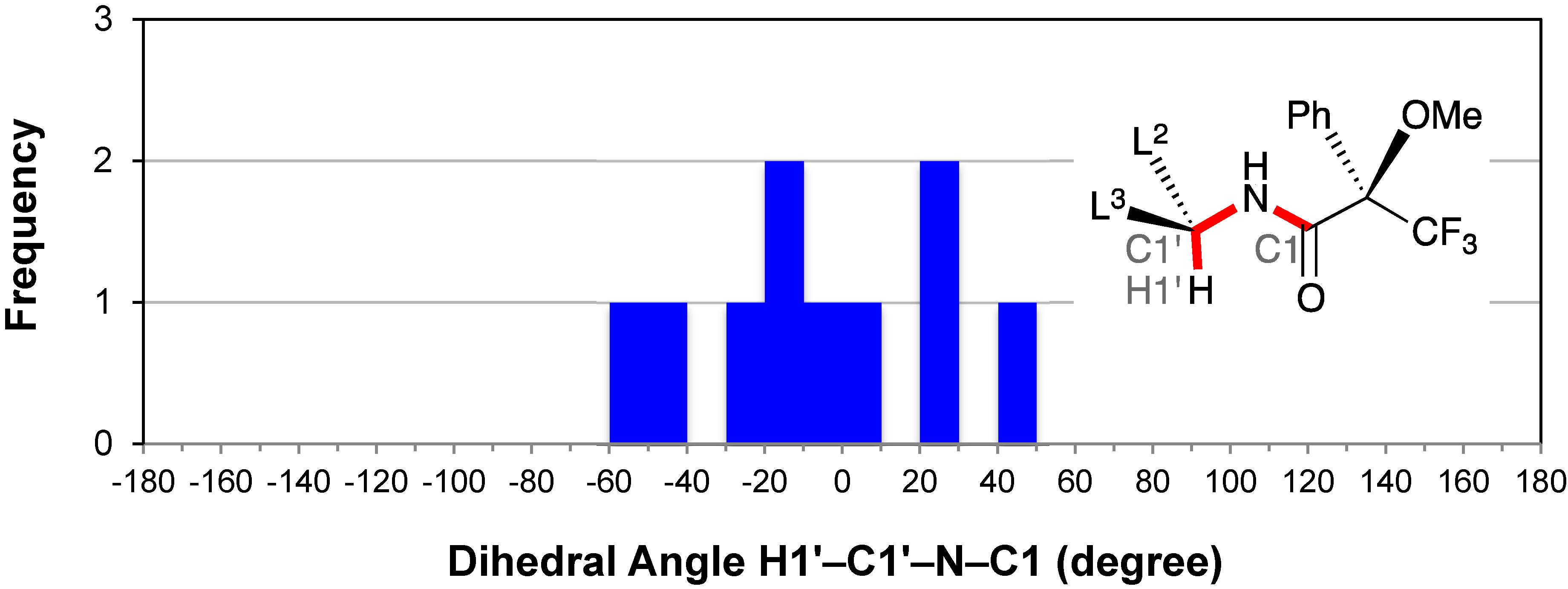
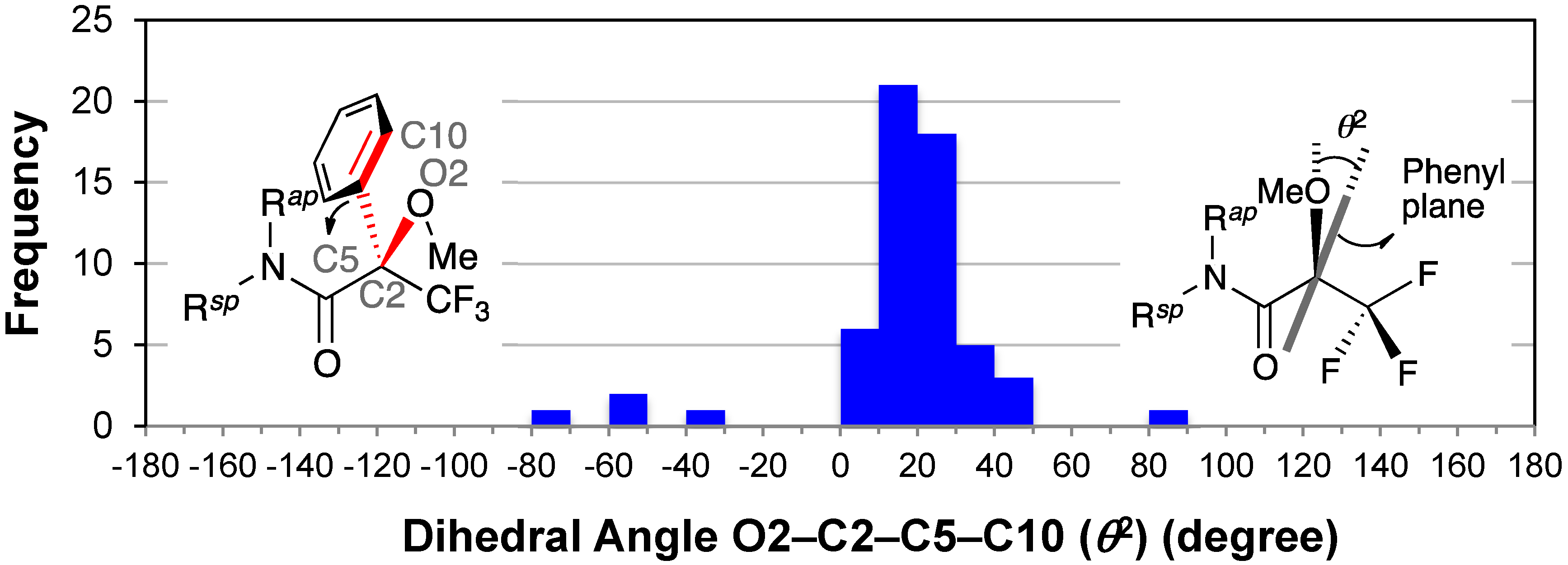
2.7. Dihedral Angle between the Methoxy Group and Phenyl Group: O2–C2–C5–C10
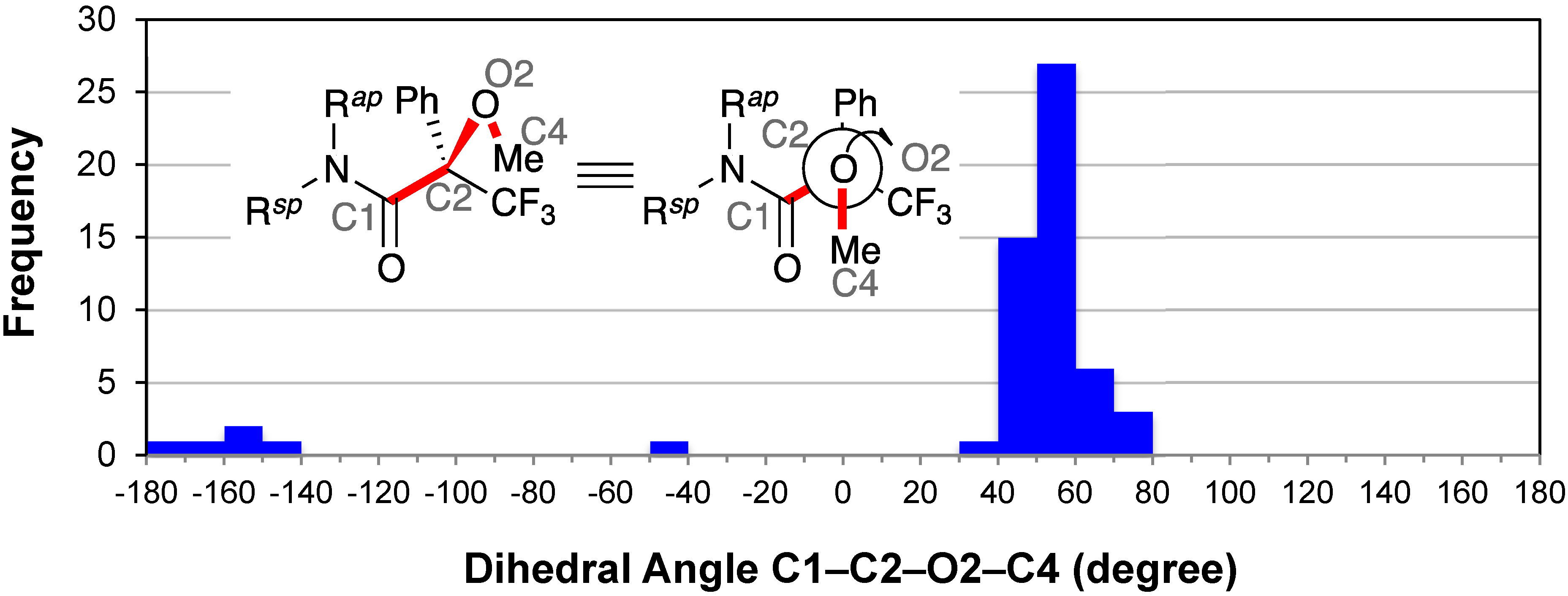
2.8. Conformation of the Methoxy Group: C1–C2–O2–C4
3. Experimental Section
3.1. Database Study of MTPA Amide
3.2. Caution
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Seco, J.M.; Quiñoá, E. ; Riguera. R. Assignment of the absolute configuration of polyfunctional compounds by NMR using chiral derivatizing agents. Chem. Rev. 2012, 112, 4603–4641. [Google Scholar] [CrossRef] [PubMed]
- Dale, J.A.; Dull, D.L.; Mosher, H.S. α-Methoxy-α-trifluoromethylphenylacetic acid, a versatile reagent for the determination of enantiomeric composition of alcohols and amines. J. Org. Chem. 1969, 34, 2543–2549. [Google Scholar] [CrossRef]
- Dale, J.A.; Mosher, H.S. Nuclear magnetic resonance enantiomer reagents. Configurational correlations via nuclear magnetic resonance chemical shifts of diastereomeric mandelate, O-methylmandelate, and α-methoxy-α-trifluoromethylphenylacetate (MTPA) esters. J. Am. Chem. Soc. 1973, 95, 512–519. [Google Scholar] [CrossRef]
- Sullivan, G.R.; Dale, J.A.; Mosher, H.S. Correlation of configuration and 19F chemical shifts of α-methoxy-α-trifluoromethylphenylacetate derivatives. J. Org. Chem. 1973, 38, 2143–2147. [Google Scholar] [CrossRef]
- Ohtani, I.; Kusumi, T.; Kashman, Y.; Kakisawa, H. High-field FT NMR application of Mosher’s method. The absolute configurations of marine terpenoids. J. Am. Chem. Soc. 1991, 113, 4092–4096. [Google Scholar] [CrossRef]
