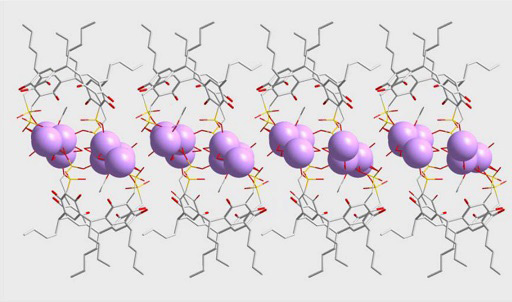
Synthesis and Characterization of Two Sulfonated Resorcinarenes: A New Example of a Linear Array of Sodium Centers and Macrocycles
Abstract
:1. Introduction
2. Results and Discussion

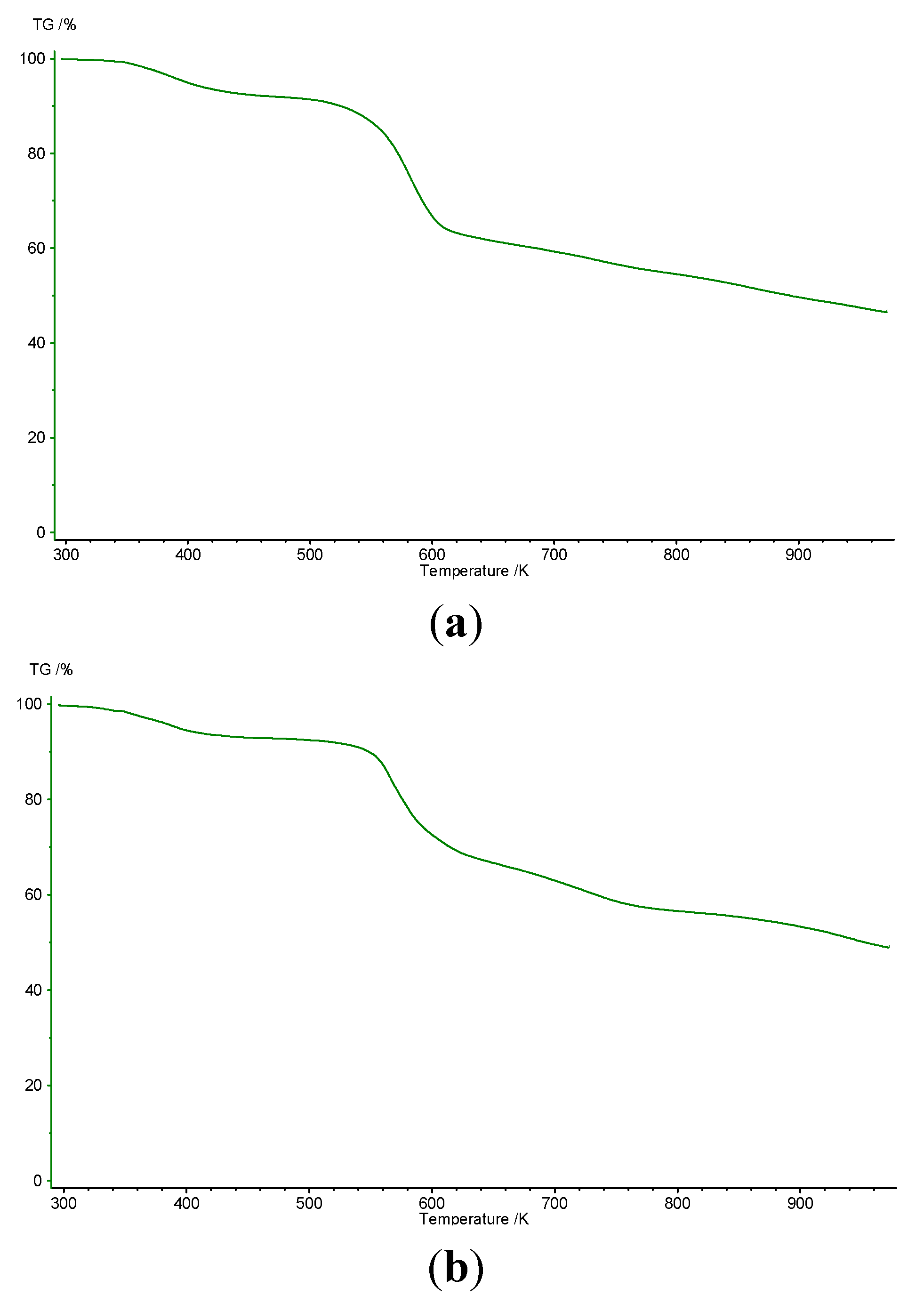
| Resorcinarene | Step | Temperature Range (°C) | Δmexp (%) | Δmcalc (%) |
|---|---|---|---|---|
| 2a | 1 | 85–140 | 4.40 | 4.38 |
| 2 | 270–350 | |||
| 2b | 1 | 97–147 | 4.39 | 4.33 |
| 2 | 267–347 |
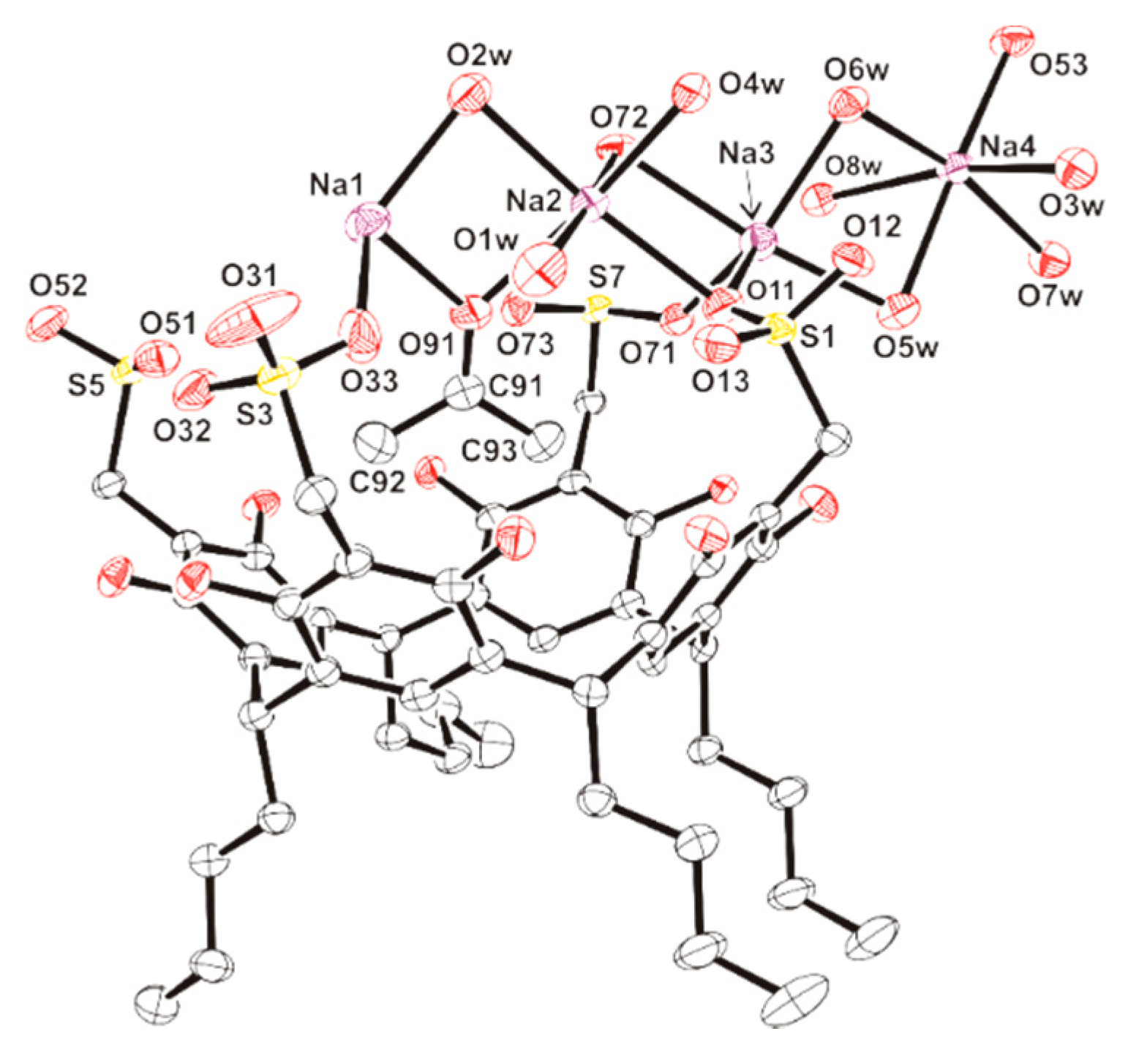
| Atoms | Length (Å) | Atoms | Length (Å) |
| C(14)···C(54) | 5.198(6) | C(20)···C(60) | 7.152(5) |
| C(34)···C(74) | 5.162(4) | C(40)···C(80) | 7.249(5) |
| Na(1)-O(33) | 2.281(3) | Na(1)-O(91) | 2.375(3) |
| Na(1)-O(1w) | 2.863(4) | Na(1)-O(2w) | 2.359(3) |
| Na(1)-O(3w)a | 2.313(3) | Na(1)-O(7w)a | 2.342(3) |
| Na(2)-O(11) | 2.414(3) | Na(2)-O(91) | 2.337(3) |
| Na(2)-O(1w) | 2.729(4) | Na(2)-O(2w) | 2.401(3) |
| Na(2)-O(4w) | 2.350(3) | Na(2)-O(72) | 2.327(3) |
| Na(3)-O(11) | 2.386(3) | Na(3)-O(71) | 2.349(3) |
| Na(3)-O(5w) | 2.370(3) | Na(3)-O(6w) | 2.434(3) |
| Na(3)-O(8w) | 2.887(3) | Na(3)-O(72) | 2.514(3) |
| Na(4)-O(3w) | 2.355(3) | Na(4)-O(5w) | 2.472(3) |
| Na(4)-O(6w) | 2.363(3) | Na(4)-O(7w) | 2.368(3) |
| Na(4)-O(8w) | 2.495(3) | Na(4)-O(53) | 2.369(3) |
| Atoms | Angles (deg) | Atoms | Angles (deg) |
| O(33)-Na(1)-O(2w) | 132.1(1) | O(33)-Na(1)-O(91) | 94.5(1) |
| O(33)-Na(1)-O(1w) | 60.6(1) | O(33)-Na(1)-O(3w)a | 95.7(1) |
| O(33)-Na(1)-O(7w)a | 126.0(1) | O(91)-Na(1)-O(1w) | 69.6(1) |
| O(91)-Na(1)-O(2w) | 86.9(1) | O(91)-Na(1)-O(3w)a | 169.4(1) |
