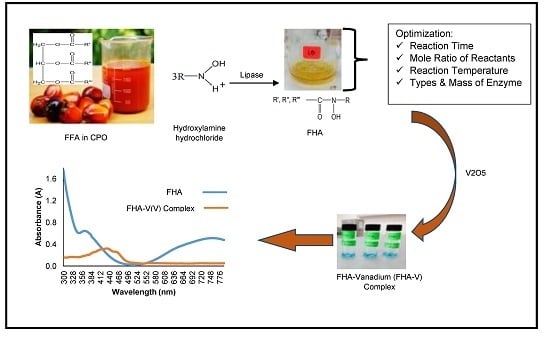
Study on the Spectrophotometric Detection of Free Fatty Acids in Palm Oil Utilizing Enzymatic Reactions
Abstract
:1. Introduction

2. Results and Discussion
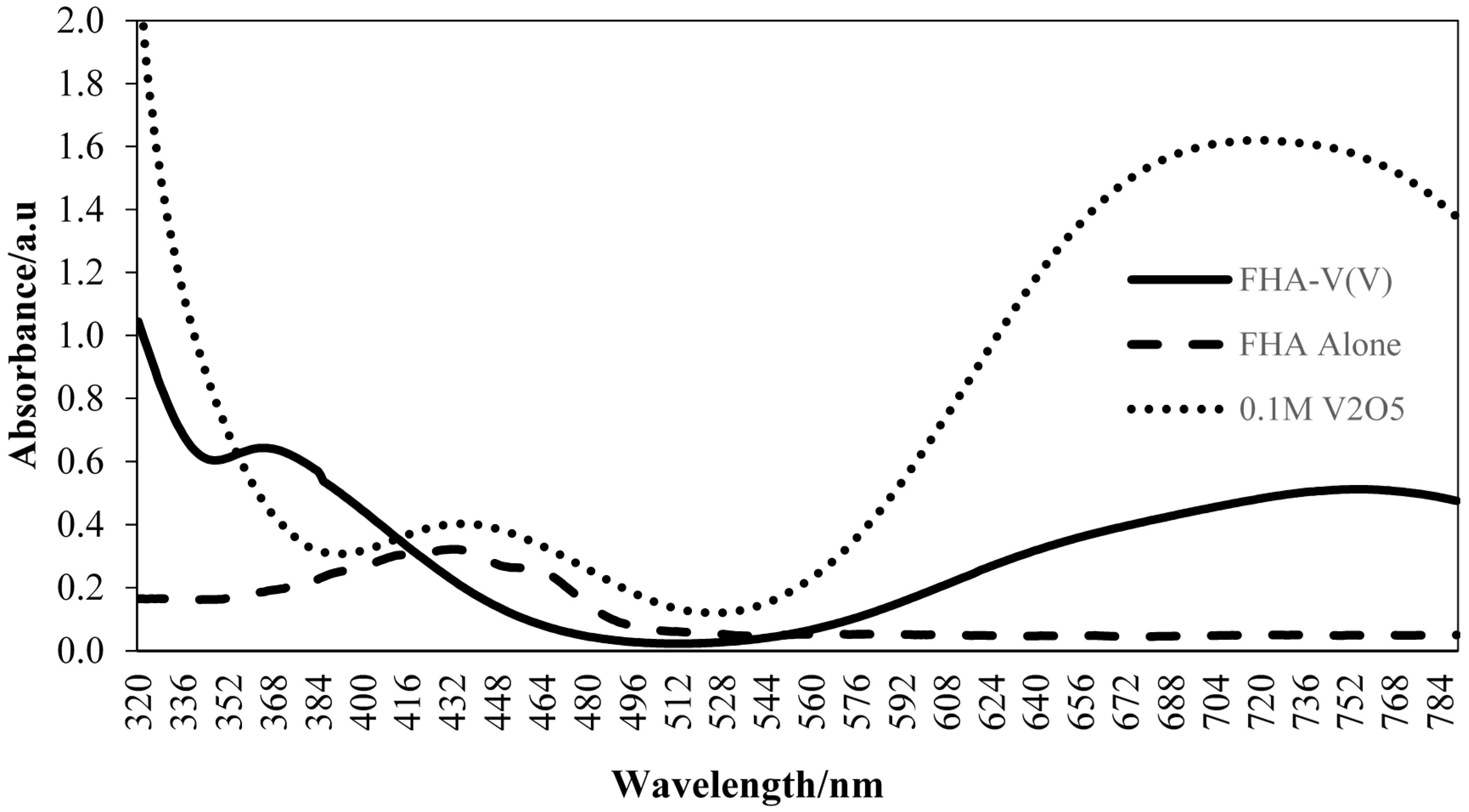
2.1. Detection of Free Fatty Acids (FFAs) Based on Enzymatic Aminolysis Reactions
