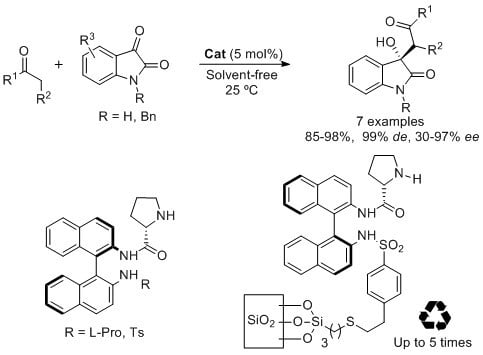
Enantioselective Solvent-Free Synthesis of 3-Alkyl-3-hydroxy-2-oxoindoles Catalyzed by Binam-Prolinamides
Abstract
:1. Introduction
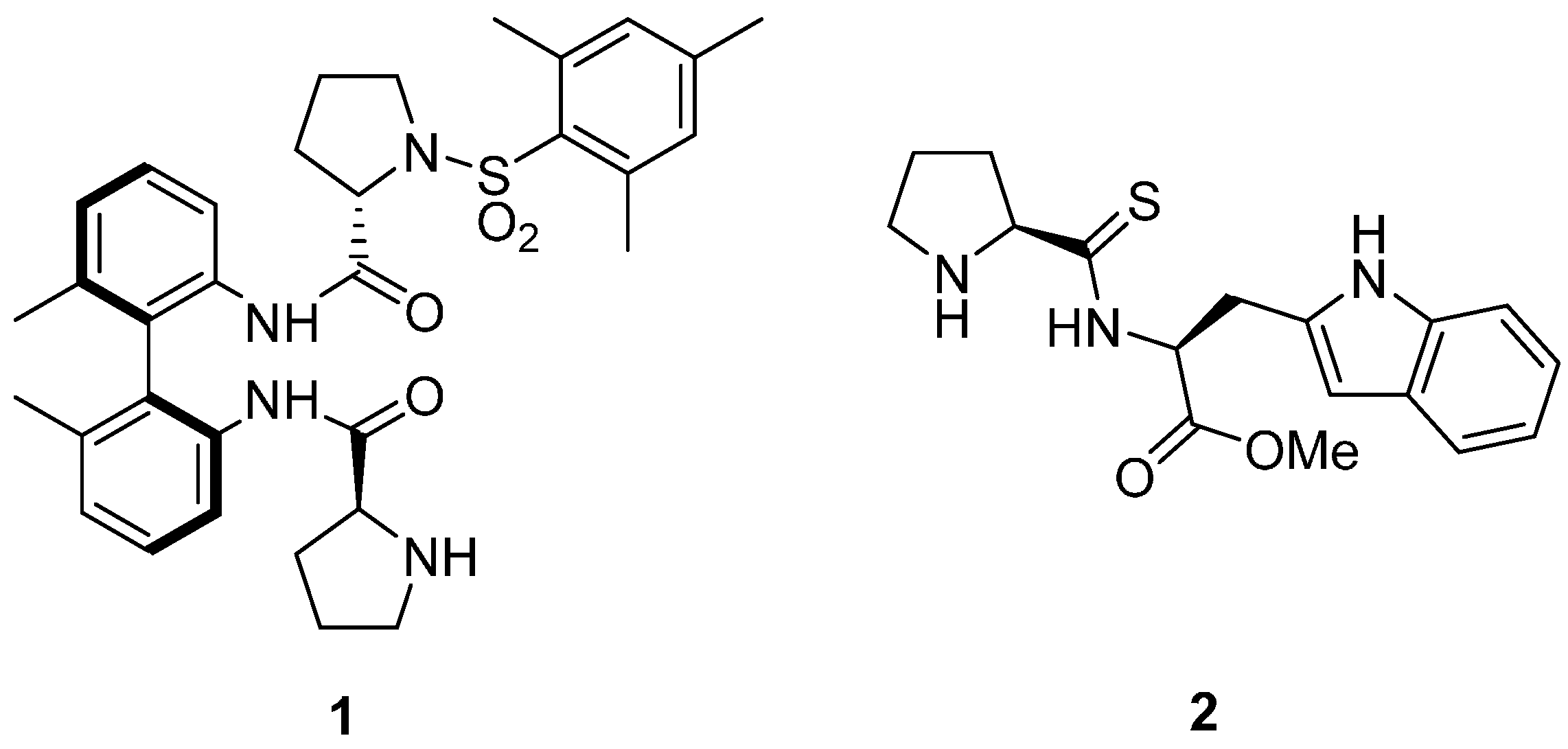
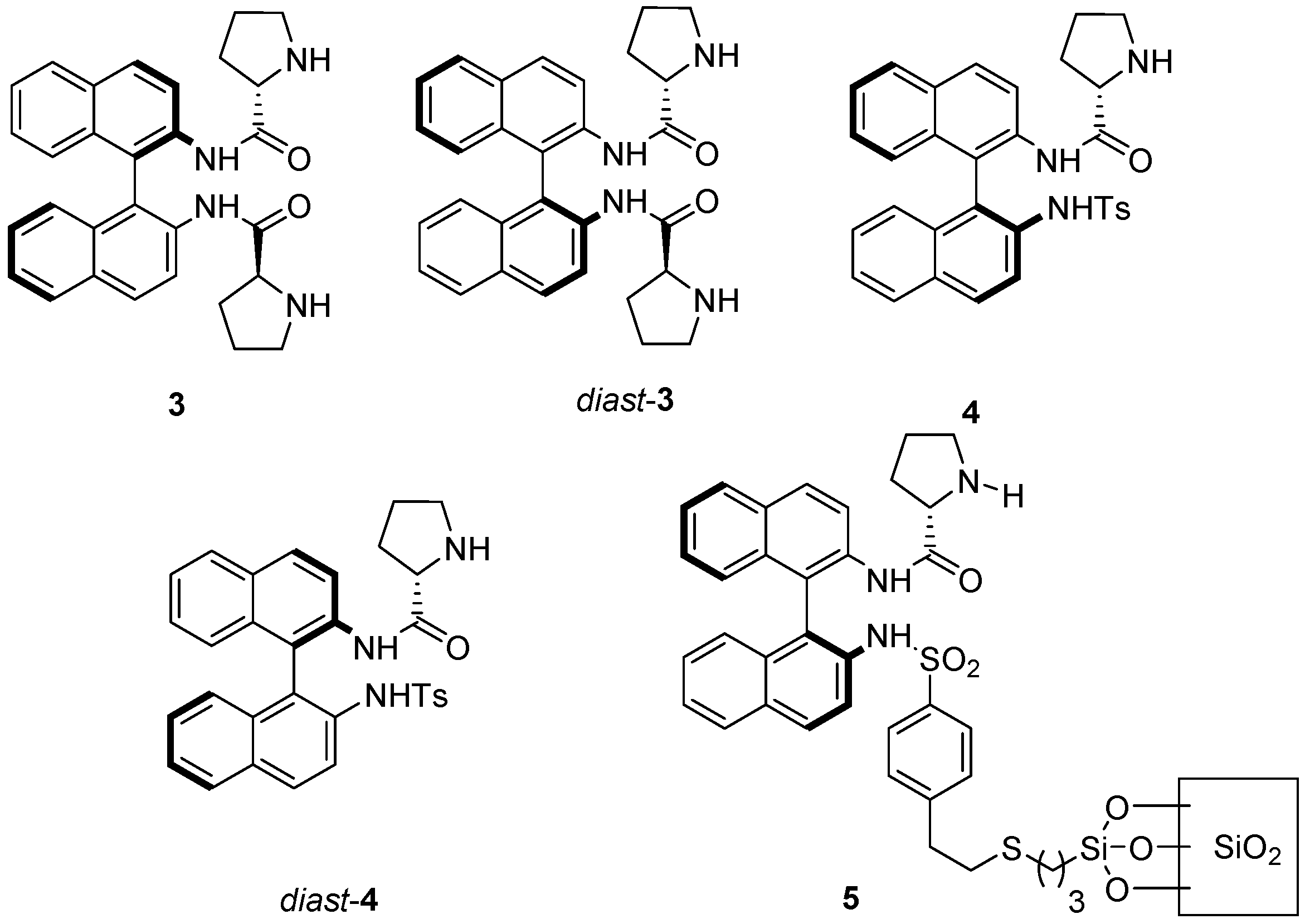
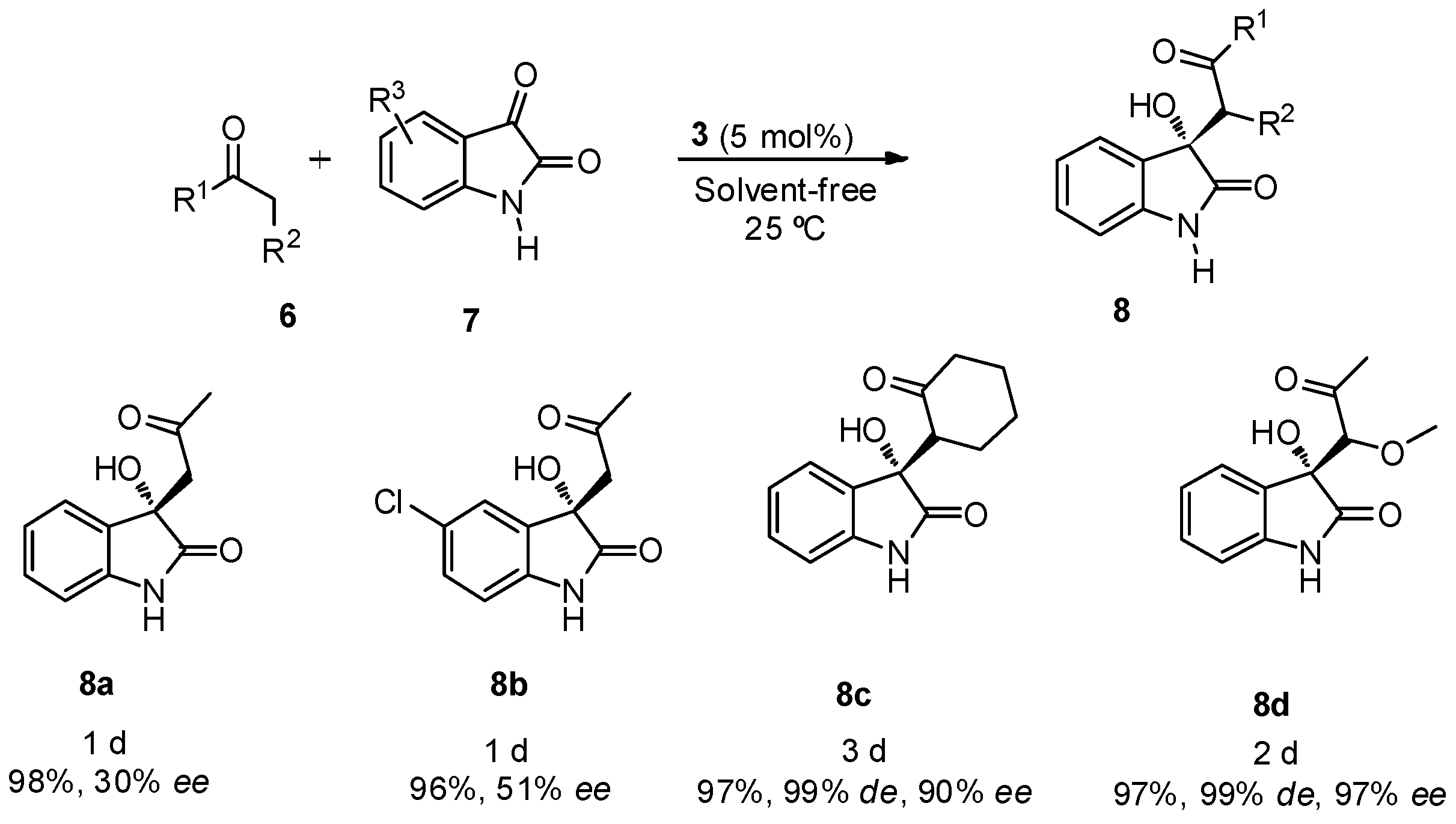
2. Results and Discussion
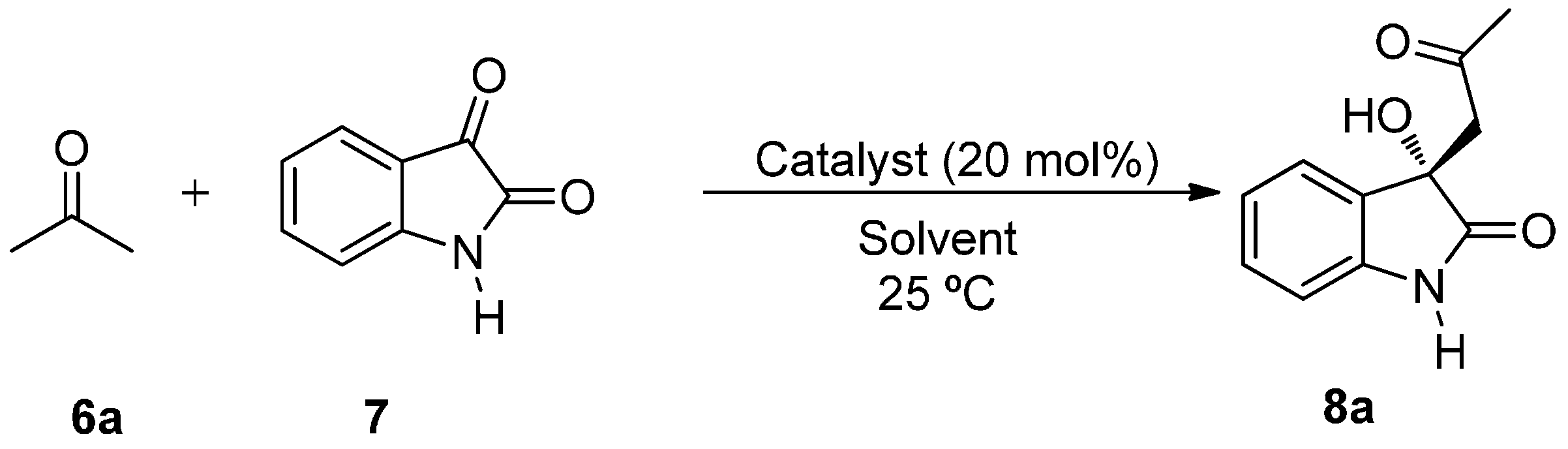
| Entry | Solvent a | Cat. (mol %) | Ketone (equiv.) | T a (°C) | t | Conv (%) b | ee (%) c |
|---|---|---|---|---|---|---|---|
| 1 | DMSO | 3(20) | 3 | 25 | 3 days | <50 | 12 |
| 2 | THF | 3 (20) | 3 | 25 | 3 days | <30 | 21 |
| 3 | Hexane | 3 (20) | 3 | 25 | 3 days | <20 | 14 |
| 4 | H2O:DMF | 3 (20) | 3 | 25 | 3 days | <20 | 0 |
| 5 | DMF | 3 (20) | 3 | 25 | 3 days | <20 | 0 |
| 6 | H2O | 3 (20) | 3 | 25 | 3 days | <30 | 11 |
| 7 | – | 3 (20) | 3 | 25 | 6 h | >99 | 29 |
| 8 | – | 3 (20) | 3 | 0 | 1 days | 83 | 27 |
| 9 | – | 3 (20) | 3 | −20 | 3 days | 74 | 31 |
| 10 | – | 3 (20) | 3 | −50 | 3 days | <50 | 33 |
| 11 | – | 3 (20) | 3 | −70 | 3 days | <30 | 30 |
| 12 d | – | 3 (20) | 3 | 25 | 6 h | >99 | 22 |
| 13 d,e | – | 3 (20) | 3 | 25 | 4 h | >99 | 17 |
| 14 e | – | 3 (20) | 3 | 25 | 4 h | >99 | 20 |
| 15 | – | 3 (5) | 3 | 25 | 1 days | >99 | 31 |
| 16 | – | 3 (10) | 3 | 25 | 1 days | >99 | 28 |
| 17 | – | 3 (5) | 2 | 25 | 1 days | >99 | 30 |
| 18 | – | 3 (5) | 1 | 25 | 1 days | 82 | 27 |
| 19 | Acetone | 3 (5) | excess | 25 | 1 days | >99 | 22 |
| 20 | – | diast-3 (5) | 2 | 25 | 1 days | >99 | 20 |
| 21 | – | 4 (5) | 2 | 25 | 3 days | <30 | 24 |
| 22 | – | diast-4 (5) | 2 | 25 | 3 days | <30 | 29 |
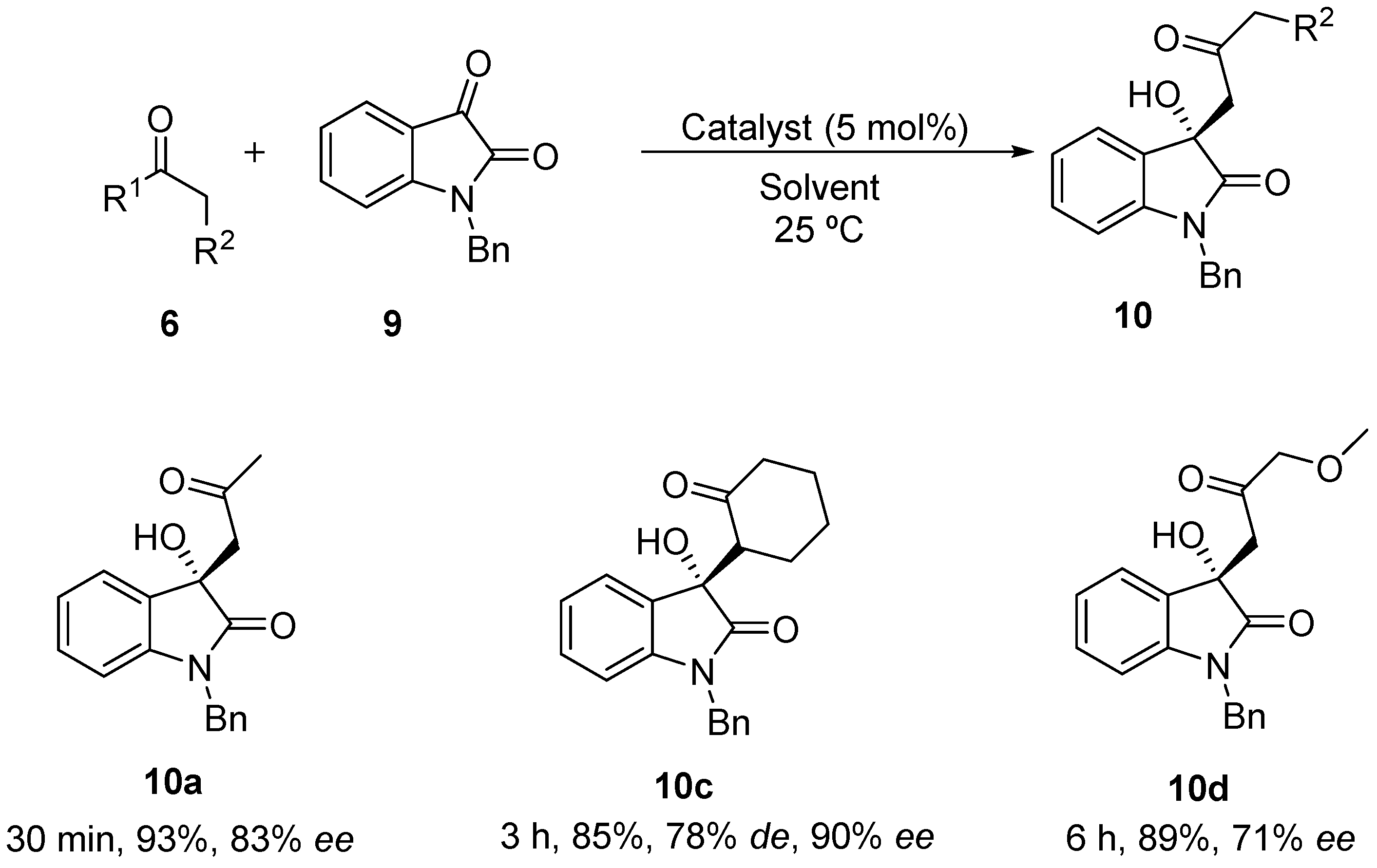
| Entry | R1,R2 | Cat. (mol %) | Product | Ketone (equiv.) | T a (°C) | t (min) | Conv (%) a,b | de (%) c | ee (%) d |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Me,H | 3 | 10a | 2 | 25 | 5 | >99 | - | 0 |
| 2 | Me,H | diast-3 | 10a | 2 | 25 | 5 | >99 | - | 0 |
| 3 | Me,H | 4 | 10a | 2 | 25 | 30 | >99 (93) | - | 83 |
| 4 | Me,H | diast-4 | 10a | 2 | 25 | 90 | >99 | - | 75 |