| O(91)-Na(1)-O(7w)a | 87.6(1) | O(1w)-Na(1)-O(2w) | 75.5(1) |
| O(1w)-Na(1)-O(3w)a | 118.2(1) | O(1w)-Na(1)-O(7w)a | 157.1(1) |
| O(2w)-Na(1)-O(3w)a | 88.4(1) | O(2w)-Na(1)-O(7w)a | 101.9(1) |
| O(3w)a-Na(1)-O(7w)a | 84.2(1) | O(11)-Na(2)-O(72) | 90.8(1) |
| O(11)-Na(2)-O(91) | 100.0(1) | O(11)-Na(2)-O(1w) | 93.0(1) |
| O(11)-Na(2)-O(2w) | 166.2(1) | O(11)-Na(2)-O(4w) | 86.8(1) |
| O(72)-Na(2)-O(91) | 104.9(1) | O(72)-Na(2)-O(2w) | 99.1(1) |
| O(72)-Na(2)-O(1w) | 175.8(1) | O(72)-Na(2)-O(4w) | 93.8(1) |
| O(91)-Na(2)-O(1w) | 72.6(1) | O(91)-Na(2)-O(2w) | 86.8(1) |
| O(91)-Na(2)-O(4w) | 159.9(1) | O(1w)-Na(2)-O(2w) | 77.5(1) |
| O(1w)-Na(2)-O(4w) | 88.3(1) | O(2w)-Na(2)-O(4w) | 83.0(1) |
| O(11)-Na(3)-O(72) | 87.1(1) | O(11)-Na(3)-O(71) | 108.1(1) |
| O(11)-Na(3)-O(5w) | 99.8(1) | O(11)-Na(3)-O(6w) | 112.0(1) |
| O(11)-Na(3)-O(8w) | 168.9(1) | O(71)-Na(3)-O(72) | 84.2(1) |
| O(71)-Na(3)-O(5w) | 97.7(1) | O(71)-Na(3)-O(6w) | 139.0(1) |
| O(71)-Na(3)-O(8w) | 70.0(1) | O(72)-Na(3)-O(5w) | 171.8(1) |
| O(72)-Na(3)-O(6w) | 89.5(1) | O(72)-Na(3)-O(8w) | 103.5(1) |
| O(5w)-Na(3)-O(6w) | 83.8(1) | O(5w)-Na(3)-O(8w) | 70.0(1) |
| O(6w)-Na(3)-O(8w) | 72.3(1) | O(53)-Na(4)-O(3w) | 91.4(1) |
| O(53)-Na(4)-O(5w) | 175.5(1) | O(53)-Na(4)-O(6w) | 101.1(1) |
| O(53)-Na(4)-O(7w) | 92.0(1) | O(53)-Na(4)-O(8w) | 103.3(1) |
| O(3w)-Na(4)-O(5w) | 89.8(1) | O(3w)-Na(4)-O(6w) | 94.9(1) |
| O(3w)-Na(4)-O(7w) | 82.7(1) | O(3w)-Na(4)-O(8w) | 165.2(1) |
| O(5w)-Na(4)-O(6w) | 83.1(1) | O(5w)-Na(4)-O(7w) | 83.9(1) |
| O(5w)-Na(4)-O(8w) | 75.6(1) | O(6w)-Na(4)-O(7w) | 166.8(1) |
| O(6w)-Na(4)-O(8w) | 81.1(1) | O(7w)-Na(4)-O(8w) | 98.0(1) |
