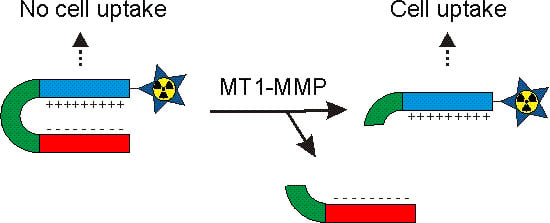
Development of Radiolabeled Membrane Type-1 Matrix Metalloproteinase Activatable Cell Penetrating Peptide Imaging Probes
Abstract
:1. Introduction
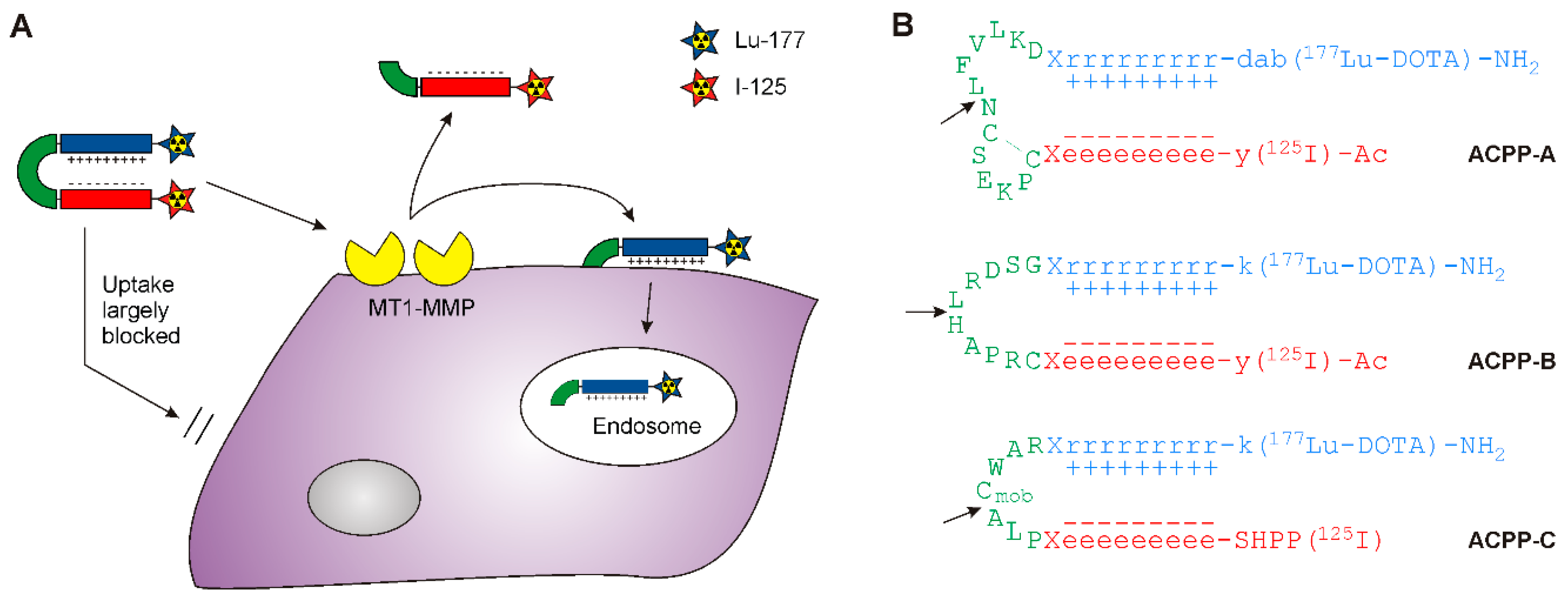
2. Results and Discussion
2.1. Probe Synthesis
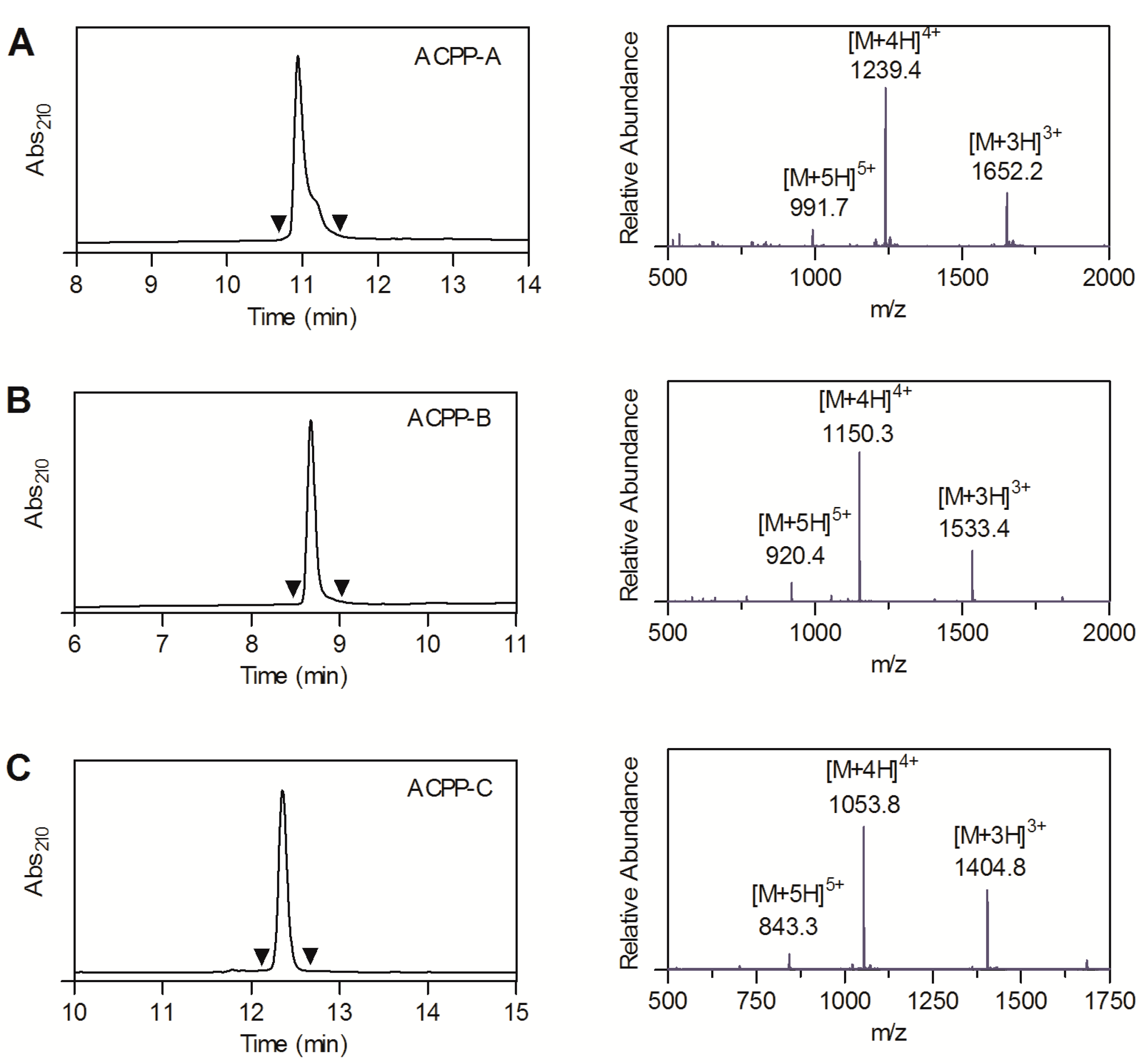
2.2. MT1-MMP Sensitivity of ACPP Probes
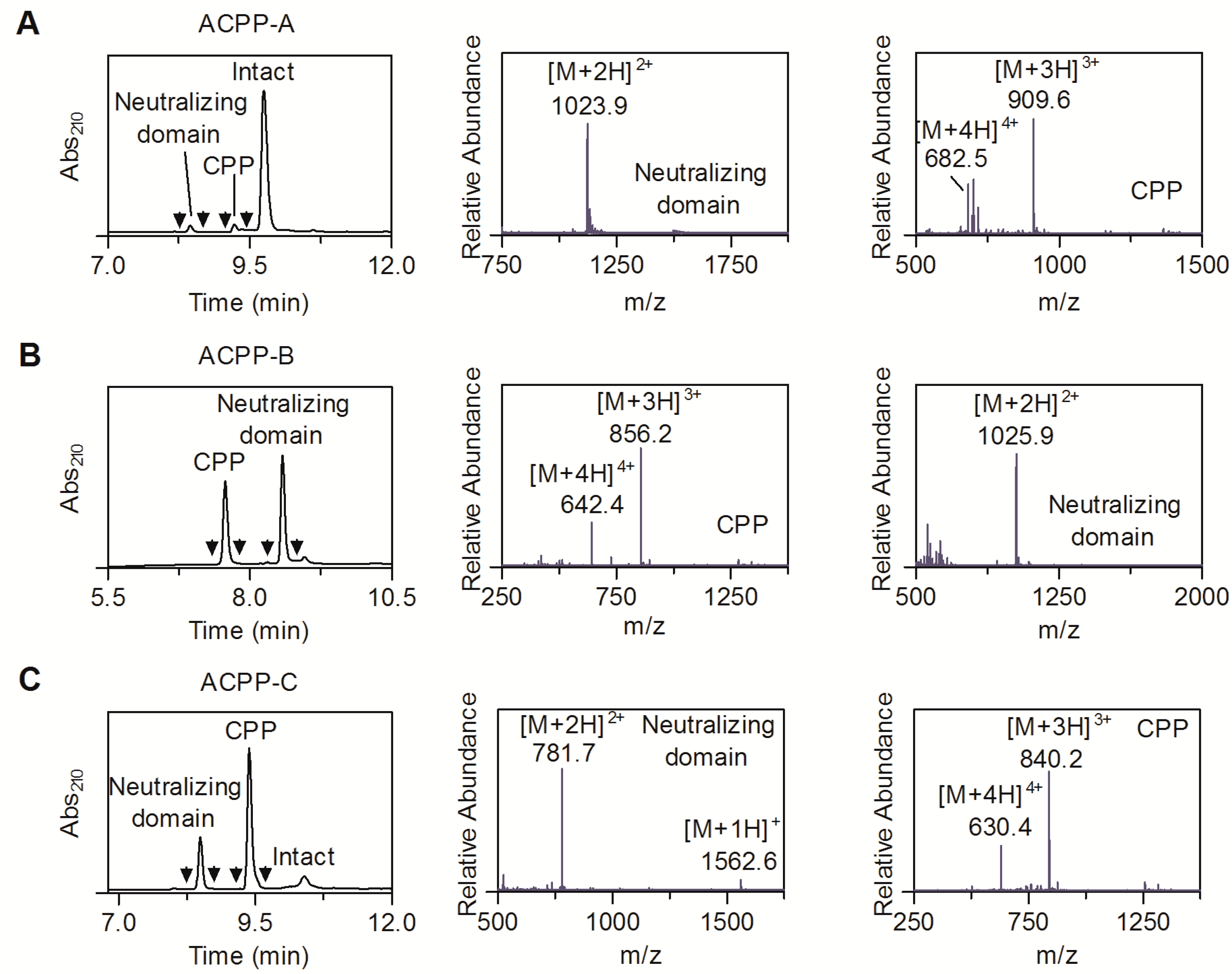
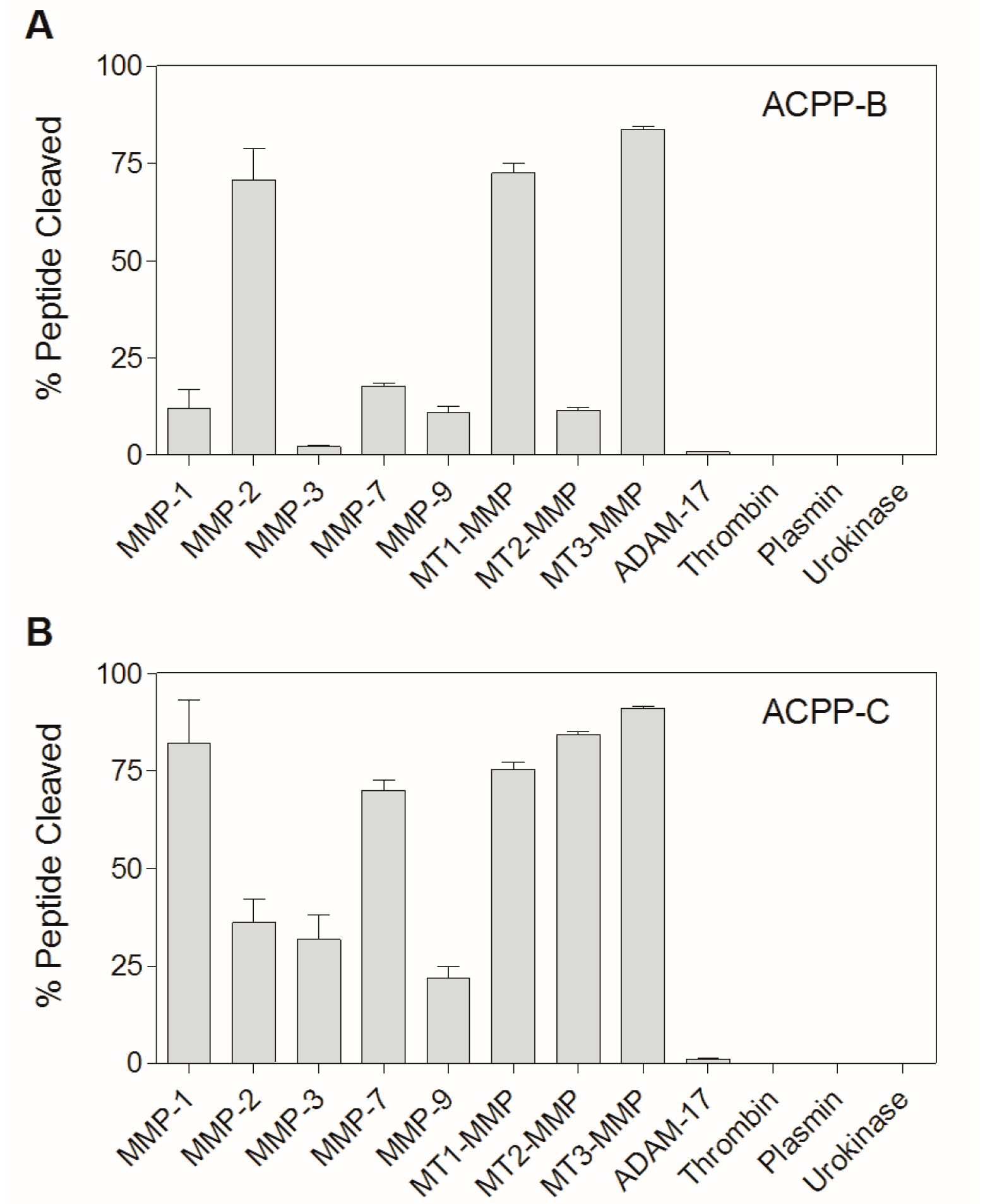
2.3. Enzyme Specificity of ACPP-B & ACPP-C
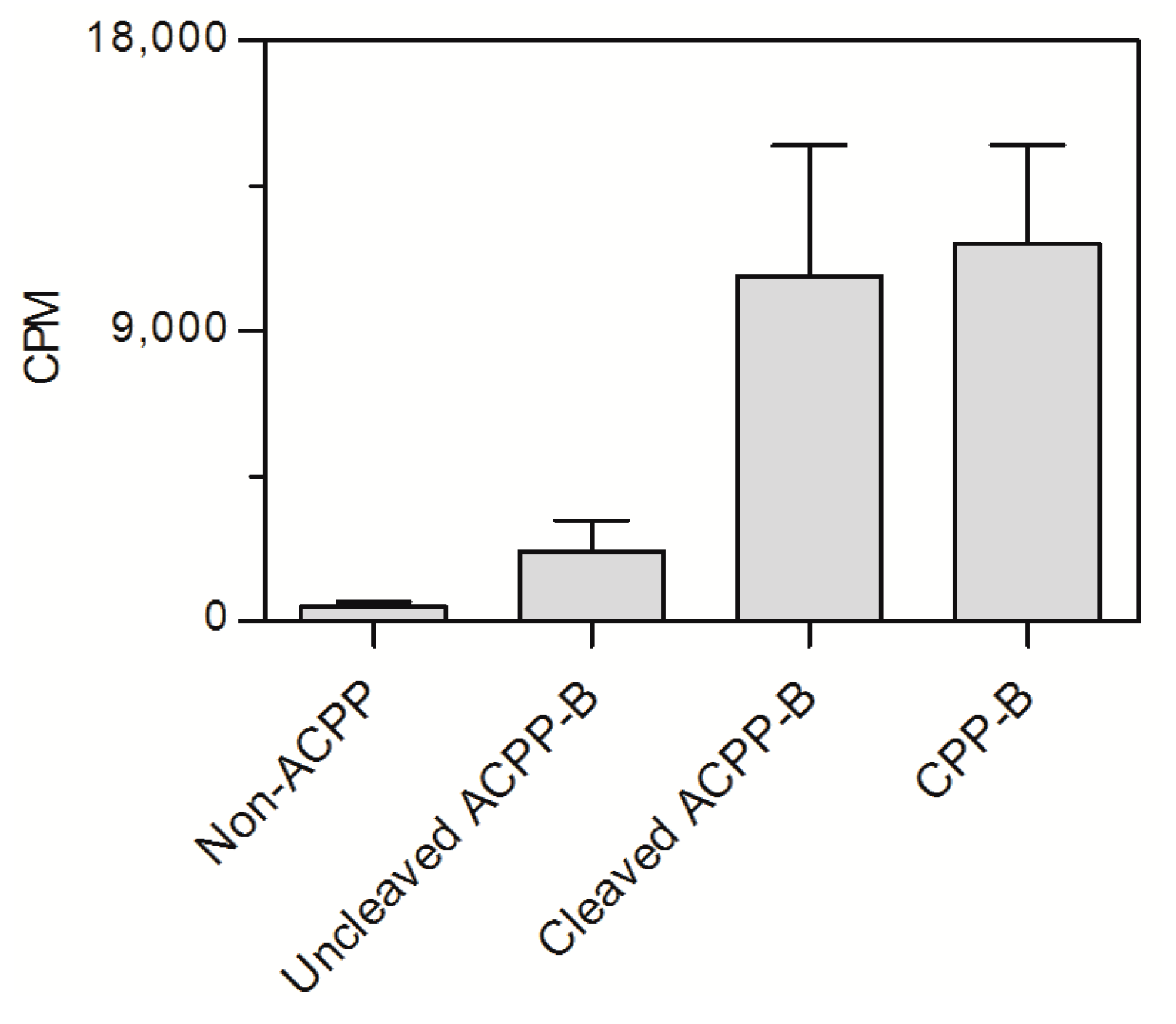
2.4. In Vitro Cell Assay ACPP-B
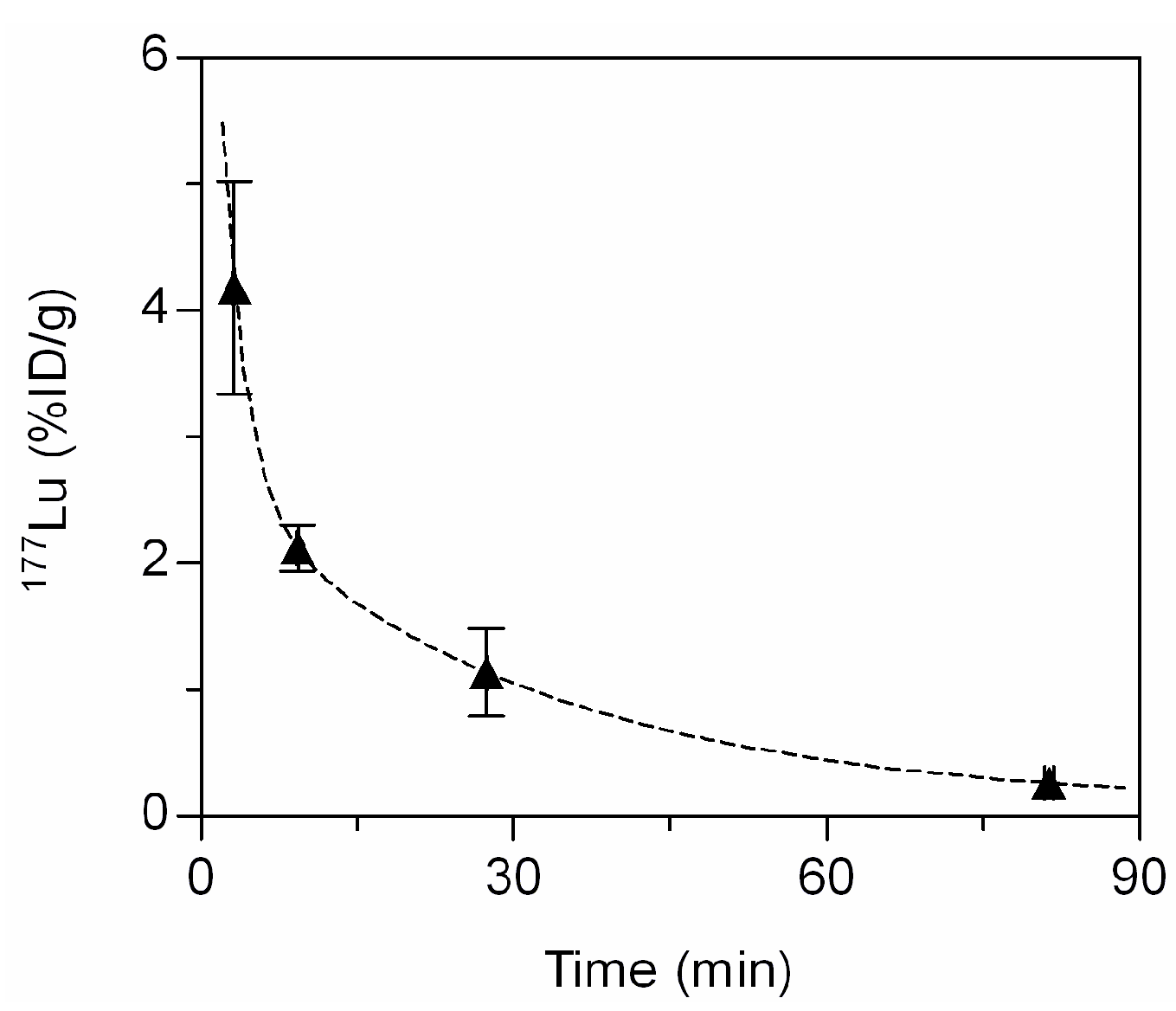
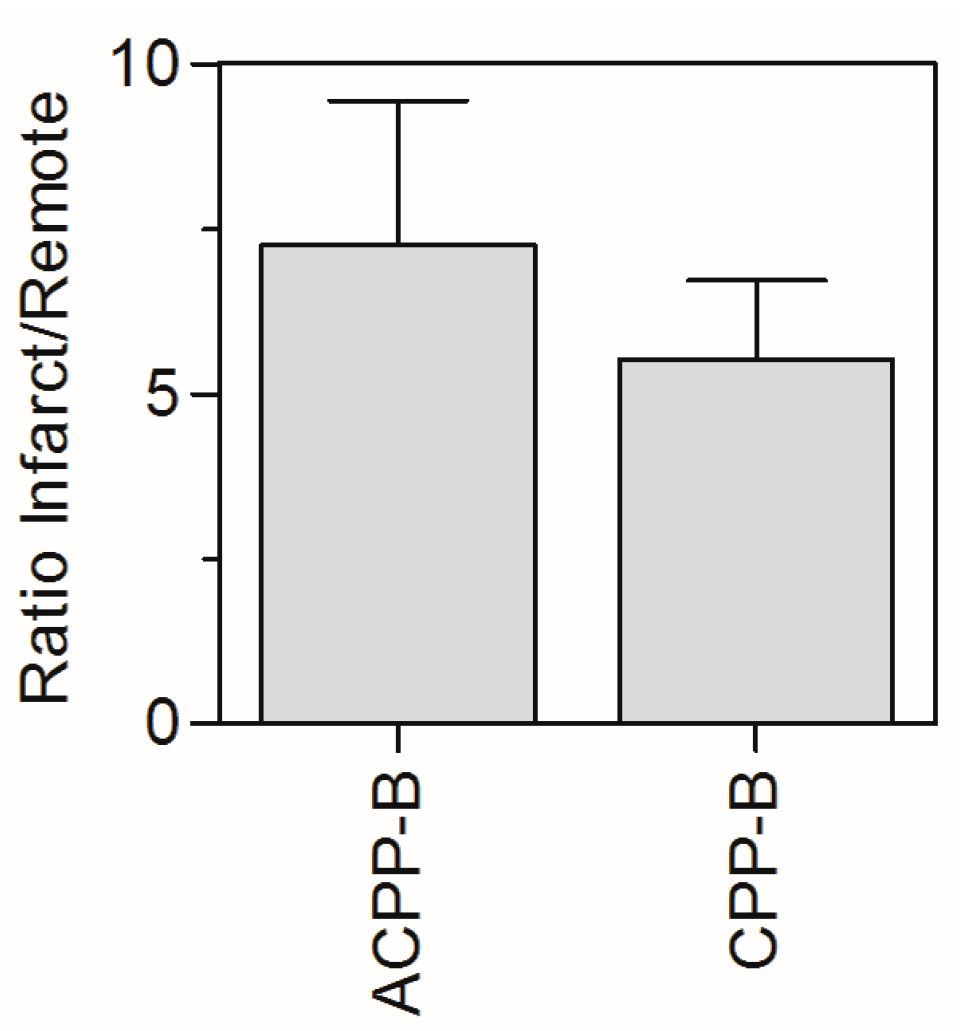
2.5. In Vivo Biodistribution
| 177Lu-ACPP-B ( n = 5) | 177Lu-ACPP-2/9 ( n = 3) | |
|---|---|---|
| Blood | 0.08 ± 0.03 | 0.09 ± 0.03 |
| Heart, infarct | 0.75 ± 0.15 | 3.60 ± 0.68 |
| Heart, remote | 0.11 ± 0.02 | 0.35 ± 0.13 |
| Muscle | 0.09 ± 0.07 | 0.48 ± 0.14 |
| Lung | 0.38 ± 0.09 | 1.09 ± 0.30 |
| Spleen | 0.77 ± 0.13 | 2.72 ± 1.48 |
| Liver | 6.76 ± 0.91 | 28.5 ± 5.33 |
| Kidney | 100.4 ± 13.0 | 59.9 ± 8.10 |
| Fat | 0.43 ± 0.28 | 0.33 ± 0.08 |
| Thigh bone | 1.92 ± 0.73 | 2.47 ± 1.02 |
| Brain | 0.01 ± 0.01 | 0.02 ± 0.01 |
3. Experimental Section
3.1. Materials
3.2. Probe Synthesis
3.3. MT1-MMP Sensitivity
3.4. Enzyme Assay
3.4.1. Radiolabeling
3.4.2. Cell Culture
3.4.3. In Vitro Cell Incubation with Radiolabeled ACPP Probes
3.4.4. Animal Studies
3.4.5. Mouse Model of Myocardial Infarction (MI)
3.4.6. Blood Kinetics and Biodistribution