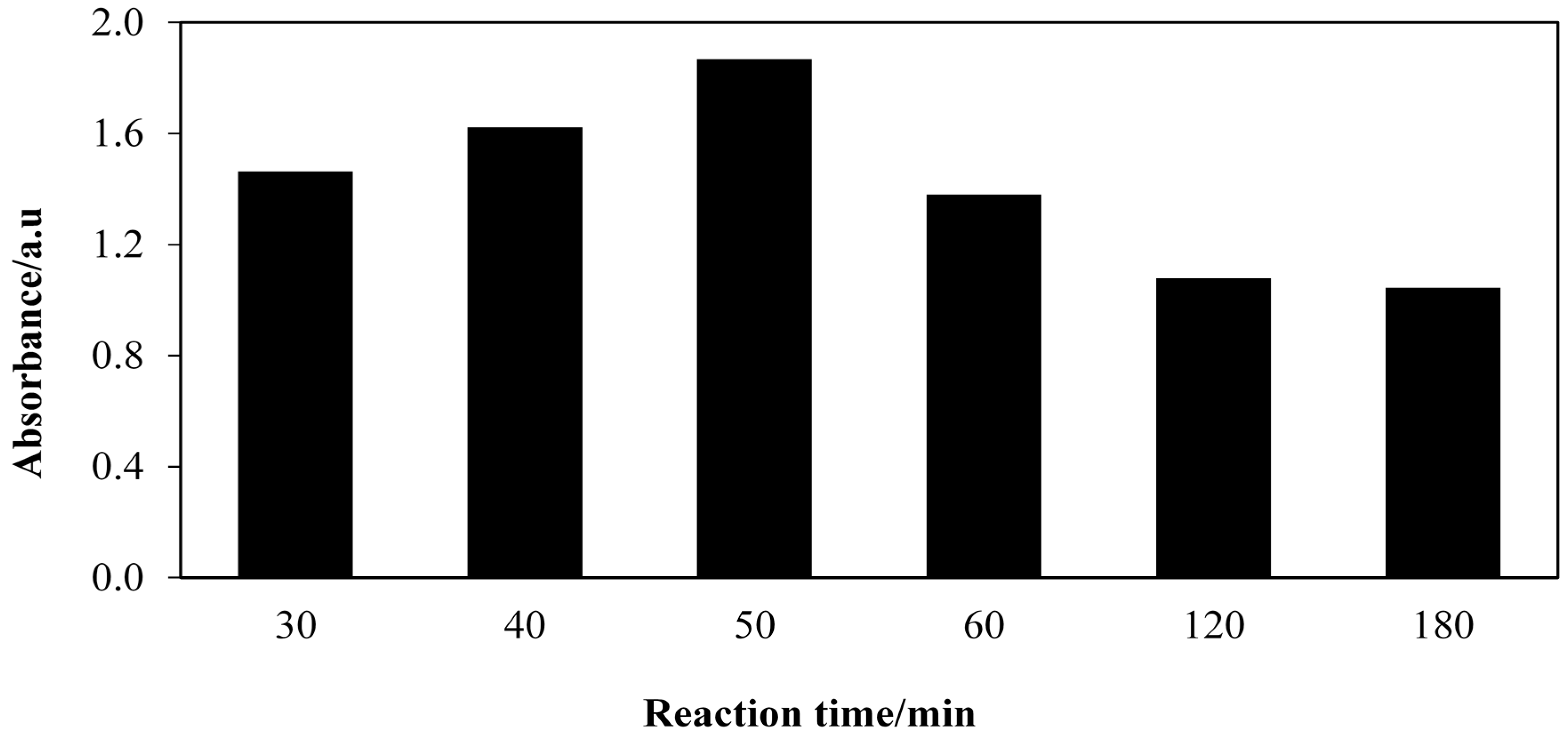
2.2. Effect of Aminolysis Reaction Time
2.3. Effect of Mole Ratio of Reactants
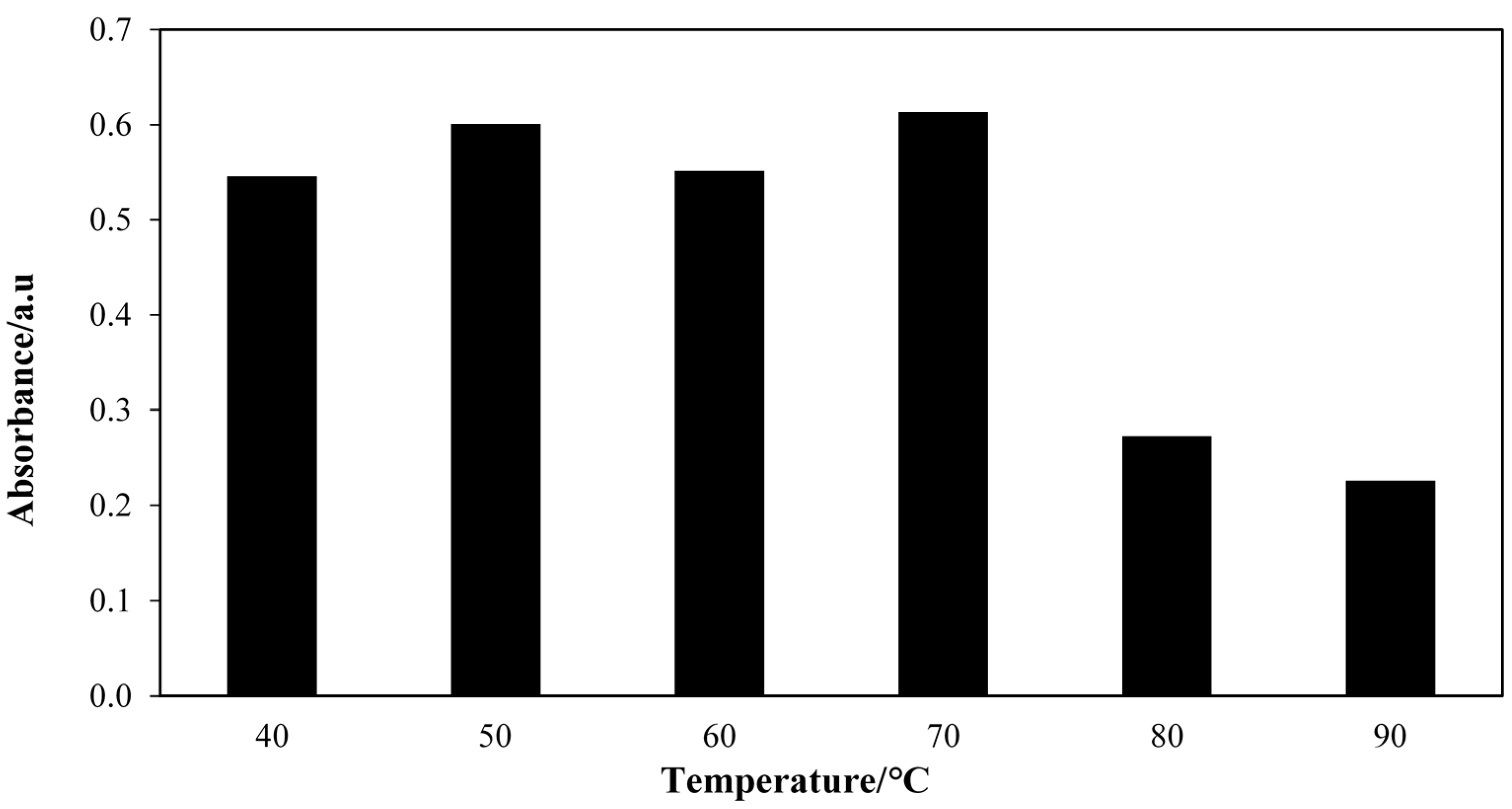
2.4. Effect of Temperature