| 5 e | Me,H | 4 | 10a | 2 | 25 | 30 | >99 | - | 70 |
| 6 f | Me,H | 4 | 10a | 2 | 25 | 30 | >99 | - | 59 |
| 7 e,f | Me,H | 4 | 10a | 2 | 25 | 30 | >99 | - | 63 |
| 8 | (CH2)4 | 3 | 10c | 2 | 25 | 90 | 90 (85) | 78 | 90 |
| 9 | Me,OMe | 3 | 10d | 2 | 25 | 360 | 92 (89) | - | 71 |
3. Experimental Section
3.1. General Information
3.2. Procedure for the Addition of Ketones to Isatins
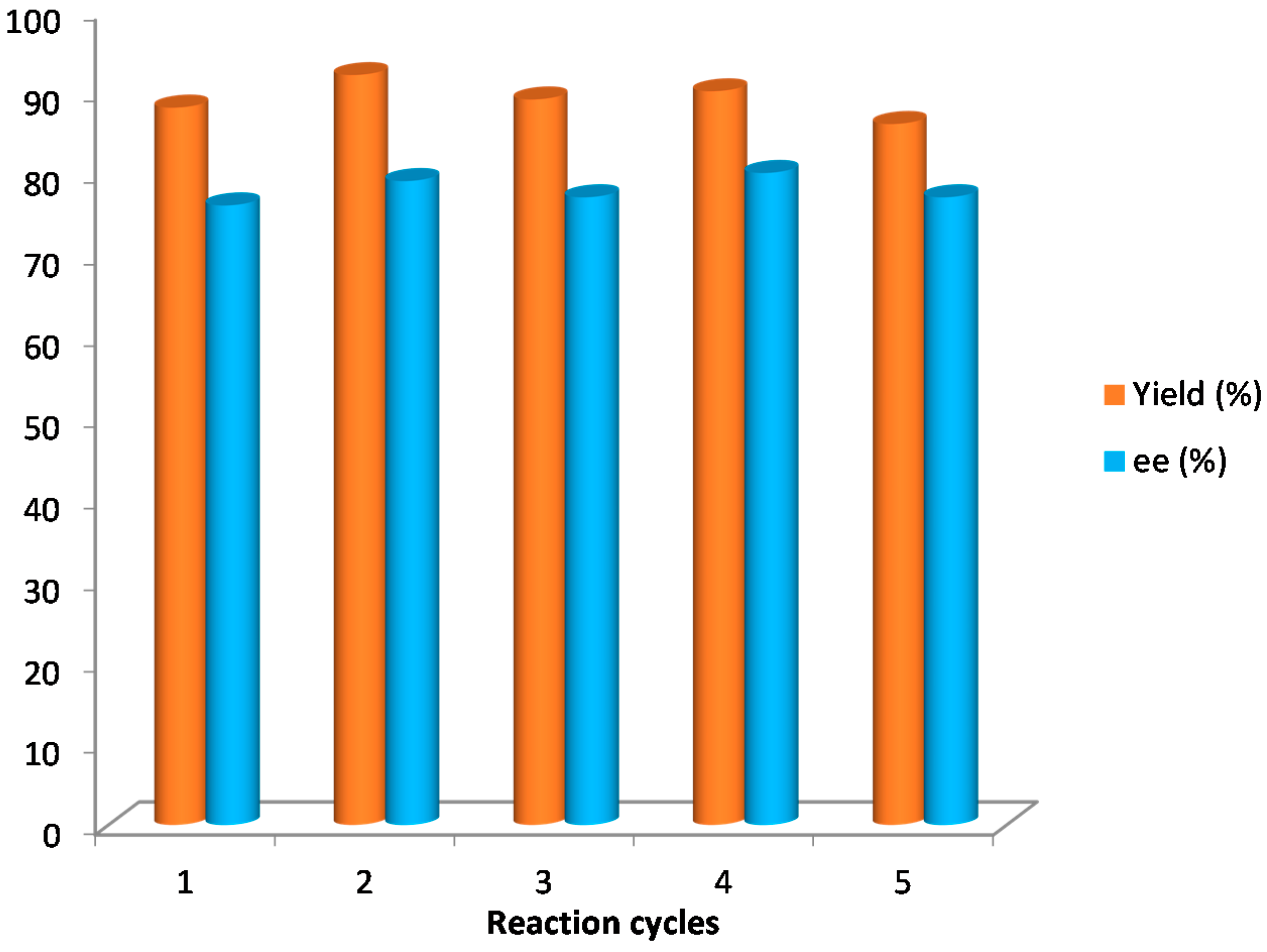
3.3. Procedure for Catalysis Recover and Reuse
3.4. Physical, Analytical and Spectal Data
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Da Silva, J.F.M.; Garden, S.J.; Pinto, A.C. The Chemistry of Isatins: A Review from 1975 to 1999. J. Braz. Chem. Soc. 2001, 12, 273–324. [Google Scholar] [CrossRef]
- Liu, Y.C.; Zhang, R.; Wu, Q.Y.; Chen, Q.; Yang, G.F. Recent Developments in the Synthesis and Applications of Isatins. Org. Prep. Proc. Int. 2014, 46, 317–362. [Google Scholar] [CrossRef]
- Grewal, A.S. Isatin derivatives with several biological activities. Int. J. Pharm. Res. 2014, 6, 1–7. [Google Scholar]
- Khan, N.; Khan, Z.; Ahmed, W.; Khan, N.; Hameed, Z. Recent Pharmacological Advancements in Isatin Chemistry. Int. J. Chem. Sci. 2014, 12, 1596–1606. [Google Scholar]
- Phogat, P.; Singh, P. A Mini Review on Central Nervous System Potential of Isatin Derivatives. Cent. Nerv. Syst. Agents Med. Chem. 2015, 15, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Heiran, R.; Herrera, R.P.; Marqués-López, E. Isatin as a Strategic Motif for Asymmetric Catalysis. ChemCatChem 2013, 5, 2131–3148. [Google Scholar] [CrossRef]
- Flores, M.; Peña, J.; García-García, P.; Garrido, N.M.; Díez, D. Enantioselective Organocatalytic Reactions of Isatin. Curr. Org. Chem. 2013, 17, 1957–1985. [Google Scholar] [CrossRef]
- Guillena, G.; Nájera, C.; Ramón, D.J. Enantioselective Direct Aldol Reaction: The Blossoming of Modern Organocatalysis. Tetrahedron Asymmetry 2007, 18, 2249–2293. [Google Scholar] [CrossRef]