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Van Duijnhoven, S.M.J.; Robillard, M.S.; Hermann, S.; Kuhlmann, M.T.; Schäfers, M.; Nicolay, K.; Grüll, H. Imaging of MMP activity in postischemic cardiac remodelling using radiolabeled MMP-2/9 activatable peptide probes. Mol. Pharm. 2014, 11, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Felsen, C.N.; Savariar, E.N.; Whitney, M.; Tsien, R.Y. Detection and monitoring of localized matrix metalloproteinase upregulation in a murin model of asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 306, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Whitney, M.; Savariar, E.N.; Friedman, B.; Levin, R.A.; Crisp, J.L.; Glasgow, H.L.; Lefkowitz, R.; Adams, S.R.; Steinbach, P.; Nashi, N.; et al. Ratiometric activatable cell-penetrating peptides provide rapid in vivo readout of thrombin activation. Angew. Chem. Int. Ed. Engl. 2013, 52, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Strongin, A.Y.; Collier, I.; Bannikov, G.; Marmer, B.L.; Grant, G.A.; Goldberg, G.I. Mechanism of cell surface activation of 72-kDa type IV collagenase: Isolation of the activated form of the membrane metalloprotease. J. Biol. Chem. 1995, 270, 5331–5338. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.A.; Gaillard, W.F., 2nd; Rivers, W.T.; Koval, C.N.; Stroud, R.E.; Mukherjee, R.; Spinale, F.G. Heterogeneity in MT1-MMP activity with ischemia-reperfusion and previous myocardial infarction: Relation to regional myocardial function. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1947–H1958. [Google Scholar] [CrossRef] [PubMed]
