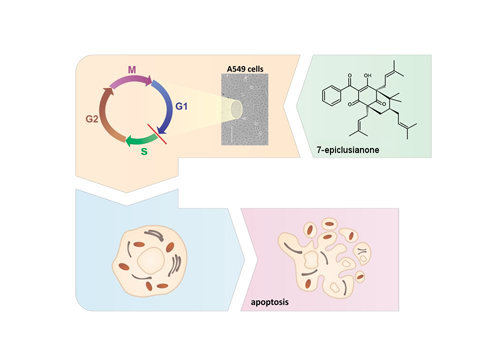
7-Epiclusianone, a Benzophenone Extracted from Garcinia brasiliensis (Clusiaceae), Induces Cell Cycle Arrest in G1/S Transition in A549 Cells
Abstract
:1. Introduction
2. Results and Discussion
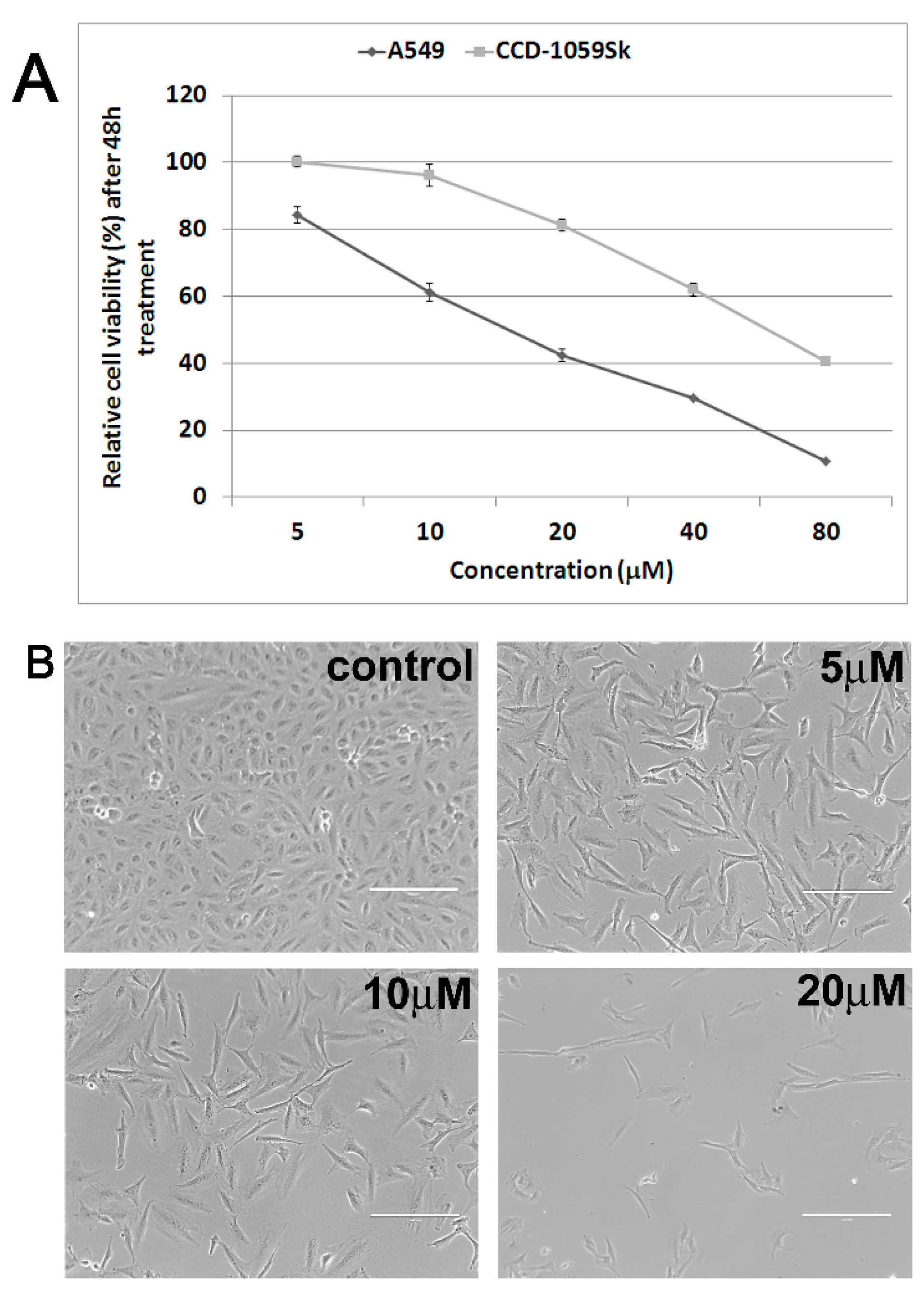
2.1. Antiproliferative and Cytotoxic Activity
| Cell Cycle Phases | Control | 5 µM | 10 µM |
|---|---|---|---|
| Sub-G1 | 0.90 ± 0.14 | 0.73 ± 0.17 | 1.79 ± 0.16 a |
| G0/G1 | 62.10 ± 1.06 | 73.83 ± 1.97 a | 75.20 ± 1.70 a |
| S | 19.77 ± 2.34 | 9.84 ± 0.80 a | 5.53 ± 0.43 a |
| G2/M | 17.23 ± 1.64 | 15.60 ± 1.55 a | 17.48 ± 1.94 |
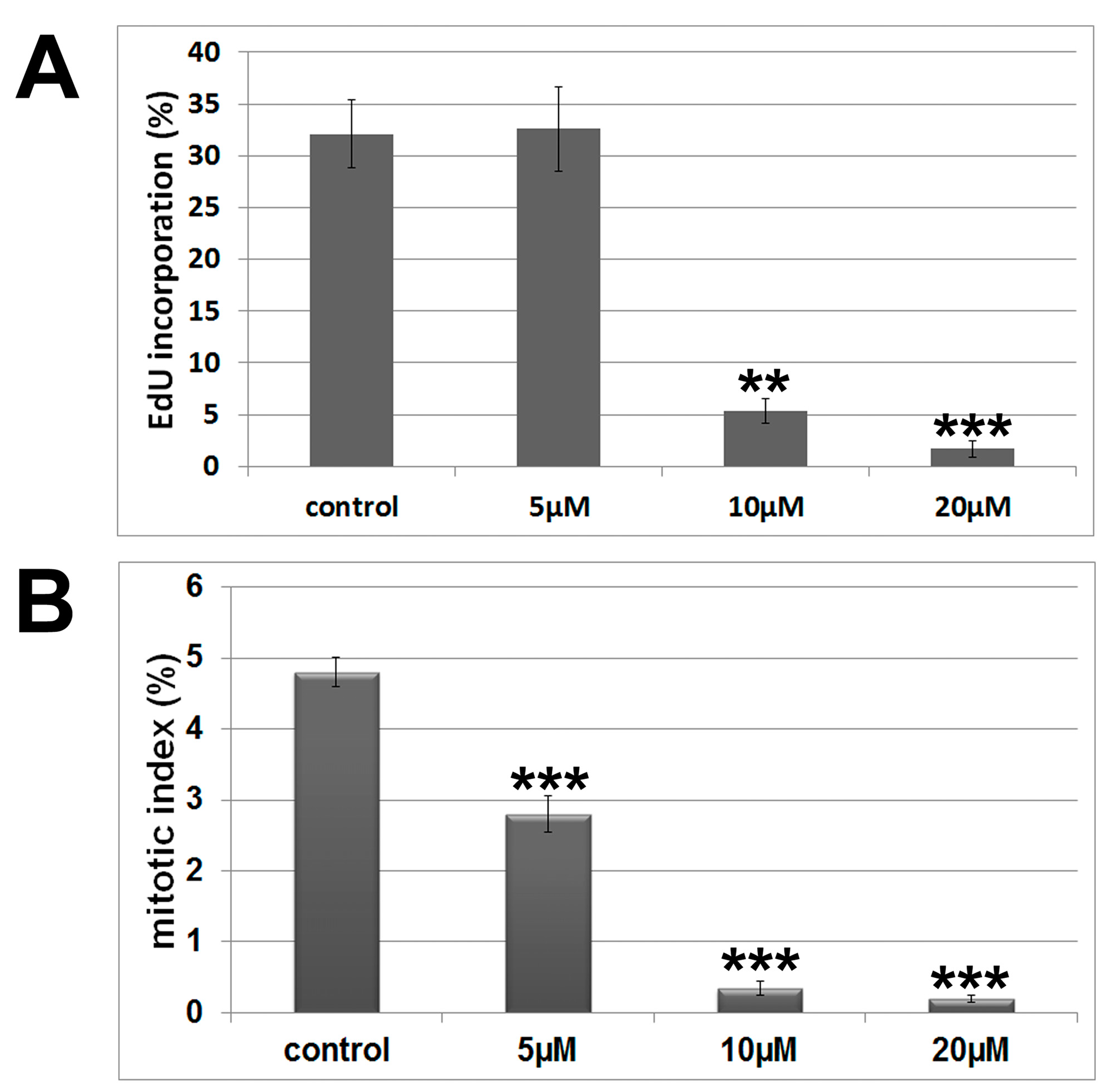
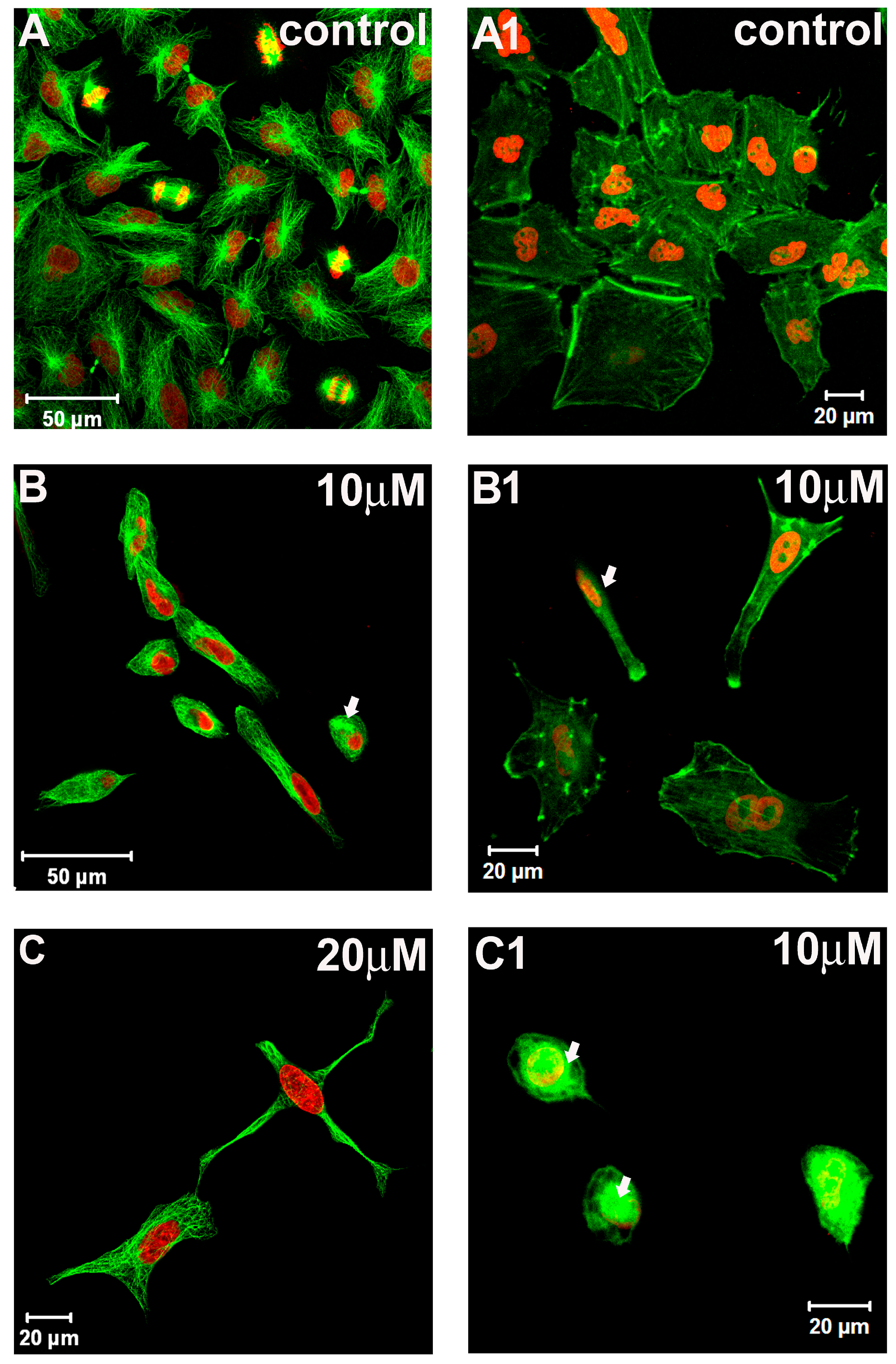
2.2. Cytoskeleton and Cell Morphology
3. Experimental Section
3.1. General Procedures
3.2. Isolation of 7-Epiclusianone from G. Braziliensis

3.3. Cell Culture Conditions
3.4. Cell Viability
3.5. Cell Cycle Analysis
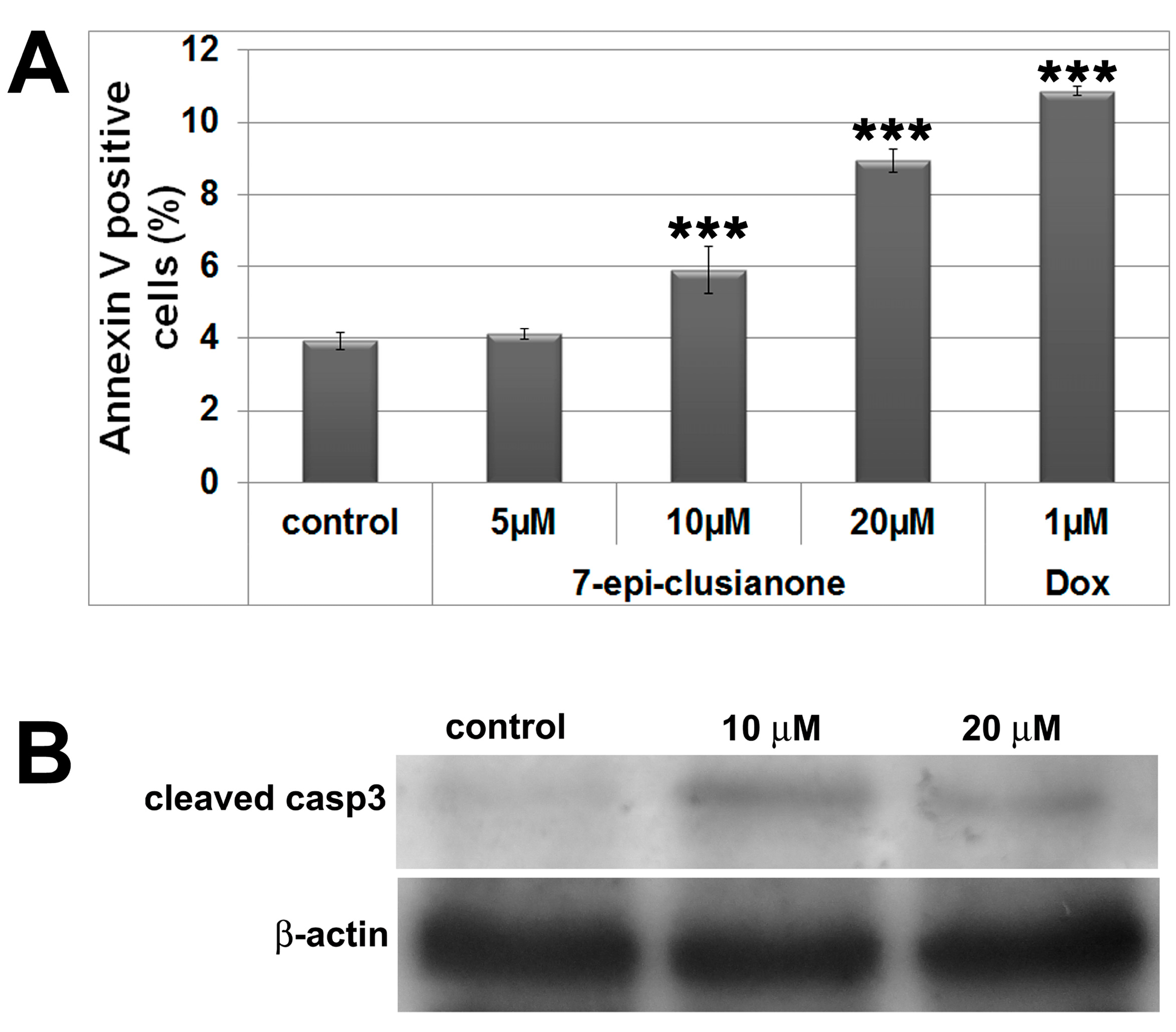
3.6. Annexin V Assay
3.7. Cytoskeleton Elements: Microtubules and F-Actin
3.8. Mitotic Index
3.9. S-phase Determination Using EdU Assay
3.10. Immunoblot
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jemal, A.; Center, M.M.; DeSantis, C.; Ward, E.M. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1893–1907. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Heymach, J.V.; Lippman, S.M. Lung cancer. N. Engl. J. Med. 2008, 359, 1367–1380. [Google Scholar] [CrossRef] [PubMed]