- Geary, L.M.; Hultin, P.G. The State of the Art in Asymmetric Induction: The Aldol Reaction as a Case Study. Tetrahedron Asymmetry 2009, 20, 131–173. [Google Scholar] [CrossRef]
- Zlotin, S.G.; Kucherenko, A.S.; Beletskaya, I.P. Organocatalysis of Asymmetric Aldol Reaction. Catalysts and Reagents. Russ. Chem. Rev. 2009, 78, 737–784. [Google Scholar] [CrossRef]
- Trost, B.; Brindle, C.S. The Direct Catalytic Asymmetric Aldol Reaction. Chem. Soc. Rev. 2010, 39, 1600–1632. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.M.; Asadi, S. Recent applications of organocatalysts in asymmetric aldol reactions. Tetrahedron Asymmetry 2012, 23, 1431–1465. [Google Scholar] [CrossRef]
- Guillena, G. Modern Methods in Stereoselective Aldol Reactions; Mahrwald, R., Ed.; Wiley-VCH: Weinheim, Germany, 2013; pp. 155–268. [Google Scholar]
- Chen, G.; Wang, Y.; He, H.; Gao, S.; Yang, X.; Hao, X. l-Proline-Catalyzed Asymmetric Aldol Condensation of N-Substituted Isatins with Acetone. Heterocycles 2006, 68, 2327–2333. [Google Scholar] [CrossRef]
- Corrêa, R.J.; Garden, S.J.; Angelici, G.; Tomasini, C. A DFT and AIM Study of the Proline-Catalyzed Asymmetric Cross-Aldol Addition of Acetone to Isatins: A Rationalization for the Reversal of Chirality. Eur. J. Org. Chem. 2008, 2008, 736–744. [Google Scholar] [CrossRef]
- Luppi, G.; Cozzi, P.G.; Monari, M.; Kaptein, B.; Broxterman, Q.B.; Tomasini, C. Dipeptide-Catalyzed Asymmetric Aldol Condensation of Acetone with (N-Alkylated) Isatins. J. Org. Chem. 2005, 70, 7418–7421. [Google Scholar] [CrossRef] [PubMed]
- Angelici, G.; Corrêa, R.J.; Garden, S.J.; Tomasini, C. Water influences the enantioselectivity in the proline or prolinamide-catalyzed aldol addition of acetone to isatins. Tetrahedron Lett. 2009, 50, 814–817. [Google Scholar] [CrossRef]
- Chen, J.R.; Liu, X.P.; Zhu, X.Y.; Li, L.; Qiao, Y.F.; Zhang, J.M.; Xiao, W.J. Organocatalytic asymmetric aldol reaction of ketones with isatins: Straightforward stereoselective synthesis of 3-alkyl-3-hydroxyindolin-2-ones. Tetrahedron 2007, 63, 10437–10444. [Google Scholar] [CrossRef]