- Tatti, O.; Vehviläinen, P.; Lehti, K.; Keski-Oja, J. MT1-MMP releases latent TGF-beta1 from endothelial cell extracellular matrix via proteolytic processing of LTBP-1. Exp. Cell Res. 2008, 314, 2501–2514. [Google Scholar] [CrossRef] [PubMed]
- Ogura, S.; Ohdaira, T.; Hozumi, Y.; Nagai, H. Metastasis-related factors expressed in pT1 pN0 breast cancer: Assessment of recurrence risk. J. Surg. Oncol. 2007, 96, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Rozanov, D.V.; Savinov, A.Y.; Williams, R.; Liu, K.; Golubkov, V.S.; Krajewski, S.; Strongin, A.Y. Molecular signature of MT1-MMP: Transactivation of the downstream universal gene network in cancer. Cancer Res. 2008, 68, 4086–4096. [Google Scholar] [CrossRef] [PubMed]
- Tsunezuka, Y.; Kinoh, H.; Takina, T.; Watanabe, Y.; Okada, Y.; Shinagawa, A.; Sato, H.; Seiki, M. Expression of membrane-type matrix metalloproteinase 1 (MT1-MMP) in tumor cells enhances pulmonary metastasis in an experimental metastasis assay. Cancer Res. 1996, 56, 5678–5683. [Google Scholar] [PubMed]
- Schneider, F.; Sukhova, G.K.; Aikawa, M.; Canner, J.; Gerdes, N.; Tang, S.M.; Shi, G.P.; Apte, S.S.; Libby, P. Matrix metalloproteinase-14 deficiency in bone marrow-derived cells promotes collagen accumulation in mouse atherosclerotic plaques. Circulation 2008, 117, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Deschamps, A.M.; Yarbrough, W.M.; Squires, C.E.; Allen, R.A.; McClister, D.M.; Dowdy, K.B.; McLean, J.E.; Mingoia, J.T.; Sample, J.A.; Mukherjee, R.; et