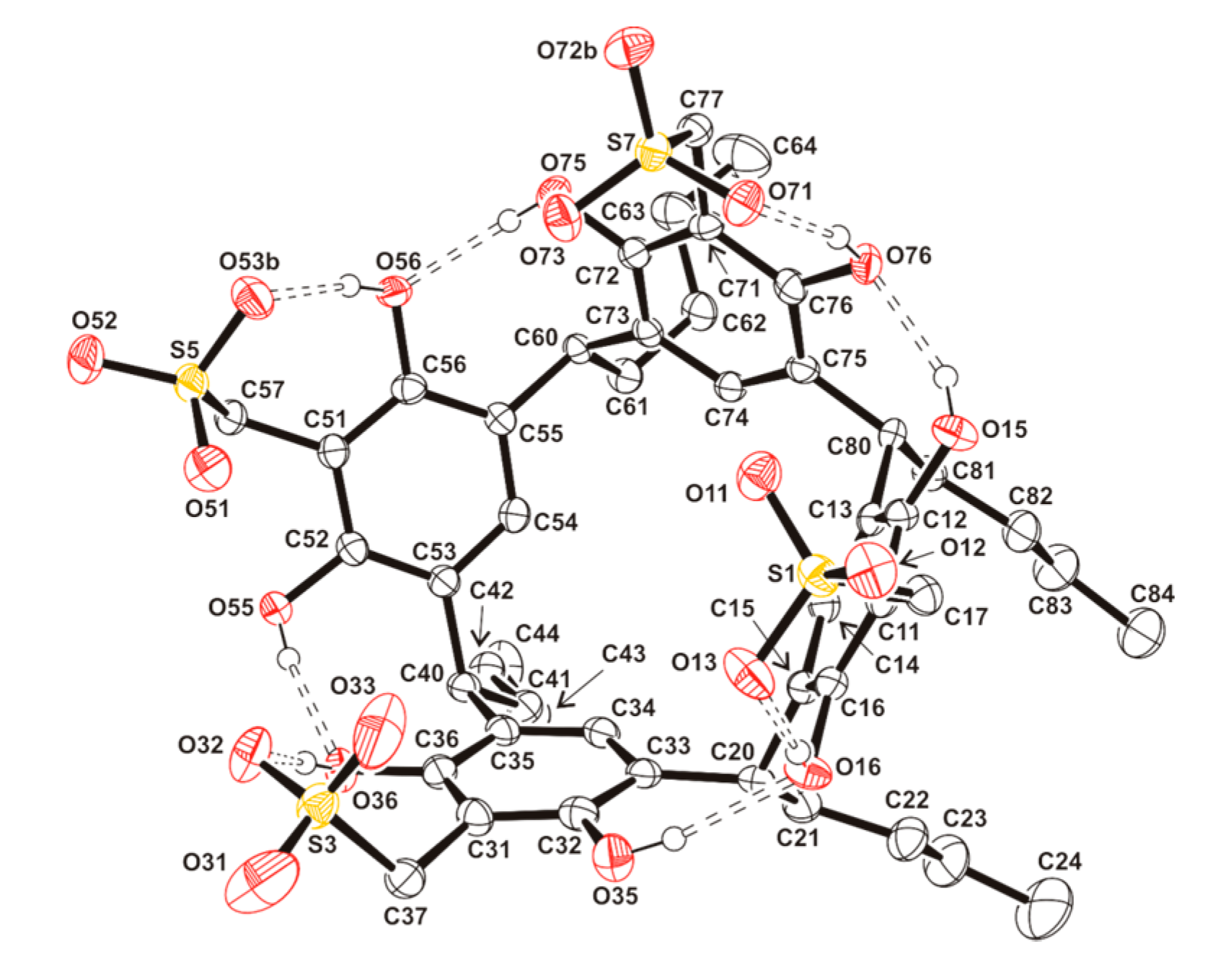
| D-H···A | D···A/Å | H···A/Å | D-H···A/° |
|---|---|---|---|
| O(15)-H(15)···O(76) | 2.780(4) | 1.98(5) | 155(4) |
| O(16)-H(16)···O(13) | 2.667(3) | 1.86(5) | 163(5) |
| O(35)-H(35)···O(16) | 2.823(4) | 1.99(5) | 170(5) |
| O(36)-H(36)···O(32) | 2.699(4) | 1.83(4) | 168(4) |
| O(55)-H(55)···O(36) | 2.791(4) | 2.02(5) | 166(5) |
| O(56)-H(56)···O(53)b | 2.765(3) | 1.87(5) | 169(4) |
| O(75)-H(75)···O(56) | 2.825(4) | 2.04(5) | 175(5) |
| O(76)-H(76)···O(71) | 2.721(3) | 1.89(5) | 164(4) |
| O(1w)-H(1B)···O(13) | 2.665(5) | 1.82(4) | 162(3) |
| O(2w)-H(2B)···O(52)c | 2.718(4) | 1.92(3) | 173(3) |
| O(3w)-H(3A)···O(4w)d | 2.756(4) | 2.03(3) | 149(3) |
| O(3w)-H(3B)···O(1w)d | 2.720(5) | 1.98(4) | 152(3) |
| O(4w)-H(4A)···O(12)d | 2.756(4) | 2.03(3) | 149(3) |
| O(4w)-H(4B)···O(31)e | 2.724(5) | 1.92(3) | 170(2) |
| O(5w)-H(5A)···O(51)f | 2.803(4) | 2.03(3) | 160(3) |
| O(5w)-H(5B)···O(32)f | 2.849(4) | 2.05(4) | 166(3) |
| O(6w)-H(6A)···O(73)b | 2.889(4) | 2.27(5) | 134(4) |
| O(6w)-H(6B)···O(12)d | 2.832(4) | 2.03(3) | 172(3) |
| O(7w)-H(7A)···O(51)f | 2.728(4) | 1.93(5) | 166(5) |
| O(7w)-H(7B)···O(8w)g | 2.812(3) | 2.01(2) | 172(2) |
| O(8w)-H(8A)···O(73)b | 2.796(4) | 2.03(4) | 158(4) |
| O(8w)-H(8B)···O(52)f | 2.968(4) | 2.25(4) | 148(3) |
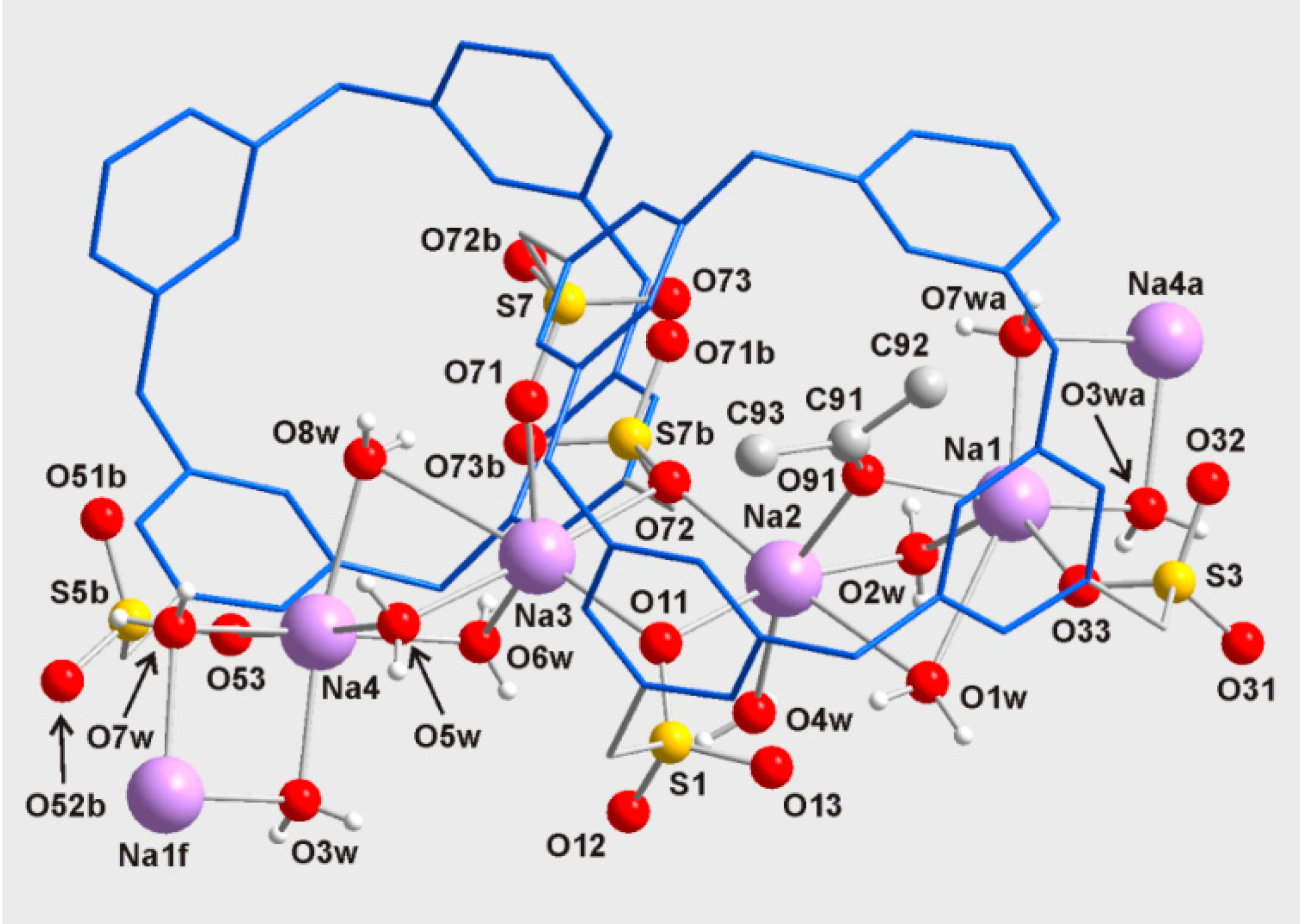
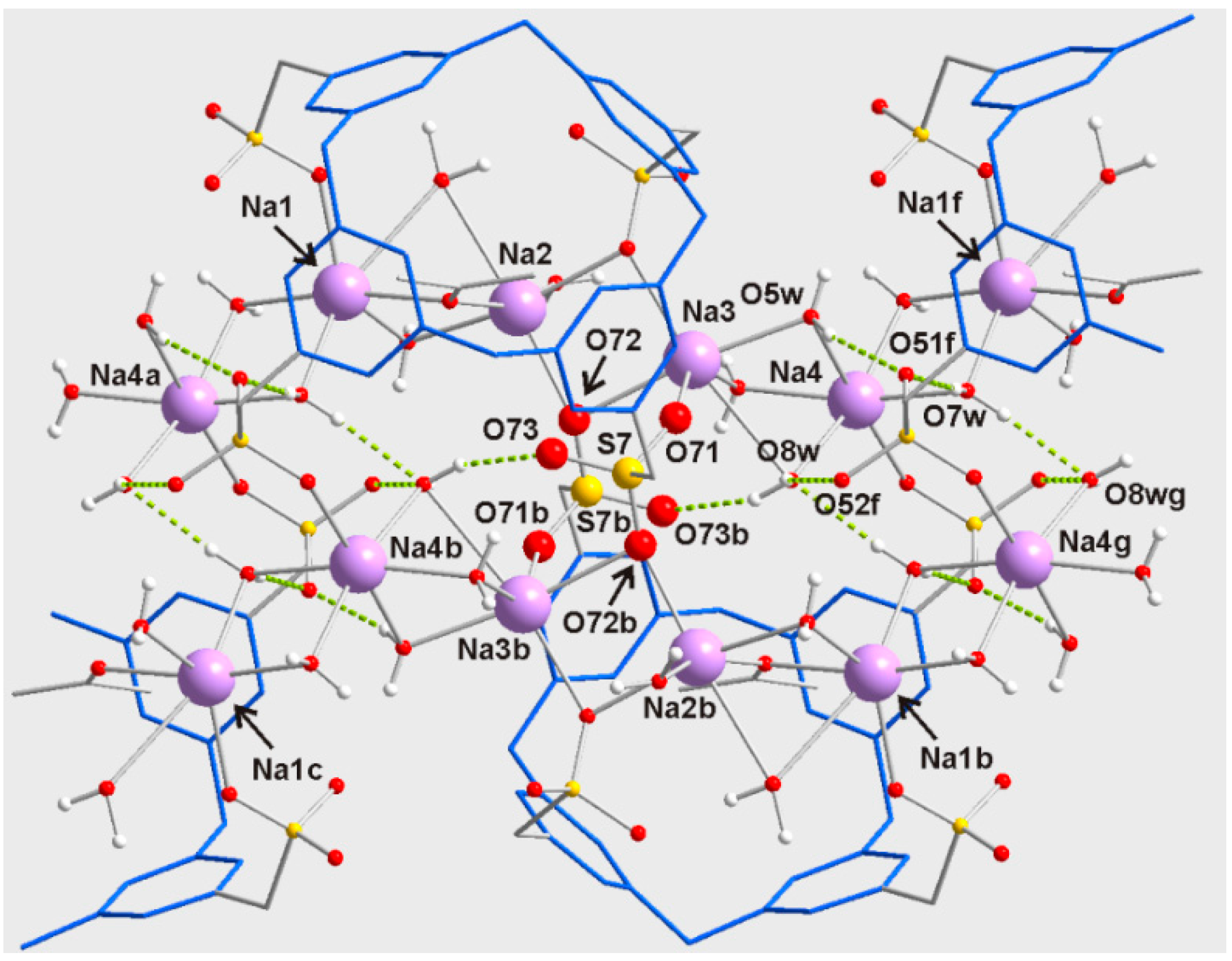
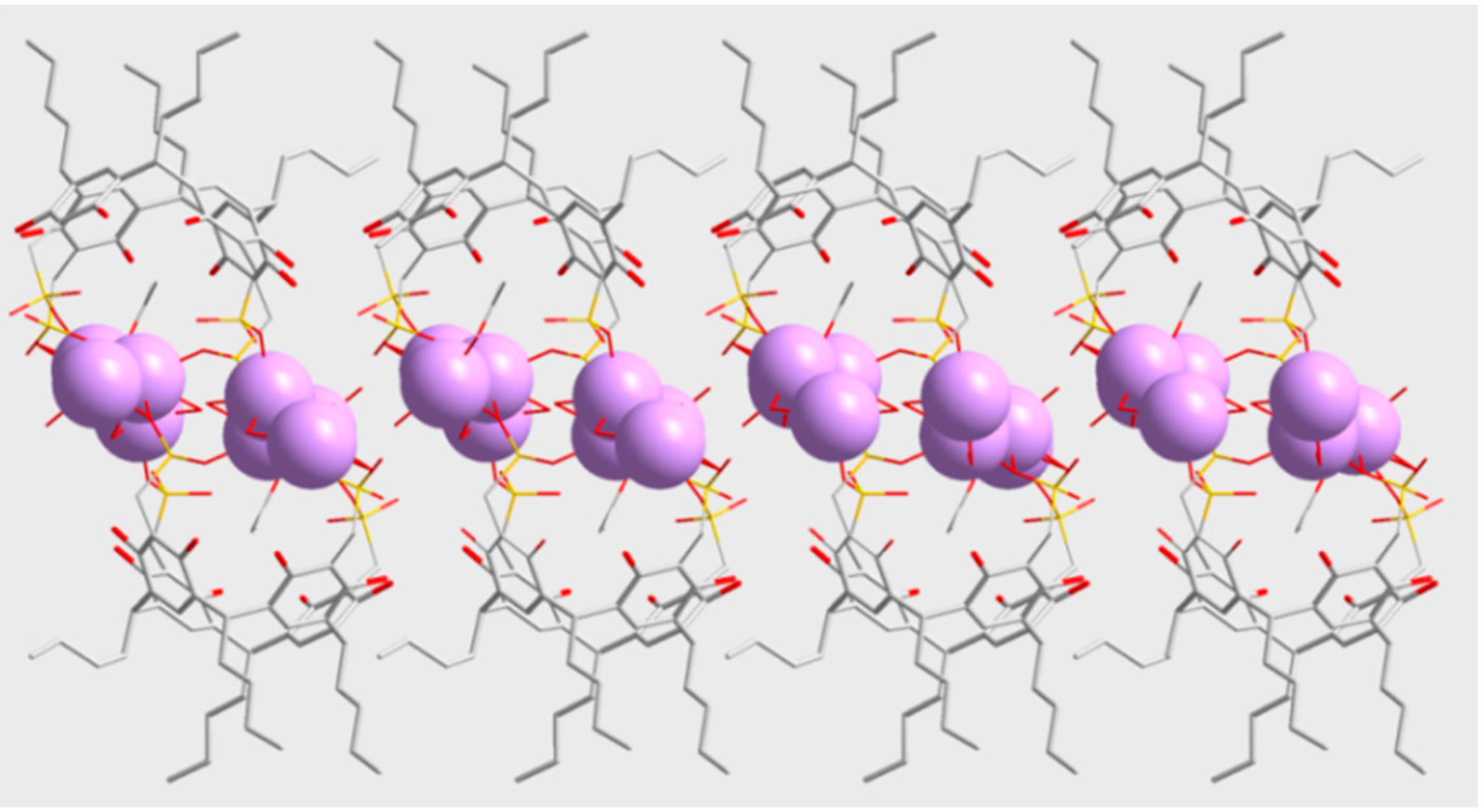
3. Experimental Section
3.1. General Information
3.2. General Synthetic Method for Resorcinarenes 1a and 1b
3.3. General Method for Sulfonation of Resorcinarenes
3.4. X-ray Crystallography