2.5. Effect of Enzyme
2.5.1. Effect of Enzyme Mass

2.5.2. Effect of Enzyme Type
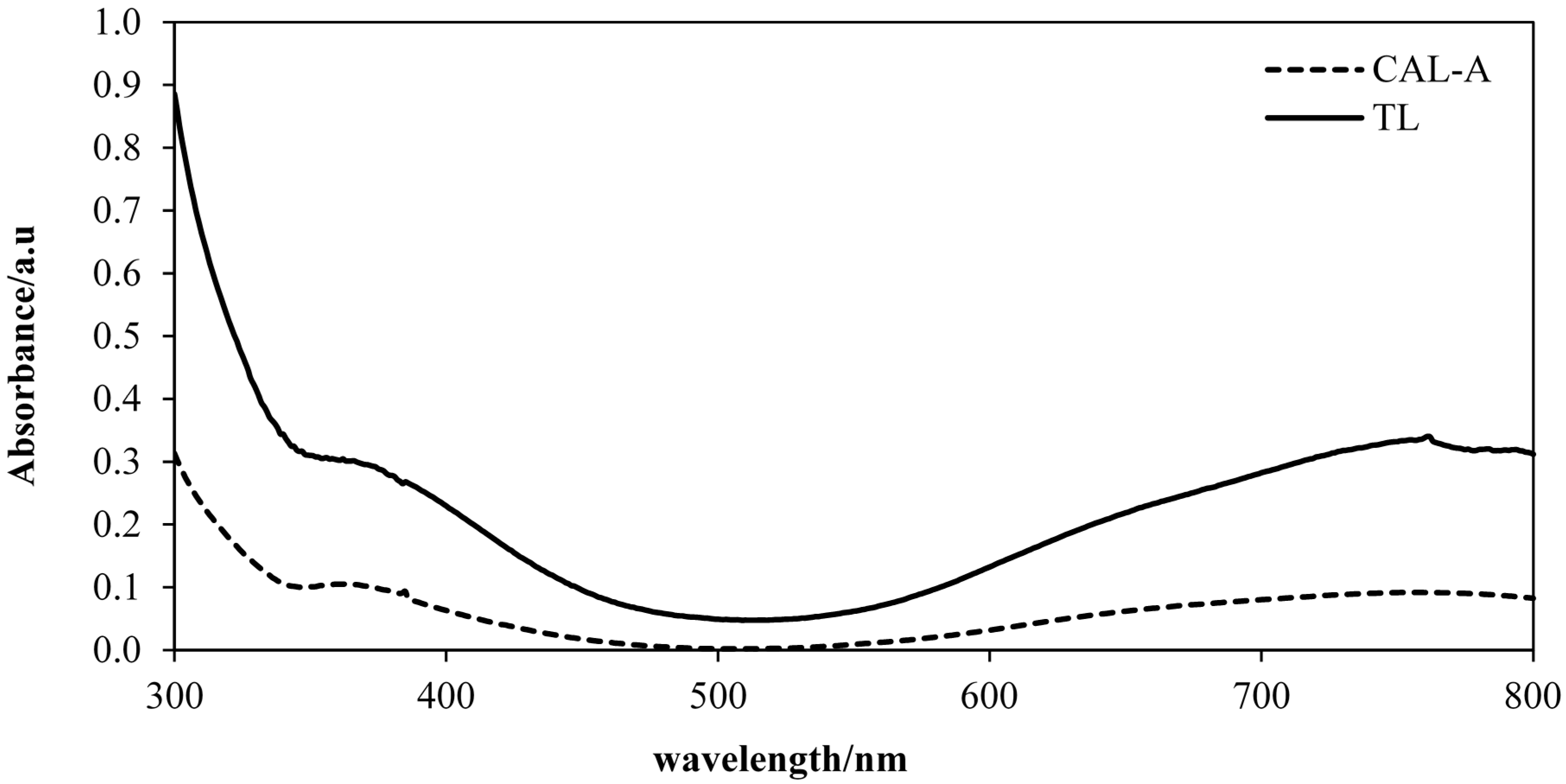

2.6. Effect of Different Concentrations of V(V)