- Kusumi, T.; Fukushima, T.; Ohtani, I.; Kakisawa, H. Elucidation of the absolute configurations of amino acids and amines by the modified Mosher’s method. Tetrahedron Lett. 1991, 32, 2939–2942. [Google Scholar] [CrossRef]
- Ohtani, I. I.; Hotta, K.; Ichikawa, Y.; Isobe, M. Application of modified Mosher’s method to α-aromatic secondary alcohols. Exception of the rule and conformational analyses. Chem. Lett. 1995, 24, 513–514. [Google Scholar] [CrossRef]
- Kusumi, T. Determination of the absolute configuration of organic compounds by means of NMR spectroscopy—Modified Mosher’s method. J. Syn. Org. Chem. Jpn. 1993, 51, 462–470. [Google Scholar] [CrossRef]
- Ichikawa, A.; Ono, H.; Mikata, Y. Crystal structures of Mosher’s salt and ester elucidated by X-ray crystallography. CrystEngComm 2013, 15, 8088–8096. [Google Scholar]
- Harada, N. Determination of absolute configurations by X-ray crystallography and 1H NMR anisotropy. Chirality 2008, 20, 691–723. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, S.; Naito, J.; Yamamoto, Y.; Kasai, Y.; Fujita, T.; Noro, K.; Shimanuki, K.; Akagi, M.; Watanabe, M.; Matsumoto, T.; Watanabe, M.; Ichikawa, A.; Harada, N. Crystalline-state conformational analysis of MαNP esters, powerful resolution and chiral 1H NMR anisotropy tools. Eur. J. Org. Chem. 2007, 1827–1840. [Google Scholar] [CrossRef]
- Kasai, Y.; Sugio, A.; Sekiguchi, S.; Kuwahara, S.; Matsumoto, T.; Watanabe, M.; Ichikawa, A.; Harada, N. Conformational analysis of MαNP esters, powerful chiral resolution and 1H NMR anisotropy tools—Aromatic geometry and solvent effects on ∆δ values. Eur. J. Org. Chem. 2007, 1811–1826. [Google Scholar] [CrossRef]