| Crystal Parameters | Data/Values |
|---|---|
| CCDC a deposition number | 1050083 |
| Empirical formula | C54H88Na4O30S4 |
| Moiety formula | C51H82Na4O29S4, C3H6O |
| Formula weight | 1437.44 |
| Temperature | 200(2) K |
| Wavelength | 0.71073 Å |
| Crystal system | Triclinic |
| Space group | P-1 |
| Unit cell dimensions | a = 12.055(2) Å, α = 80.28(1)° |
| b = 12.384(1) Å, β = 88.26(1)° | |
| c = 23.563(3) Å, γ = 72.81(1)° | |
| Volume | 3311.7(8) Å3 |
| Z | 2 |
| Dcal | 1.442 g·cm−3 |
| Absorption coefficient | 0.256 mm−1 |
| F(000) | 1520 |
| Crystal size | 0.16 × 0.10 × 0.10 mm3 |
| range | 3.06° to 27.50° |
| Index ranges (h, k, l) | −15 to 15 |
| −16 to 16 | |
| −30 to 30 | |
| Reflections collected | 99438 |
| Unique data | 15,183, R(int) = 0.107 |
| Observed data (I > 2σ(I)) | 8986 |
| Goodness-of-fit on F2 | 1.040 |
| Final R indices (I > 2σ(I)) | R1 = 0.066, wR2 = 0.136 |
| R indices (all data) | R1 = 0.134, wR2 = 0.167 |
| Largest diffraction peak and hole | 1.219 and −0.572 e·Å−3 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Li, N.; Harrison, R.G.; Lamb, J.D. Aplications of resorcinarene derivatives in chemical separations. J. Incl. Phenom. Macrocycl. Chem. 2014, 78, 39–60. [Google Scholar] [CrossRef]
- Tan, H.M.; Soh, S.F.; Zhao, J.; Yong, E.L.; Gong, Y. Preparation and application of methylcalix[4]resorcinarene-bonded silica particles as chiral stationary phase in high-performance liquid chromatography. Chyrality 2011, 23, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Harrison, R.G.; Lamb, J.D. Anion separation and preconcentration with cyclen and cyclen-resorcinarene