- Nakamura, S.; Hara, N.; Nakashima, H.; Kubo, K.; Shibata, N.; Toru, T. Enantioselective Synthesis of (R)-Convolutamydine A with New N-Heteroarylsulfonylprolinamides. Chem. Eur. J. 2008, 14, 8079–8081. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Zhang, S.; Liu, L.; Duan, W.; Wang, W. Organocatalytic Enantioselective Cross-Aldol Reactions of Aldehydes with Isatins: Formation of Two Contiguous Quaternary Centered 3-Substituted 3-Hydroxyindol-2-ones Chem. Asian J. 2009, 4, 1664–1667. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.B.; Du, X.L.; Cun, L.F.; Zhang, X.M.; Yuan, W.C. Highly enantioselective aldol reaction of acetaldehyde and isatins only with 4-hydroxydiarylprolinol as catalyst: Concise stereoselective synthesis of (R)-convolutamydines B and E, (−)-donaxaridine and (R)-chimonamidine. Tetrahedron 2010, 66, 1441–1446. [Google Scholar] [CrossRef]
- Pearson, A.J.; Panda, S. N-Prolinylanthranilamide Pseudopeptides as Bifunctional Organocatalysts for Asymmetric Aldol Reactions. Org. Lett. 2011, 13, 5548–5551. [Google Scholar] [CrossRef] [PubMed]
- Kinsella, M.; Duggan, P.G.; Lennon, C.M. Screening of simple N-aryl and N-heteroaryl pyrrolidine amide organocatalysts for the enantioselective aldol reaction of acetone with isatin. Tetrahedron Asymmetry 2011, 22, 1423–1433. [Google Scholar] [CrossRef]
- Pearson, A.J.; Panda, S.; Bunge, S.D. Synthesis of a Potential Intermediate for TMC-95A via an Organocatalyzed Aldol Reaction. J. Org. Chem. 2013, 78, 9921–9928. [Google Scholar] [CrossRef] [PubMed]
- Bañón-Caballero, A.; Guillena, G.; Najera, C. Solvent-Free Enantioselective Organocatalyzed Aldol Reactions. Mini-Rev. Org. Chem. 2014, 11, 118–128. [Google Scholar] [CrossRef]
- Zhang, F.; Li, C.; Qi, C. Highly diastereo- and enantioselective direct aldol reaction under solvent-free conditions. Tetrahedron Asymmetry 2013, 24, 380–388. [Google Scholar] [CrossRef]