al. Trafficking of the membrane type-1 matrix metalloproteinase in ischemia and reperfusion: Relation to interstitial membrane type-1 matrix metalloproteinase activity. Circulation 2005, 111, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Spinale, F.G.; Escobar, G.P.; Mukherjee, R.; Zavadzkas, J.A.; Saunders, S.M.; Jeffords, L.B.; Leone, A.M.; Beck, C.; Bouges, S.; Stroud, R.E. Cardiac-restricted overexpression of membrane type-1 matrix metalloproteinase in mice: Effects on myocardial remodeling with aging. Circ. Heart Fail. 2009, 2, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Skavdahl, M.; Steenbergen, C.; Clark, J.; Myers, P.; Demianenko, T.; Mao, L.; Rockman, H.A.; Korach, K.S.; Murphy, E. Estrogen receptor-beta mediates male-female differences in the development of pressure overload hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H469–H476. [Google Scholar] [CrossRef] [PubMed]
- Spinale, F.G.; Mukherjee, R.; Zavadzkas, J.A.; Koval, C.N.; Bouges, S.; Stroud, R.E.; Dobrucki, L.W.; Sinusas, A.J. Cardiac restricted overexpression of membrane type-1 matrix metalloproteinase causes adverse myocardial remodeling following myocardial infarction. J. Biol. Chem. 2010, 285, 30316–30327. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, H.; Wang, L.; Wang, Y.; Jiang, K.; Li, C.; Ma, Q.; Gao, S.; Wang, L.; Li, W.; et al. High-affinity peptide against MT1-MMP for in vivo tumor imaging. J. Control Release 2011, 150, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Chang, Y.; Lee, Y.; Park, J.; Kim, I.; Bae, S.; Lee, G.; Kim, T.; Kim, J.; Kim, K.; et al. Targeting of membrane-type1-matrix metalloproteinase (MT1-MMP) using superparamagnetic nanoparticles in human liver cells. Coll. Surf. A Physicochem. Eng. Aspects. 2008, 313–314, 647–650. [Google Scholar] [CrossRef]
- Van Steenkiste, M.; Oltenfreiter, R.; Frankenne, F.; Vervoort, L.; Maguoi, E.; Noel, A.; Foidart, J.M.; van de Wiele, C.; de Vos, F. Membrane type 1 matrix metalloproteinase detection in tumors, using the iodinated endogenous [123I]-tissue inhibitor 2 of metalloproteinases as imaging agent. Cancer Biother. Radiopharm. 2010, 25, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Kuge, Y.; Takai, N.; Ogawa, Y.; Temma, T.; Zhao, Y.; Nishigori, K.; Ishino, S.; Kamihashi, J.; Kiyono, Y.; Shiomi, M.; et al. Imaging with radiolabelled anti-membrane type 1 matrix metalloproteinase (MT1-MMP) antibody: Potentials for characterizing atherosclerotic plaques. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, M.; Lu, S.; Li, X.Y.; Xu, J.; Seong, J.; Giepmans, B.N.; Shyy, J.Y.; Weiss, S.J.; Wang, Y. Visualization of polarized membrane type 1 matrix metalloproteinase activity in live cells by fluorescence resonance energy transfer imaging. J. Biol. Chem. 2008, 283, 17740–17748. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, M.; Huang, H.; Shaner, N.C.; Remacle, A.G.; Shiryaev, S.A.; Strongin, A.Y.; Tsien, R.Y.; Wang, Y. Simultaneous visualization of protumorigenic Src and MT1-MMP activities with fluorescence resonance energy transfer. Cancer Res. 2010, 70, 2204–2212. [Google Scholar] [CrossRef] [PubMed]