derivatives. J. Chromatogr. Sci. 2009, 47, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wu, Y.; Lu, K.; Ye, B. A simple but highly sensitive and selective calixarene-based sensor for serotonin. Electrochim. Acta 2013, 87, 756–762. [Google Scholar] [CrossRef]
- Cortez-Maya, S.; Hernandez-Ortega, S.; Ramirez-Apan, T.; Lijanova, I.V.; Martínez-Garcia, M. Synthesis of 5-aryl-1,4-benzodiazepine derivatives attached in resorcinarene-PANAM dendrimers and their anti-cancer activity. Bioorg. Med. Chem. 2012, 20, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Lijanova, I.V.; Moggio, I.; Arias, E.; Klimova, T.; Martínez-Garía, M. Resorcinarene-dendrimers with stilbene moieties for optoelectronics. Tetrahedron 2008, 64, 10258–10266. [Google Scholar] [CrossRef]
- Jain, V.K.; Kanaiva, P.H.; Bhojak, N. Synthesis, spectral characterization of azo dyes derived from calix[4]resorcinarene and their application in dyeing of fibers. Fiber. Polym. 2008, 9, 720–726. [Google Scholar] [CrossRef]
- Kazakova, E.K.; Morozova, J.E.; Mironova, D.A.; Konovalov, A.I. Sorption of azo dyes from aqueous solutions by tetradodecyloxybenzylcalix[4]resorcinarene derivatives. J. Incl. Phenom. Macrocycl. Chem. 2012, 74, 467–472. [Google Scholar] [CrossRef]
- O’farrell, C.M.; Chudomel, J.M.; Collins, J.M.; Dignam, C.F.; Wenzel, T.J. Water-soluble calix[4]resorcinarenes with hydroxyproline groups as chiral NMR solvating agents. J. Org. Chem. 2008, 73, 2843–2851. [Google Scholar] [CrossRef] [PubMed]
- Beyeh, N.K.; Weimann, D.P.; Kaufmann, L.; Schalley, C.A.; Rissanen, K. Ion-pair recognition of tetramethylammonium salts by halogenated resorcinarenes. Chem. Eur. J. 2012, 18, 5552–5557. [Google Scholar] [CrossRef] [PubMed]