- Hernández, J.G.; García-López, V.; Juaristi, E. Solvent-free asymmetric aldol reaction organocatalyzed by (S)-proline-containing thiodipeptides under ball-milling conditions. Tetrahedron 2012, 68, 92–97. [Google Scholar] [CrossRef]
- Guillena, G.; Hita, M.C.; Nájera, C. BINAM-prolinamides as recoverable catalysts in the direct aldol condensation. Tetrahedron Asymmetry 2006, 17, 729–733. [Google Scholar] [CrossRef]
- Gryko, D.; Kowalczyk, B.; Zawadzki, L. Bisprolinediamides with the binaphthyl backbone as organocatalysts for the direct asymmetric aldol reaction. Synlett 2006, 1059–1062. [Google Scholar] [CrossRef]
- Guillena, G.; Hita, M.C.; Nájera, C. High acceleration of the direct aldol reaction cocatalyzed by BINAM-prolinamides and benzoic acid in aqueous media. Tetrahedron Asymmetry 2006, 17, 1493–1497, (Corrigendum: Tetrahedron Asymmetry 2007, 18, 1031). [Google Scholar] [CrossRef]
- Guizzetti, S.; Benaglia, M.; Pignataro, L.; Puglisi, A. A multifunctional proline-based organic catalyst for enantioselective aldol reactions. Tetrahedron Asymmetry 2006, 17, 2754–2760. [Google Scholar] [CrossRef]
- Ma, G.N.; Zhang, Y.P.; Shi, M. l-Proline diamides with an axially chiral binaphthylene backbone as efficient- organocatalysts for direct asymmetric aldol reactions: The effect of acetic acid. Synthesis 2007, 197–208. [Google Scholar] [CrossRef]
- Guizzetti, S.; Benaglia, M.; Raimondi, L.; Celentano, G. Enantioselective direct aldol reaction “on water” promoted by chiral organic catalysts. Org. Lett. 2007, 9, 1247–1250. [Google Scholar] [CrossRef] [PubMed]
- Guillena, G.; Hita, M.C.; Nájera, C. Organocatalyzed direct aldol condensation using l-proline and BINAM-prolinamides: Regio-, diastereo-, and enantioselective controlled synthesis of 1,2-diols. Tetrahedron Asymmetry 2006, 17, 1027–1031, (Corrigendum: Tetrahedron Asymmetry 2007, 18, 1030). [Google Scholar] [CrossRef]
- Guillena, G.; Hita, M.C.; Nájera, C. Highly selective direct aldol reaction organocatalyzed by (S)-BINAM-l-prolinamide and benzoic acid using α-chalcogen-substituted ketones as donors. ARKIVOC 2007, iv, 260–269, (Corrigendum: ARKIVOC 2007, i, 146–147). [Google Scholar] [CrossRef]