- Wistuba, I.I.; Gazdar, A.F. Lung cancer preneoplasia. Annu. Rev. Pathol. 2006, 1, 331–348. [Google Scholar] [CrossRef] [PubMed]
- Lauand, C.; Rezende-Teixeira, P.; Cortez, B.A.; Niero, E.L.D.O.; Machado-Santelli, G.M. Independent of ErbB1 gene copy number, EGF stimulates migration but is not associated with cell proliferation in non-small cell lung cancer. Cancer Cell Int. 2013, 13, 1–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, A. Strategies for discovering drugs from previously unexplored natural products. Drug Discov. Today 2000, 5, 294–300. [Google Scholar] [CrossRef]
- Santos, M.H.; Nagem, T.J.; de Oliveira, T.T.; Braz, R. 7-Epiclusianone, the new tetraprenylated benzophenone and others chemical constituents from the fruits of Rheedia gardneriana. Quím. Nova 1999, 5, 654–660. [Google Scholar] [CrossRef]
- Neves, J.S.; Coelho, L.P.; Cordeiro, R.S.B.; Veloso, M.P.; Silva, P.M.R.; Dos Santos, M.H.; Martins, M.A. Antianaphylactic properties of 7-epiclusianone, a tetraprenylated benzophenone isolated from Garcinia brasiliensis. Planta Med. 2007, 73, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.S.B.; Murata, R.M.; Yatsuda, R.; Dos