- Ichikawa, A.; Ono, H.; Mikata, Y. Naphthyl groups in chiral recognition: structures of salts and esters of 2-methoxy-2-naphthylpropanoic acids. Chem. Asian J. 2012, 7, 2294–2304. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, A.; Ono, H.; Echigo, T.; Mikata, Y. Crystal structures and chiral recognition of the diastereomeric salts prepared from 2-methoxy-2-(1-naphthyl)propanoic acid. CrystEngComm 2011, 13, 4536–4548. [Google Scholar] [CrossRef]
- Ichikawa, A.; Ono, H.; Takenaka, M.; Mikata, Y. Crystal conformations and molecular packing of (S)-2-methoxy-2-(1-naphthyl)propanoic acid and a diastereomeric amide prepared from (R)-2-methoxy-2-(1-naphthyl)propanoic acid. CrystEngComm 2010, 12, 2261–2268. [Google Scholar] [CrossRef]
- Ichikawa, A.; Ono, H.; Mikata, Y. Characteristic conformations and molecular packings in crystal structures of diastereomeric esters prepared from (S)-2-methoxy-2-(1-naphthyl)propanoic acid. Tetrahedron Asymmetry 2008, 19, 2693–2698. [Google Scholar] [CrossRef]
- Ichikawa, A.; Ono, H. Preparation of single-enantiomer biofunctional molecules with (S)-2-methoxy-2-(1-naphthyl)propanoic acid. Biosci. Biotechnol. Biochem. 2008, 72, 2418–2422. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, A.; Ono, H. Preparation of Single-enantiomer 2-methyl-4-heptanol, a pheromone of Metamasius hemipterus, using (S)-2-methoxy-2-(1-naphthyl)propionic acid. J. Chromatogr. A 2006, 1117, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, A.; Ono, H. Preparation of single-enantiomer semiochemicals using 2-methoxy-2-(1-naphthyl)propionic acid and 2-methoxy-2-(9-phenanthryl)propionic acid. Tetrahedron Asymmetry 2005, 16, 2559–2568. [Google Scholar] [CrossRef]