- Guillena, G.; Hita, M.C.; Nájera, C. α-Chloroacetone as a donor in the BINAM-l-prolinamide organocatalyzed aldol reaction: Application to the enantioselective synthesis of α,β-epoxy ketones. Tetrahedron Asymmetry 2007, 18, 1272–1277. [Google Scholar] [CrossRef]
- Kucherenko, A.S.; Syutkin, D.E.; Zlotinivat, S.G. Asymmetric aldol condensation in an ionic liquid-water system catalyzed by (S)-prolinamide der ives. Russ. Chem. Bull. 2008, 57, 591–594. [Google Scholar] [CrossRef]
- Moles, F.J.N.; Guillena, G.; Nájera, C. Aqueous organocatalyzed aldol reaction of glyoxylic acid for the enantioselective synthesis of α-hydroxy-γ-keto acids. RSC Adv. 2014, 4, 9963–9966. [Google Scholar] [CrossRef]
- Moles, F.J.N.; Bañón-Caballero, A.; Guillena, G.; Nájera, C. Enantioselective aldol reactions with aqueous 2,2-dimethoxyacetaldehyde organocatalyzed by binam-prolinamides under solvent-free conditions. Tetrahedron Asymmetry 2014, 25, 1323–1330. [Google Scholar] [CrossRef] [Green Version]
- Moles, F.J.N.; Guillena, G.; Nájera, C. Glyoxylic Acid versus Ethyl Glyoxylate for the Aqueous EnantioselectiveSynthesis of α-Hydroxy-β-Keto Acids and Esters by the N-Tosyl-(Sa)-binam-prolinamide-Organocatalyzed Aldol Reaction. Synthesis 2015. [Google Scholar] [CrossRef]
- Moles, F.J.N.; Guillena, G.; Nájera, C. Aqueous enantioselective aldol reaction of methyl- and phenylglyoxal organocatalyzed by N-Tosyl-(Sa)-binam-l-prolinamide. Synlett 2015, 26, 656–660. [Google Scholar] [CrossRef]
- Guillena, G.; Hita, M.C.; Nájera, C.; Viózquez, S.F. Solvent-free asymmetric direct aldol reactions organocatalysed by recoverable (Sa)-binam-l-prolinamide. Tetrahedron Asymmetry 2007, 18, 2300–2304. [Google Scholar] [CrossRef]
- Guillena, G.; Hita, M.C.; Nájera, C.; Viózquez, S.F. A highly efficient solvent-free asymmetric direct aldol reaction organocatalyzed by recoverable (S)-binam-l-prolinamides. ESI-MS evidence of the enamine-iminium formation. J. Org. Chem. 2008, 73, 5933–5943. [Google Scholar] [CrossRef] [PubMed]