Santos, M.H.; Nagem, T.J.; Alencar, S.M.; Koo, H.; Rosalen, P.L. Antimicrobial activity of Rheedia brasiliensis and 7-epiclusianone against Streptococcus mutans. Phytomedicine 2008, 15, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Murata, R.M.; Almeida, L.S.B.; Yatsuda, R.; Dos Santos, M.H.; Nagem, T.J.; Rosalen, P.L.; Koo, H. Inhibitory effects of 7-epiclusianone on glucan synthesis, acidogenicity and biofilm formation by Streptococcus mutans. FEMS Microbiol. Lett. 2008, 282, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Naldoni, F.J.; Claudino, A.L.R.; Cruz, J.W.; Chavasco, J.K.; Silva, P.M.F.; Veloso, M.P.; Dos Santos, M.H. Antimicrobial activity of benzophenones and extracts from the fruits of Garcinia brasiliensis. J. Med. Food 2009, 12, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Coelho, L.P.; Fra, M.; Lu, A.; Pires, D.A.; Henrique, M.; Aure, M. 7-Epiclusianone, a tetraprenylated benzophenone, relaxes airway smooth muscle through activation of the nitric oxide-cGMP pathway. J. Pharmacol. Exp. Ther. 2008, 327, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Santa-Cecília, F.V.; Freitas, L.S.; Vilela, F.C.; Veloso, C.D.C.; Rocha, C.Q.; Moreira, M.E.C.; Dias, D.F.; Giusti-Paiva, A.; Dos Santos, M.H. Antinociceptive and anti-inflammatory properties of 7-epiclusianone, a prenylated benzophenone from Garcinia brasiliensis. Eur. J. Pharmacol. 