3. Experimental Section
3.1. Materials and Reagents
3.2. Apparatus
3.3. General Procedure
3.4. Correlation Study for the Developed Method with the MPOB Standard Titration Method
3.4.1. Preparation of Crude Palm Oil (CPO) Stock Samples
3.4.2. Preparation of Stock Palmitic Acid Solution (100 a.d.)
| Concentration Required, a.d. | Volume of Palmitic Acid Stock, mL | Volume of CPO Stock, mL | Total Volume Solution, mL |
|---|---|---|---|
| 0.5 | 0.05 | 9.95 | 10.00 |
| 1.0 | 0.10 | 9.90 | 10.00 |
| 2.0 | 0.20 | 9.80 | 10.00 |
| 5.0 | 0.50 | 9.50 | 10.00 |
| 10.0 | 1.00 | 9.00 | 10.00 |
| 20.0 | 2.00 | 8.00 | 10.00 |
| 40.0 | 4.00 | 6.00 | 10.00 |
| 80.0 | 8.00 | 2.00 | 10.00 |
| 100.0 | 10.00 | 0.00 | 10.00 |
3.4.3. MPOB Standard Titrimetric Method
3.4.4. Preparation of Samples for the Correlation Study
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cornelius, J.A. Some technical factors influencing the quality of palm kernels. J. Sci. Food Agric. 1966, 7, 57–61. [Google Scholar] [CrossRef]
- Saad, B.; Ling, C.W.; Jab, M.S.; Lim, B.P.; Ali, A.S.M.; Wai, W.T.; Saleh, M.I. Determination of free fatty acids in palm oil samples using non-aqueous flow injection titrimetric method. J. Food Chem. 2007, 102, 1407–1414. [Google Scholar] [CrossRef]
- Keurentjes, J.; Doornbusch, G.; Van’t-Riet, K. The removal of fatty acids from edible oil. Removal of the dispersed phase of a water-in-oil dispersion by a hydrophilic membrane. Sep. Sci. Technol. 1991, 26, 409–423. [Google Scholar] [CrossRef]
- Raita, M.; Laothanachareon, T.; Champreda, V.; Laosiripojana, N. Biocatalytic esterification of palm oil fatty acids for biodiesel production using glycine-based cross-linked protein coated microcrystalline lipase. J. Mol. Catal. B Enzym. 2011, 73, 74–79. [Google Scholar]
- Ariffin, A. The effect of specific quality parameters of crude palm oil (CPO) on the recovery and quality of the intended final palm oil products. In Proceedings of the Malaysian Palm Oil Board (MPOB), Kuching, Malaysia, 14 August 2006.