- Bañón-Caballero, A.; Guillena, G.; Nájera, C. Solvent-Free Enantioselective Friedlander Condensation with Wet 1,1′-Binaphthalene-2,2′-diamine-Derived Prolinamides as Organocatalysts. J. Org. Chem. 2013, 78, 5349–5356. [Google Scholar] [CrossRef] [PubMed]
- Viózquez, S.F.; Bañón- Caballero, A.; Guillena, G.; Nájera, C; Gómez-Bengoa, E. Enantioselective direct aldol reaction of α-keto esters catalyzed by (Sa)-binam-d-prolinamide under quasi solvent-free conditions. Org. Biomol. Chem. 2012, 10, 4029–4035. [Google Scholar] [CrossRef] [PubMed]
- Guillena, G.; Nájera, C.; Viózquez, S.F. N-Tosyl-(Sa)-binam-l-prolinamide as highly efficient bifunctional organocatalyst for the general enantioselective solvent-free aldol reaction. Synlett 2008, 3031–3035. [Google Scholar] [CrossRef]
- Bradshaw, B.; Etxebarria-Jardí, G.; Bonjoch, J.; Viózquez, S.F.; Guillena, G.; Nájera, C. Efficient solvent-free Robinson annulation protocols for the highly enantioselective synthesis of the Wieland-Miescher ketone and analogues. Adv. Synth. Catal. 2009, 351, 2482–2490. [Google Scholar] [CrossRef]
- Viózquez, S.F.; Guillena, G.; Nájera, C.; Bradshaw, B.; Etxebarria-Jardí, G.; Bonjoch, J. (Sa,S)-N-[2′-(4-Methylphenylsulfonamido)-1,1′-binaphthyl-2-yl]pyrrolidine-2-carboxamide: An organocatalyst for the direct aldol reaction. Org. Synth. 2011, 88, 317–329. [Google Scholar]
- Bradshaw, B.; Etxebarria-Jardí, G.; Bonjoch, J.; Viózquez, S.F.; Guillena, G.; Nájera, C. Synthesis of (S)-8a-methyl-3,4,8,8a-tetrahydro-1,6-(2H,7H)-naphthalenedione via N-tosyl-(Sa)-binam-l-prolinamide organocatalysis. Org. Synth. 2011, 88, 330–341. [Google Scholar]
- Bradshaw, B.; Etxebarria-Jardí, G.; Bonjoch, J. Total synthesis of (−)-anominine. J. Am. Chem. Soc. 2010, 132, 5966–5967. [Google Scholar] [CrossRef] [PubMed]
- Bañón-Caballero, A.; Guillena, G.; Nájera, C. Solvent-free direct enantioselective aldol reaction using polystyrene-supported N-sulfonyl-(Ra)-binam-d-prolinamide as a catalyst. Green Chem. 2010, 12, 1599–1606. [Google Scholar] [CrossRef]