- Ichikawa, A.; Ono, H.; Harada, N. Stereochemical studies of chiral resolving agents, M9PP and H9PP acids. Chirality 2004, 16, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, A.; Ono, H.; Harada, N. Synthesis and analytical properties of (S)-(+)-2-methoxy-2-(9-phenanthryl)propionic acid. Tetrahedron Asymmetry 2003, 14, 1593–1597. [Google Scholar] [CrossRef]
- Ichikawa, A.; Ono, H.; Hiradate, S.; Watanabe, M.; Harada, N. Absolute configurations of 2-methoxy-2-(1-naphthyl)propionic acid and 2-methoxy-2-(2-naphthyl)propionic acid as determined by the phenylglycine methyl ester (PGME) method. Tetrahedron Asymmetry 2002, 13, 1167–1172. [Google Scholar] [CrossRef]
- Ichikawa, A.; Hiradate, S.; Sugio, A.; Kuwahara, S.; Watanabe, M.; Harada, N. Absolute configuration of 2-methoxy-2-(2-naphthyl)propionic acid as determined by the 1H NMR anisotropy method. Tetrahedron Asymmetry 2000, 11, 2669–2675. [Google Scholar] [CrossRef]
- Ichikawa, A.; Hiradate, S.; Sugio, A.; Kuwahara, S.; Watanabe, M.; Harada, N. Absolute configuration of 2-hydroxy-2-(1-naphthyl)propionic acid as determined by the 1H NMR anisotropy method. Tetrahedron Asymmetry 1999, 10, 4075–4078. [Google Scholar] [CrossRef]
- Hirayama, N. Chapter 10: Comparison of Structures. In Introduction to X-ray Analysis for Chemistry and Pharmacy, 2nd ed.; Maruzen Publishing Co. Ltd.: Tokyo, Japan, 2006; pp. 95–106. [Google Scholar]
- Definitions of dihedral angle are as follows: 0° to ±30°, synperiplanar (sp); ±150° to 180°, antiperiplanar (ap) IUPAC. Compendium of Chemical Terminology, 2nd ed.; (the “Gold Book”); McNaught, A.D., Wilkinson, A., Eds.; Blackwell Scientific Publications: Oxford, UK, 1997. [Google Scholar] XML on-line corrected version: http://goldbook.iupac.org (2006-) Created by Nic, M.; Jirat, J.; Kosata B. Updates compiled by Jenkins, A.