2011, 670, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Murata, R.M.; Yatsuda, R.; Dos Santos, M.H.; Kohn, L.K.; Martins, F.T.; Nagem, T.J.; Alencar, S.M.; de Carvalho, J.E.; Rosalen, P.L. Antiproliferative effect of benzophenones and their influence on cathepsin activity. Phytother. Res. 2010, 24, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Salic, A.; Mitchison, T.J. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc. Natl. Acad. Sci. USA 2007, 105, 2415–2420. [Google Scholar] [CrossRef] [PubMed]
- Protiva, P.; Hopkins, M.E.; Baggett, S.; Yang, H.; Lipkin, M.; Holt, P.R.; Kennelly, E.J.; Bernard, W.I. Growth inhibition of colon cancer cells by polyisoprenylated benzophenones is associated with induction of the endoplasmic reticulum response. Int. J. Cancer 2008, 123, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Parasramka, M.A.; Gupta, S.V. Garcinol inhibits cell proliferation and promotes apoptosis in pancreatic adenocarcinoma cells. Nutr. Cancer. 2011, 63, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Bartek, J.; Lukas, J. DNA damage checkpoints: From initiation to recovery or adaptation. Curr. Opin. Cell Biol. 2007, 19, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Farnebo, M.; Bykov, V.J.; Wiman, K.G. The p53 tumor suppressor: a master regulator of diverse cellular processes and therapeutic target in cancer. Biochem. Biophys. Res. Commun. 