- Bañón-Caballero, A.; Guillena, G.; Nájera, C. Cross-linked-polymer-supported N-{2′-[(Arylsulfonyl)amino][1,1′-binaphthalen]-2-yl}prolinamide as organocatalyst for the direct aldol intermolecular reaction under solvent-free conditions. Helv. Chim. Acta 2012, 95, 1831–1841. [Google Scholar] [CrossRef]
- Uozumi, Y.; Sakurai, F. Asymmetric aldol reaction with BINAM-sulfonyl polymeric organocatalyst. Synfacts 2013, 9, 114. [Google Scholar] [CrossRef]
- Bañón-Caballero, A.; Guillena, G.; Nájera, C.; Faggi, E.; Sebastián, R.M.; Vallribera, A. Recoverable silica-gel supported binam-prolinamides as organocatalysts for the enantioselective solvent-free intra- and intermolecular aldol reaction. Tetrahedron 2013, 69, 1307–1315. [Google Scholar] [CrossRef]
- Uozumi, Y.; Sakurai, F. Silica-supported prolinamide for solvent-free asymmetric aldol reaction. Synfacts 2013, 9. [Google Scholar] [CrossRef]
- Li, L.; Gou, S.; Liu, F. Highly stereoselective direct aldol reactions catalyzed by a bifunctional chiral diamine. Tetrahedron Asymmetry 2014, 25, 193–197. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bañn-Caballero, A.; Flores-Ferrándiz, J.; Guillena, G.; Nájera, C. Enantioselective Solvent-Free Synthesis of 3-Alkyl-3-hydroxy-2-oxoindoles Catalyzed by Binam-Prolinamides. Molecules 2015, 20, 12901-12912. https://doi.org/10.3390/molecules200712901
Bañn-Caballero A, Flores-Ferrándiz J, Guillena G, Nájera C. Enantioselective Solvent-Free Synthesis of 3-Alkyl-3-hydroxy-2-oxoindoles Catalyzed by Binam-Prolinamides. Molecules. 2015; 20(7):12901-12912. https://doi.org/10.3390/molecules200712901
Chicago/Turabian StyleBañn-Caballero, Abraham, Jesús Flores-Ferrándiz, Gabriela Guillena, and Carmen Nájera. 2015. "Enantioselective Solvent-Free Synthesis of 3-Alkyl-3-hydroxy-2-oxoindoles Catalyzed by Binam-Prolinamides" Molecules 20, no. 7: 12901-12912. https://doi.org/10.3390/molecules200712901