- CCDC 199868: Haberhauer, G.; Rominger, F. Straightforward synthesis of a novel class of rigid bicyclic dipeptidomimetics from simple dipeptides: Fused imidazole amino acids. Synlett 2003, 780–784. [Google Scholar] [CrossRef]
- CCDC 222942: Dondoni, A.; Massi, A.; Minghini, E.; Bertolasi, V. Multicomponent Hantzsch cyclocondensation as a route to highly functionalized 2- and 4-dihydropyridylalanines, 2- and 4-pyridylalanines, and their N-oxides: preparation via a polymer-assisted solution-phase approach. Tetrahedron 2004, 60, 2311–2326. [Google Scholar] [CrossRef]
- CCDC 603055: Lindsley, C.W.; Zhao, Z.; Leister, W.H.; O’Brien, J.; Lemaire, W.; Williams, D.L., Jr.; Chen, T.B.; Chang, R.S.L.; Burno, M.; Jacobson, M.A.; et al. Design, synthesis, and in vivo efficacy of glycine transporter-1 (GlyT1) inhibitors derived from a series of [4-phenyl-1-(propylsulfonyl) piperidin-4-yl]methyl benzamides. ChemMedChem 2006, 1, 807–811. [Google Scholar]
- CCDC 651954: Busto, E.; Gotor-Fernández, V.; Montejo-Bernardo, J.; García-Granda, S.; Gotor, V. First desymmetrization of 1,3-propanediamine derivatives in organic solvent. Development of a new route for the preparation of optically active amines. Org. Lett. 2007, 9, 4203–4206. [Google Scholar]
- CCDC 678252: Soto-Cairoli, B.; de Pomar, J.J.; Soderquist, J.A. Enantiomerically pure α-amino aldehydes from silylated α-amino acids. Org. Lett. 2008, 10, 333–336. [Google Scholar] [CrossRef] [PubMed]
- CCDC 703912: Lauzon, S.; Tremblay, F.; Gagnon, D.; Godbout, C.; Chabot, C.; Mercier-Shanks, C.; Perreault, S.; DeSève, H.; Spino, C. Sterically biased 3,3-sigmatropic rearrangement of chiral allylic azides: application to the total syntheses of alkaloids. J. Org. Chem. 2008, 73, 6239–6250. [Google Scholar]
- CCDC 734247: Busto, E.; Gotor-Fernández, V.; Montejo-Bernardo, J.; García-Granda, S.; Gotor, V. Development of a chemoenzymatic strategy for the synthesis of optically active and orthogonally protected polyamines. Tetrahedron 2009, 65, 8393–8401. [Google Scholar]
- CCDC 739753: Reznichenko, A.L.; Hampel, F.; Hultzsch, K.C. Kinetic resolution of aminoalkenes by asymmetric hydroamination: a mechanistic study. Chem. Eur. J. 2009, 15, 12819–12827. [Google Scholar] [CrossRef] [PubMed]
- CCDC 1218697: Enders, D.; Nübling, C.; Schubert, H. Asymmetric synthesis of primary amines by nucleophilic addition of alkyllithium compounds to aldehyde SAMP/RAMP hydrazones. Liebigs Ann. 1997, 1089–1100. [Google Scholar] [CrossRef]
- CCDC 1229820: Enders, D.; Funk, R.; Klatt, M.; Raabe, G.; Hovestreydt, E.R. Enantioselective synthesis of α-amino acetals and α-amino acids by nucleophilic 1,2-addition to diethoxyacetaldehyde SAMP hydrazone. Angew. Chem. Int. Ed. 1993, 32, 418–421. [Google Scholar] [CrossRef]
- CCDC 1277744: Port, A.; Virgili, A.; Alvarez-Larena, A.; Piniella, J.F. Preparation of enantiomers of 1-(1-naphthyl)-2,2-dimethylpropylamine and their behaviour as chiral solvating agents: Study of diastereochemic association by Job’s plots and intermolecular NOE measurements. Tetrahedron Asymmetry 2000, 11, 3747–3757. [Google Scholar] [CrossRef]
- CCDC 140352: Tietze, L.F.; Zhou, Y.; Töpken, E. Synthesis of simple enantiopure tetrahydro-β-carbolines and tetrahydroisoquinolines. Eur. J. Org. Chem. 2000, 2247–2252. [Google Scholar] [CrossRef]
- CCDC 167289: Golubev, A.S.; Schedel, H.; Radics, G.; Sieler, J.; Burger, K. Stereoselective syntheses of 4-fluoro- and 4,4-difluoropipecolic acids. Tetrahedron Lett. 2001, 42, 7941–7944. [Google Scholar] [CrossRef]