2010, 396, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Nedialkov, P.T.; Zheleva-Dimitrova, D.; Momekov, G.; Karlov, K.; Girreser, U.; Kitanov, G.M. Elegaphenone and 7-epiclusianone, the major cytotoxic constituents of Hypericum elegans. Nat. Prod. Res. 2011, 18, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Stanton, R.A.; Gernet, K.M.; Nettles, J.H.; Aneja, R. Drugs that target dynamic microtubules: A new molecular perspective. Med. Res. Rev. 2011, 31, 443–481. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, G.; TenDyke, K.; Towle, M.J.; Cheng, H.; Liu, J.; Marsh, J.P.; Schiller, S.E.; Spyvee, M.R.; Yang, H.; Seletsky, B.M.; et al. Tubulin-based antimitotic mechanism of E7974, a novel analogue of the marine sponge natural product hemiasterlin. Mol. Cancer Ther. 2009, 8, 2852–2860. [Google Scholar] [CrossRef] [PubMed]
- Niero, E.L.; Machado-Santelli, G.M. Cinnamic acid induces apoptotic cell death and cytoskeleton disruption in human melanoma cells. J. Exp. Clin. Cancer Res. 2013, 32, 31–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, A.K.; Sá-Júnior, P.L.; Pasqualoto, K.F.M.; Azevedo, R.A.; Câmara, D.A.D.; Costa, A.S.; Figueiredo, C.R.; Matsuo, A.L.; Massaoka, M.H.; Auada, A.V.V.; et