- Che Man, Y.B.; Moh, M.H.; van de Voort, F.R. Determination of free fatty acids in crude palm oil and refined-bleached-deodorized palm olein using fourier transform infrared spectroscopy. J. Am. Oil Chem. Soc. 1999, 76, 485–490. [Google Scholar]
- Purseglove, J.W. Tropical Crops-Monocotyledons; Longman: London, UK, 1985. [Google Scholar]
- Ainie, K.; Siew, W.L.; Tan, Y.A.; Noraini, I.; Mohtar, Y.; Tang, T.S.; Nuzul, A.I. A Compendium of Test on Palm Oil Products, Palm Kernel Products, Fatty Acids, Food Related Products and Others; Malaysian Palm Oil Board: Selangor, Malaysia, 2004. [Google Scholar]
- Che Man, Y.B.; Setiowaty, G. Application of fourier transform infrared spectroscopy to determine free fatty acid contents in palm olein. J. Food Chem. 1999, 66, 109–114. [Google Scholar] [CrossRef]
- Che Man, Y.B.; Moh, M.H. Determination of free fatty acids in palm oil by near-infrared reflectance spectroscopy. J. Am. Oil Chem. Soc. 1998, 75, 557–564. [Google Scholar]
- Zaidul, I.S.M.; Norulaini, N.A.N.; Omar, A.K.M.; Smith, R.L., Jr. Supercritical carbon dioxide (SC-CO2) extraction of palm kernel oil from palm kernel. J. Food Eng. 2007, 79, 1007–1014. [Google Scholar]
- Li, G.; You, J.; Suo, Y.; Song, C.; Sun, Z.; Xia, L.; Zhao, X.; Shi, J. A developed pre-column derivatization method for the determination of free fatty acids in edible oils by reversed-phase HPLC with fluorescence detection and its application to Lycium barbarum seed oil. Food Chem. 2011, 125, 1365–1372. [Google Scholar]
- Servat, F.; Montet, D.; Pina, M.; Gaizy, P.; Arnaud, A.; Ledon, H.; Marcou, L.; Graille, J. Synthesis of fatty hydroxamic acids catalysed by the lipase of Mucormiehei. J. Am. Oil Chem. Soc. 1990, 67, 646–649. [Google Scholar] [CrossRef]
- Suhendra, D.; Yunus, W.M.Z.W.; Haron, M.J.; Basri, M.; Silong, S. Enzymatic synthesis of fatty hydroxamic acids from palm oil. J. Oleo Sci. 2005, 54, 33–38. [Google Scholar] [CrossRef]
- Vaysse, L.; Dubreucq, E.; Pirat, J.L.; Galzy, P. Fatty hydroxamic acid biosynthesis in aqueous medium in the presence of the lipase-acyltransferase from Candida parapsilopsis. J. Biotechnol. 1997, 53, 41–46. [Google Scholar] [CrossRef]
- Jahangerian, H.; Haron, M.J.; Silong, S.; Yusof, N.A. Enzymatic synthesis of phenyl fattyhydroxamic acids from canola and palm oils. J. Oleo Sci. 2011, 60, 281–286. [Google Scholar] [CrossRef]
- Al-Mulla, E.A.J.; Yunus, W.M.Z.W.; Ibrahim, N.A.B.; Rahman, M.Z.A. Enzymatic synthesis of palm olein-based fatty thiohydroxamic acids. J. Oleo Sci. 2010, 59, 569–573. [Google Scholar] [CrossRef] [PubMed]
- González-Navarro, H.; Braco, L. Improving lipase activity in solvent-free media by interfacial activation-based molecular bioimprinting. J. Mol. Catal. B Enzym. 1996, 3, 111–119. [Google Scholar] [CrossRef]
- Vallikivi, I.; Lille, Ü.; Lookene, A.; Metsala, A.; Sikk, P.; Tõugu, V.; Vija, H.; Villo, L.; Parve, O. Lipase action on some non-triglyceride substrates. J. Mol. Catal. B Enzym. 2003, 22, 279–298. [Google Scholar] [CrossRef]
- Idris, A.; Bukhari, A. Immobilized Candida Antarctica lipase B: Hydration, stripping off and application in ring opening polyester synthesis. J. Biotechnol. Adv. 2012, 30, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Tongboriboon, K.; Cheirsilp, B.; H-Kittikun, A. Mixed lipases for efficient enzymatic synthesis of biodiesel from used palm oil and ethanol in a solvent-free system. J. Mol. Catal. B Enzym. 2010, 67, 52–59. [Google Scholar] [CrossRef]
- Shao, P.; Meng, X.; He, J.; Sun., P. Analysis of immobilized Candida rugosa lipase catalyzed preparation of biodiesel from rapeseed soapstock. J. Food Bioprod. Process 2008, 86, 283–289. [Google Scholar] [CrossRef]