- CCDC 241708: Cordes, M.; Franke, D. Studies toward nitrogen-containing natural products using radical cyclizations of chiral vinylogous amides. Synlett 2004, 1917–1920. [Google Scholar] [CrossRef]
- CCDC 251663: Roszkowski, P.; Wojtasiewicz, K.; Leniewski, A.; Maurin, J.K.; Lis, T.; Czarnocki, Z. Enantioselective synthesis of 1-substituted tetrahydro-β-carboline derivatives via the asymmetric transfer hydrogenation. J. Mol. Catal. A Chem. 2005, 232, 143–149. [Google Scholar]
- CCDC 288331: Peltier, H.M.; Ellman, J.A. N-Sulfinyl metalloenamine conjugate additions: Asymmetric synthesis of piperidines. J. Org. Chem. 2005, 70, 7342–7345. [Google Scholar] [CrossRef] [PubMed]
- CCDC 296547: Peeters, O.M.; Polavarapu, P.L.; Zhang, P.; Gera, L.; Gal, J. (+)-1-[(R)-3,3,3-Trifluoro-2-methoxy-2-phenylpropionyl]-4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy}phenyl)piperazine methanol solvate. Acta Cryst. 2006, E62, o191–o192. [Google Scholar]
- CCDC 604432: Roszkowski, P.; Maurin, J.K.; Czarnocki, Z. Enantioselective synthesis of (R)-(−)-praziquantel (PZQ). Tetrahedron Asymmetry 2006, 17, 1415–1419. [Google Scholar] [CrossRef]
- CCDC 605818: Gribkov, D.V.; Hultzsch, K.C.; Hampel, F. 3,3′-Bis(trisarylsilyl)-substituted binaphtholate rare earth metal catalysts for asymmetric hydroamination. J. Am. Chem. Soc. 2006, 128, 3748–3759. [Google Scholar] [CrossRef] [PubMed]
- CCDC 638938: Laurent, S.A.L.; Boissier, J.; Coslédan, F.; Gornitzka, H.; Robert, A.; Meunier, B. Synthesis of “trioxaquantel”® derivatives as potential new antischistosomal drugs. Eur. J. Org. Chem. 2008, 895–913. [Google Scholar] [CrossRef]
- CCDC 675390: Zhang, Z.; Bender, C.F.; Widenhoefer, R.A. Gold(I)-catalyzed dynamic kinetic enantioselective intramolecular hydroamination of allenes. J. Am. Chem. Soc. 2007, 129, 14148–14149. [Google Scholar] [CrossRef] [PubMed]
- CCDC 706349: Nicolaou, K.C.; Dalby, S.M.; Li, S.; Suzuki, T.; Chen, D.Y.K. Total synthesis of (+)-haplophytine. Angew. Chem. Int. Ed. 2009, 48, 7616–7620. [Google Scholar] [CrossRef] [PubMed]
- CCDC 707825: Zhong, Y.L.; Krska, S.W.; Zhou, H.; Reamer, R.A.; Lee, J.; Sun, Y.; Askin, D. Catalytic asymmetric synthesis of an HIV integrase inhibitor. Org. Lett. 2009, 11, 369–372. [Google Scholar] [CrossRef] [PubMed]
- CCDC 766837: Zhu, H.; Plewe, M.B.; Rheingold, A.L.; Moore, C.; Yanovsky, A. (3aS,7aS)-5-[(S)-3,3,3-Trifluoro-2-methoxy-2-phenylpropanoyl]-2,3,4,5,6,7-hexahydro-1H-pyrrolo-[3,4-c]pyridin-3(2H)-one monohydrate. Acta Cryst. 2010, E66, o175–o176. [Google Scholar]
- CCDC 830079: Chapurina, Y.; Ibrahim, H.; Guillot, R.; Kolodziej, E.; Collin, J.; Trifonov, A.; Schulz, E.; Hannedouche, J. Catalytic, enantioselective intramolecular hydroamination of primary amines tethered to di- and trisubstituted alkenes. J. Org. Chem. 2011, 76, 10163–10172. [Google Scholar]
- CCDC 1104875: Nishi, T.; Ishibashi, K.; Nakajima, K.; Iio, Y.; Fukazawa, T. An efficient synthesis of enantiomerically pure 2-[(2R)-arylmorpholin-2-yl]ethanols, key intermediates of tachykinin receptor antagonist. Tetrahedron Asymmetry 1998, 9, 3251–3262. [Google Scholar] [CrossRef]
- CCDC 1105464: Nishi, T.; Nakajima, K.; Iio, Y.; Ishibashi, K.; Fukazawa, T. Practical methods for the preparation of spiro[benzo[c]thiophene-1(3H),4′-piperidine]-(2S)-oxide by resolution and asymmetric sulfoxidation. Tetrahedron Asymmetry 1998, 9, 2567–2570. [Google Scholar] [CrossRef]
- CCDC 1267151: Uematsu, N.; Fujii, A.; Hashiguchi, S.; Ikariya, T.; Noyori, R. Asymmetric transfer hydrogenation of imines. J. Am. Chem. Soc. 1996, 118, 4916–4917. [Google Scholar]