al. Cytotoxic effects of dillapiole on MDA-MB-231 cells involve the induction of apoptosis through the mitochondrial pathway by inducing an oxidative stress while altering the cytoskeleton network. Biochimie 2014, 99, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Piccinelli, A.L.; Cuesta-Rubio, O.; Chica, M.B.; Mahmood, N.; Pagano, B.; Pavone, M.; Barone, V.; Rastrelli, L. Structural revision of clusianone and 7-epiclusianone and anti-HIV activity of polyisoprenylated benzophenones. Tetrahedron 2005, 61, 8206–8211. [Google Scholar] [CrossRef]
- Derogis, P.B.; Martins, F.T.; Souza, T.C.; Moreira, M.E.C.; Souza Filho, J.D.; Doriguetto, A.C.; Souza, K.R.; Veloso, M.P.; Dos Santos, M.H. Complete assignment of the 1H- and 13C-NMR spectra of garciniaphenone and ketoenol equilibrium statements for prenylated benzophenones. Magn. Res. Chem. 2008, 46, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Overbeeke, R.; Steffens-Nakken, H.; Vermes, I.; Reutelingsperger, C.; Haanen, C. Early features of apoptosis detected by four different flow cytometry assays. Apoptosis 1998, 3, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Ionta, M.; Rosa, M.C.; Almeida, R.B.; Freitas, V.M.; Rezende-Teixeira, P.; Machado-Santelli, G.M. Retinoic acid and cAMP inhibit rat hepatocellular carcinoma cell proliferation and enhance cell differentiation. Braz. J. Med. Biol. Res. 2012, 45, 721–29. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionta, M.; Ferreira-Silva, G.A.; Niero, E.L.; Costa, É.D.; Martens, A.A.; Rosa, W.; Soares, M.G.; Machado-Santelli, G.M.; Lago, J.H.G.; Santos, M.H. 7-Epiclusianone, a Benzophenone Extracted from Garcinia brasiliensis (Clusiaceae), Induces Cell Cycle Arrest in G1/S Transition in A549 Cells. Molecules 2015, 20, 12804-12816. https://doi.org/10.3390/molecules200712804
Ionta M, Ferreira-Silva GA, Niero EL, Costa ÉD, Martens AA, Rosa W, Soares MG, Machado-Santelli GM, Lago JHG, Santos MH. 7-Epiclusianone, a Benzophenone Extracted from Garcinia brasiliensis (Clusiaceae), Induces Cell Cycle Arrest in G1/S Transition in A549 Cells. Molecules. 2015; 20(7):12804-12816. https://doi.org/10.3390/molecules200712804
Chicago/Turabian StyleIonta, Marisa, Guilherme A. Ferreira-Silva, Evandro L. Niero, Éderson D'Martin Costa, Adam A. Martens, Welton Rosa, Marisi G. Soares, Gláucia M. Machado-Santelli, João Henrique G. Lago, and Marcelo H. Santos. 2015. "7-Epiclusianone, a Benzophenone Extracted from Garcinia brasiliensis (Clusiaceae), Induces Cell Cycle Arrest in G1/S Transition in A549 Cells" Molecules 20, no. 7: 12804-12816. https://doi.org/10.3390/molecules200712804