- Watkins, G.A.; Jones, E.F.; Scott Shell, M.; VanBrocklin, H.F.; Pan, M.H.; Hanrahan, S.M.; Feng, J.J.; He, J.; Sounni, N.E.; Dill, K.A.; et al. Development of an optimized activatable MMP-14 targeted SPECT imaging probe. Bioorg. Med. Chem. 2009, 17, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Kridel, S.J.; Sawai, H.; Ratnikov, B.I.; Chen, E.I.; Li, W.; Godzik, A.; Strongin, A.Y.; Smith, J.W. A unique substrate binding mode discriminates membrane type-1 matrix metalloproteinase from other matrix metalloproteinases. J. Biol. Chem. 2002, 277, 23788–23793. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, F.; Ma, Y.; Liu, G.; Kim, K.; Fang, X.; Lee, S.; Chen, X. In vivo optical imaging of membrane-type matrix metalloproteinase (MT-MMP) activity. Mol. Pharm. 2011, 8, 2331–2338. [Google Scholar] [CrossRef] [PubMed]
- Van Duijnhoven, S.M.J.; Robillard, M.S.; Nicolay, K.; Grüll, H. Tumor targeting of MMP-2/9 activatable cell-penetrating imaging probes is caused by tumor-independent activation. J. Nucl. Med. 2011, 52, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Van Duijnhoven, S.M.J.; Robillard, M.S.; Nicolay, K.; Grüll, H. In vivo biodistribution of radiolabeled MMP-2/9 activatable cell-penetrating peptide probes in tumor-bearing mice. Contrast Media Mol. Imaging 2015, 10, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Giambernardi, T.A.; Grant, G.M.; Taylor, G.P.; Hay, R.J.; Maher, V.M.; McCormick, J.J.; Klebe, R.J. Overview of matrix metalloproteinase expression in cultured human cells. Matrix Biol. 1998, 16, 483–496. [Google Scholar] [CrossRef]
- Aguilera, T.A.; Olson, E.S.; Timmers, M.M.; Jiang, T.; Tsien, R.Y. Systemic in vivo distribution of activatable cell penetrating peptides is superior to that of cell penetrating peptides. Integr. Biol. 2009, 1, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Galli, C.; Mandolini, L. The role of ring strain on the ease of ring closure of bifunctional chain molecules. Eur. J. Org. Chem. 2000, 2000, 3117–3125. [Google Scholar] [CrossRef]
- Durbin, P.W.; Jeung, N.; Kullgren, B.; Clemons, G.K. Gross composition and plasma and extracellular water volumes of tissues of a reference mouse. Health Phys. 1992, 63, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhu, X.; Shundong, J.; Zhou, J.; Ozker, K.S.; Fang, W.; Molthen, R.C.; Hellman, R.S. 99mTc labeled C2A domain of Synaptotagmin I as a target-specific molecular probe for noninvasive imaging of acute myocardial infarction. J. Nucl. Med. 2006, 47, 1367–1374. [Google Scholar] [PubMed]
- Tarnavski, O.; McMullen, J.R.; Schinke, M.; Nie, Q.; Kong, S.; Izumo, S. Mouse cardiac surgery: Comprehensive techniques for the generation of mouse models of human diseases and their application for genomic studies. Physiol. Genomics 2004, 16, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Richard, J.P.; Melikov, K.; Vives, E.; Ramos, C.; Verbeure, B.; Gait, M.J.; Chernomordik, L.V.; Lebleu, B. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J. Biol. Chem. 2003, 278, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Guide for the Care and Use of Laboratory Animals; Government Printing Office: Washington, DC, USA, 1985.
- Lutgens, E.; Daemen, M.; de Muinck, E.D.; Debets, J.; Leenders, P.; Smits, J.F. Chronic myocardial infarction in the mouse: Cardiac structural and functional changes. Cardiovasc. Res. 1999, 41, 586–593. [Google Scholar] [CrossRef]
- Sample Availability: Available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Duijnhoven, S.M.J.; Robillard, M.S.; Nicolay, K.; Grüll, H. Development of Radiolabeled Membrane Type-1 Matrix Metalloproteinase Activatable Cell Penetrating Peptide Imaging Probes. Molecules 2015, 20, 12076-12092. https://doi.org/10.3390/molecules200712076
Van Duijnhoven SMJ, Robillard MS, Nicolay K, Grüll H. Development of Radiolabeled Membrane Type-1 Matrix Metalloproteinase Activatable Cell Penetrating Peptide Imaging Probes. Molecules. 2015; 20(7):12076-12092. https://doi.org/10.3390/molecules200712076
Chicago/Turabian StyleVan Duijnhoven, Sander M. J., Marc S. Robillard, Klaas Nicolay, and Holger Grüll. 2015. "Development of Radiolabeled Membrane Type-1 Matrix Metalloproteinase Activatable Cell Penetrating Peptide Imaging Probes" Molecules 20, no. 7: 12076-12092. https://doi.org/10.3390/molecules200712076