- CCDC 1280861: Fleischhauer, J.; Raabe, G.; Santos, A.G.; Schiffer, J.; Wollmer, A. Determination of the absolute configuration of 1,5-diaza-cis-decalin by comparison of measured and calculated CD-spectra. Z. Naturforsch. 1998, 53a, 896–902. [Google Scholar]
- CCDC 1294281: Takemoto, T.; Nakajima, K.; Iio, Y.; Tamura, M.; Nishi, T. Asymmetric synthesis of enantiomerically pure spiro[((2S)-hydroxy)indane-1,4′-piperidine]. Tetrahedron Asymmetry 1999, 10, 1787–1793. [Google Scholar]
- CCDC 113953: Morice, C.; Maux, P.L.; Simonneaux, G.; Toupet, L. Chiral recognition of amino esters by ruthenium porphyrin complexes and crystal structure of {5,10,15,20-tetrakis[o-(3,3,3-trifluoro-2-methoxy-2-phenylpropanoylamino)phenyl]porphyrin}bis(l-valine methyl ester)ruthenium(II) (α,α,β,β isomer). J. Chem. Soc. Dalton Trans. 1998, 4165–4172. [Google Scholar] [CrossRef]
- CCDC 1310848: Maux, P.L.; Bahri, H.; Simonneaux, G.; Toupet, L. Enantioselective oxidation of racemic phosphines with chiral oxoruthenium porphyrins and crystal structure of [5,10,15,20-tetrakis[o-((2-methoxy-2-phenyl-3,3,3-trifluoropropanoyl)amino)phenyl]porphyrinato](carbonyl)(tetrahydrofuran) ruthenium(II) (α,β,α,β isomer). Inorg. Chem. 1995, 34, 4691–4697. [Google Scholar]
- CCDC 655554: Katritzky, A.R.; Mohapatra, P.P.; Fedoseyenko, D.; Duncton, M.; Steel, P.J. 1-Benzotriazol-1-yl-3,3,3-trifluoro-2-methoxy-2-phenylpropan-1-ones: Mosher-Bt reagents. J. Org. Chem. 2007, 72, 4268–4271. [Google Scholar] [CrossRef] [PubMed]
- CCDC 1142231: Huber, R.; Knierzinger, A.; Obrecht, J.P.; Vasella, A. Nucleophilic additions to N-glycosylnitrones. Asymmetric synthesis of α-aminophosphonic acids. Helv. Chim. Acta 1985, 68, 1730–1747. [Google Scholar] [CrossRef]
- CCDC CCDC 1236701, CCDC 1236702, CCDC 1236703: Yager, K.M.; Taylor, C.M.; Smith III, A.B. Asymmetric synthesis of α-aminophosphonates via diastereoselective addition of lithium diethyl phosphite to chelating imines. J. Am. Chem. Soc. 1994, 116, 9377–9378. [Google Scholar]
- CCDC 1216345: Peng, J.; Barr, M.E.; Ashburn, D.A.; Lebioda, L.; Garber, A.R.; Martinez, R.A.; Odom, J.D.; Dunlap, R.B.; Silks III, L.A. Synthesis and characterization of acylated chiral oxazolidine-2-selones: Selone chiral derivatizing agents for the detection and quantitation of remotely disposed chiral centers. J. Org. Chem. 1995, 60, 5540–5549. [Google Scholar] [CrossRef]
- CCDC 630372: Garcia-Saez, I.; DeBonis, S.; Lopez, R.; Trucco, F.; Rousseau, B.; Thuéry, P.; Kozielski, F. Structure of human Eg5 in complex with a new monastrol-based inhibitor bound in the R configuration. J. Biol. Chem. 2007, 282, 9740–9747. [Google Scholar] [CrossRef] [PubMed]
- CCDC 143886: Enders, D.; Janeck, C.F.; Raabe, G. Asymmetric β-aminoethylation of ketones and nitriles with tosylaziridines employing the SAMP-hydrazone method. Eur. J. Org. Chem. 2000, 3337–3345. [Google Scholar] [CrossRef]
- CCDC 1261296: Mercury software could not load the CIF file: Nakagawa, K.; Somei, M. Ergot alkaloids: The first and five step total syntheses of (−)-and (+)-6,7-secoagroclavines, and syntheses of (−)- and (+)-6-nor-6-propyl-6,7-secoagroclavines ((−)-and (+)-KSU 1415). Heterocycles 1991, 32, 873–878. [Google Scholar]
- CCDC1241198: Mercury software could not load the CIF file: Eberle, M.K.; Keese, R.; Stoeckli-Evans, H. New synthesis and chirality of (−)-4,4,4,4′,4′,4′-hexafluorovaline. Helv. Chim. Acta 1998, 81, 182–186. [Google Scholar]
- Thayer, A.M. Centering on chirality. Chem. Eng. News 2007, 85, 11–19. [Google Scholar]
- Amine Based Pharmaceutical Drugs. Available online: https://www.jacobs-university.de/ses/tnugent/research/chiralamines/drugs (accessed on 3 June 2015).