- Salorinne, K.; Weimann, D.P.; Schalley, C.A.; Nissinen, M. Resorcinarene podand with amine functionalized side arms Synthesis, structure and binding properties of a neutral anion receptor. Eur. J. Org. Chem. 2009, 35, 6151–6159. [Google Scholar] [CrossRef]
- Hayashida, O.; Uchiyama, M. Cyclophane-based tetra(resorcinarene) as a host for both histone and hydrophobic molecular guests. Tetrahedron Lett. 2006, 47, 4091–4094. [Google Scholar] [CrossRef]
- Sarjono, R.E.; Siswanta, D.; Santosa, S.J.; Otho, K. Adsortion characteristics of Pb(II) and Cr(III) onto C-Methylcalix[4]resorcinarene. J. Korean Chem. Soc. 2011, 55, 454–462. [Google Scholar]
- Aoyama, Y.; Tanaka, Y.; Sugahara, S. Molecular recognition. 5. Molecular recognition of sugar via hydrogen-bonding interaction with a synthetic polyhydroxy macrocycle. J. Am. Chem. Soc. 1989, 111, 5397–5404. [Google Scholar] [CrossRef]
- Thoden van Velzen, E.U.; Engbersen, J.F.J.; Reinhoudt, D.N. Self-Assembled Monolayers of Receptor Adsorbates on Gold: Preparation and Characterization. J. Am. Chem. Soc. 1994, 116, 3597–3598. [Google Scholar] [CrossRef]
- Hedidi, M.; Hamdi, S.M.; Mazari, T.; Boutemeur, B.; Rabia, F.; Chemat, F.; Hamdi, M. Microwave-assisted synthesis of Calix[4]resorcinarenes. Tetrahedron 2006, 62, 5652–5655. [Google Scholar] [CrossRef]
- Hoegberg, A.G.S. Two stereoisomeric macrocyclic resorcinol-acetaldehyde condensation products. J. Org. Chem. 1980, 45, 4498–4500. [Google Scholar] [CrossRef]