- Hacking, M.A.P.J.; van Rantjwik, F.; Sheldon, R.A. Lipase catalysed acylation of hydroxylamine and hydrazine derivatives. J. Mol. Catal. B Enzym. 2001, 11, 315–321. [Google Scholar] [CrossRef]
- Haron, M.J.; Jahangirian, H.; Silong, S.; Yusof, N.A.; Kassim, A.; Rafiee-Moghaddam, R.; Mahdavi, B.; Peyda, M.; Abdollahi, Y.; Amin, J. Benzyl and methyl fatty hydroxamic acids based on palm kernel oil as chelating agent for liquid-liquid iron (III) extraction. Int. J. Mol. Sci. 2012, 13, 2148–2159. [Google Scholar] [CrossRef] [PubMed]
- Isha, A.; Yusof, N.A.; Ahmad, M.; Suhendra, D.; Yunus, W.M.Z.W.; Zainal, Z.A. Chemical sensor for trace V(V) ion determination based on fatty hydroxamic acid immobilized in polymethacrylate. J. Sens. Actuators B 2006, 114, 344–349. [Google Scholar] [CrossRef]
- Suhendra, D.; Yeen, K.P.; Haron, M.J.; Silong, S.; Basri, M.; Yunus, W.M.Z.W. Copper ion extraction by a mixture of fatty hydroxamic acids synthesized from commercial palm olein. Solvent Extr. Ion Exch. 2005, 23, 713–723. [Google Scholar] [CrossRef]
- Maria, P.B.D.; Carboni-Oerlemans, C.; Tuin, B.; Bargeman, G.; Meer, A.V.D.; Gemert, R.V. Biotechnological applications of Candida antarctica lipase A: State-of-the-art. J. Mol. Catal. B Enzym. 2005, 37, 36–46. [Google Scholar] [CrossRef]
- Fernandez, M.L.M.; Krieger, N.; Baron, A.M.; Zamora, P.P.; Ramos, L.P.; Mitchell, D.A. Hydrolysis and synthesis reactions catalyzed by Thermomyces lanuginosa lipase in the AOT/Isooctane reverse micellar system. J. Mol. Catal. B Enzym. 2004, 30, 43–49. [Google Scholar] [CrossRef]
- Tweddell, R.J.; Kermasha, S.; Combes, D.; Marty, A. Esterification and interesterification activities of lipases from Rhizopus niveus and Mucor meihei in three different types of organic media: A comparative study. J. Enzym. Microb. Technol. 1998, 22, 439–445. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R. Lipase from Thermomyces lanuginosus: Uses and prospects as an industrial biocatalyst. J. Mol. Catal. B Enzym. 2010, 62, 197–212. [Google Scholar] [CrossRef]
- Ali, A.S.M.; Abdurrhman, A.M. Determination of free fatty acids in palm oil samples by non-aqueous flow injection using salicyaldehyde-2,4-dinitrophenylhydrazone as colorimetric reagent. Chem. Mater. Eng. 2013, 1, 96–103. [Google Scholar]
- Sample Availability: Samples of the CPO and FHA compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azeman, N.H.; Yusof, N.A.; Abdullah, J.; Yunus, R.; Hamidon, M.N.; Hajian, R. Study on the Spectrophotometric Detection of Free Fatty Acids in Palm Oil Utilizing Enzymatic Reactions. Molecules 2015, 20, 12328-12340. https://doi.org/10.3390/molecules200712328
Azeman NH, Yusof NA, Abdullah J, Yunus R, Hamidon MN, Hajian R. Study on the Spectrophotometric Detection of Free Fatty Acids in Palm Oil Utilizing Enzymatic Reactions. Molecules. 2015; 20(7):12328-12340. https://doi.org/10.3390/molecules200712328
Chicago/Turabian StyleAzeman, Nur Hidayah, Nor Azah Yusof, Jaafar Abdullah, Robiah Yunus, Mohd Nizar Hamidon, and Reza Hajian. 2015. "Study on the Spectrophotometric Detection of Free Fatty Acids in Palm Oil Utilizing Enzymatic Reactions" Molecules 20, no. 7: 12328-12340. https://doi.org/10.3390/molecules200712328
APA StyleAzeman, N. H., Yusof, N. A., Abdullah, J., Yunus, R., Hamidon, M. N., & Hajian, R. (2015). Study on the Spectrophotometric Detection of Free Fatty Acids in Palm Oil Utilizing Enzymatic Reactions. Molecules, 20(7), 12328-12340. https://doi.org/10.3390/molecules200712328