- Hutt, A.J.; Valentová, J. The chiral switch: The development of single enantiomer drugs from racemates. Acta Fac. Pharm. Univ. Comen. 2003, Tomus L, 7–22. [Google Scholar]
- Desiraju, G.R. Crystal engineering: from molecule to crystal. J. Am. Chem. Soc. 2013, 135, 9952–9967. [Google Scholar] [CrossRef] [PubMed]
- Saigo, K.; Sakai, K. Toward efficient optical resolution by diastereomeric salt formation. J. Syn. Org. Chem. Jpn. 2011, 69, 499–505. [Google Scholar] [CrossRef]
- IUPAC Gold Book recommends that the terms s-cis and s-trans should not be applied to the N-alkyl amides. IUPAC. Compendium of Chemical Terminology, 2nd ed.; (the “Gold Book”); McNaught, A.D., Wilkinson, A., Eds.; Blackwell Scientific Publications: Oxford, UK, 1997. [Google Scholar] XML on-line corrected version: http://goldbook.iupac.org (2006-) Created by Nic, M.; Jirat, J.; Kosata, B. Updates compiled by Jenkins, A.
- Desiraju, G.R. Hydrogen bridges in crystal engineering: Interactions without borders. Acc. Chem. Res. 2002, 35, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Brand, D.J.; Steenkamp, J.A.; Brandt, E.V.; Takeuchi, Y. Conformational studies of (−)-epicatechin-Mosher ester. Tetrahedron Lett. 2007, 48, 2769–2773. [Google Scholar] [CrossRef]
- Khan, M.A.; Tavares, D.F.; Rauk, A. Magnetic non-equivalence of fluorine atoms of a trifluoromethyl group. Can. J. Chem. 1982, 60, 2451–2455. [Google Scholar] [CrossRef]
- Nishio, M. Chapter 6: CH/π hydrogen bonds. In New Edition: Introduction to Intermolecular Forces in Organic Chemistry; Kodansha Scientific: Tokyo, Japan, 2008; pp. 42–55. [Google Scholar]
- Hoye, T.R.; Renner, M.K. Application of MTPA (Mosher) Amides of secondary amines: Assignment of absolute configuration in chiral cyclic amines. J. Org. Chem. 1996, 61, 8489–8495. [Google Scholar] [CrossRef]
- Kang, C.Q.; Guo, H.Q.; Qiu, X.P.; Bai, X.L.; Yao, H.B.; Gao, L.X. Assignment of absolute configuration of cyclic secondary amines by NMR techniques using Mosher’s method: A general procedure exemplified with (−)-isoanabasine. Magn. Reson. Chem. 2006, 44, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Anslyn, E.V.; Dougherty, D.A. Chapter 6: Stereochemistry. In Modern Physical Organic Chemistry; University Science Books: Sausalito, CA, USA, 2005; pp. 297–350. [Google Scholar]
- Azumaya, I. Discovery of cis-preference of aromatic N-methylamides and its application to molecular constructions. Yakugaku Zasshi 2001, 121, 117–129. [Google Scholar] [CrossRef] [PubMed]
- The Conformational Preference of s-cis Amides. Available online: http://www.ch.imperial.ac.uk/rzepa/blog/?p=9459 (accessed on 4 June 2015).
- Shinitzky reviewed the slight differences of physico-chemical properties between enantiomers in certain cases, and reported the concept of space asymmetry. Shinitzky, M. Space asymmetry as a possible grobal feature. Chirality 2013, 25, 308–311. [Google Scholar]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ichikawa, A.; Ono, H.; Mikata, Y. Characteristic Conformation of Mosher’s Amide Elucidated Using the Cambridge Structural Database. Molecules 2015, 20, 12880-12900. https://doi.org/10.3390/molecules200712880
Ichikawa A, Ono H, Mikata Y. Characteristic Conformation of Mosher’s Amide Elucidated Using the Cambridge Structural Database. Molecules. 2015; 20(7):12880-12900. https://doi.org/10.3390/molecules200712880
Chicago/Turabian StyleIchikawa, Akio, Hiroshi Ono, and Yuji Mikata. 2015. "Characteristic Conformation of Mosher’s Amide Elucidated Using the Cambridge Structural Database" Molecules 20, no. 7: 12880-12900. https://doi.org/10.3390/molecules200712880
APA StyleIchikawa, A., Ono, H., & Mikata, Y. (2015). Characteristic Conformation of Mosher’s Amide Elucidated Using the Cambridge Structural Database. Molecules, 20(7), 12880-12900. https://doi.org/10.3390/molecules200712880