- Tundstad, L.M.; Tucker, J.A.; Dalcanale, E.; Weiser, J.; Bryant, J.A.; Sherman, J.C.; Helgeson, R.C.; Knobler, C.B.; Cram, D.J. Host-Guest Complexation. 48. Octol Building Blocks for Cavitands and Carcerands. J. Org. Chem. 1989, 54, 1305–1312. [Google Scholar] [CrossRef]
- Kazakova, E.K.; Makarova, N.A.; Ziganshina, A.U.; Muslinkina, L.A.; Muslinkin, A.A.; Habicher, W.D. Novel water-soluble tetrasulfonatomethylcalix[4]resorcinarenes. Tetrahedron Lett. 2000, 41, 10111–10115. [Google Scholar] [CrossRef]
- Pietraszkiewicz, O.; Utzig, E.; Zielenkiewics, W.; Pietraszkiewicz, M. Thermochemical investigation of some selected solvates of calix[4]resorcinarene. J. Thermal Anal. 1998, 54, 249–255. [Google Scholar] [CrossRef]
- Li, L.; Sun, J.; Zhang, L.; Yao, R.; Yan, C. Crystal structure and fluorescence sensing properties of tetramethoxyresorcinarene functionalized Schiff bases. J. Mol. Struct. 2015, 1081, 355–361. [Google Scholar] [CrossRef]
- Mcldowie, M.J.; Mecerino, M.; Skelton, B.W.; White, A.H. Facile Lewis Acid Catalized Synthesis of C4 Symetric Resorcinarenes. Org. Lett. 2000, 24, 3869–3871. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, L.; Yao, R.; Yan, C. Synthesis, crystal structures and complexing properties of tetramethoxyresorcinarene functionalized tetraacylhidrazones. J. Incl. Phenom. Macrocycl. Chem. 2014, 79, 485–494. [Google Scholar] [CrossRef]
- Tero, T.; Nisinnen, M. A perspective to resorcinarene crowns. Tetrahedron 2014, 70, 1111–1123. [Google Scholar] [CrossRef]
- Webb, H.R.; Hardie, M.J.; Raston, C.L. Scandium(III) Coordination Polymers Containing Capsules Based on Two p-Sulfonatocalix[4]arenes. Chem. Eur. J. 2001, 7, 3616–3620. [Google Scholar] [CrossRef]
- Asfari, Z.; Harrowfield, J.; Thuéry, P.; Vicens, J. Calixarenes as Polyhapto-aromatic Ligands: Alkali Metal Ions and Sulfonated Calixarenes. Supramol. Chem. 2003, 15, 69–77. [Google Scholar] [CrossRef]
- Harlow, R.L.; Simons, D.M.; Weber, P.C. Structure of sodium 4-(4-hydroxyphenylazo)benzoato tetrahydrate. Acta Crystallogr. Sect. C 1992, 48, 48–50. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- These data can be obtained, free of charge, via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, U.K.; Fax: +44 1223 336033; e-mail: [email protected]).
- Sample Availability: Samples of the compounds 1a, 1b, 2a and 2b are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanabria, E.; Esteso, M.Á.; Pérez-Redondo, A.; Vargas, E.; Maldonado, M. Synthesis and Characterization of Two Sulfonated Resorcinarenes: A New Example of a Linear Array of Sodium Centers and Macrocycles. Molecules 2015, 20, 9915-9928. https://doi.org/10.3390/molecules20069915
Sanabria E, Esteso MÁ, Pérez-Redondo A, Vargas E, Maldonado M. Synthesis and Characterization of Two Sulfonated Resorcinarenes: A New Example of a Linear Array of Sodium Centers and Macrocycles. Molecules. 2015; 20(6):9915-9928. https://doi.org/10.3390/molecules20069915
Chicago/Turabian StyleSanabria, Edilma, Miguel Ángel Esteso, Adrián Pérez-Redondo, Edgar Vargas, and Mauricio Maldonado. 2015. "Synthesis and Characterization of Two Sulfonated Resorcinarenes: A New Example of a Linear Array of Sodium Centers and Macrocycles" Molecules 20, no. 6: 9915-9928. https://doi.org/10.3390/molecules20069915