Solid Lipid Nanoparticles: A Potential Multifunctional Approach towards Rheumatoid Arthritis Theranostics
Abstract
:1. Introduction
2. Results and Discussion
2.1. Nanoparticle Characterization
| Formulation | Size (nm) | PdI | Zeta Potential (mV) | MTX Association (%) |
|---|---|---|---|---|
| SLN | 168 ± 7 | 0.234 ± 0.023 | −39 ± 4 | - |
| SLN + MTX | 172 ± 6 | 0.211 ± 0.024 | −35 ± 2 a | 98 ± 9 |
| SLN + SPIONs | 196 + 13 a | 0.238 ± 0.025 | −35 ± 2 a | - |
| SLN + MTX + SPIONs | 199 ± 13 a | 0.246 ± 0.020 | −33 ± 3 a | 102 ± 5 |
| SLN + Anti-CD64 | 172 ± 4 | 0.225 ± 0.021 | −17 ± 3 c | - |
| SLN + MTX + Anti-CD64 | 167 ± 2 | 0.200 ± 0.024 | −17 ± 2 c | 98 ± 9 |
| SLN + SPIONs + Anti-CD64 | 196 ± 3 b | 0.184 ± 0.024 | −18 ± 2 c | - |
| SLN + MTX + SPIONs + Anti-CD64 | 206 ± 3 b | 0.209 ± 0.022 | −17 ± 3 c | 102 ± 5 |
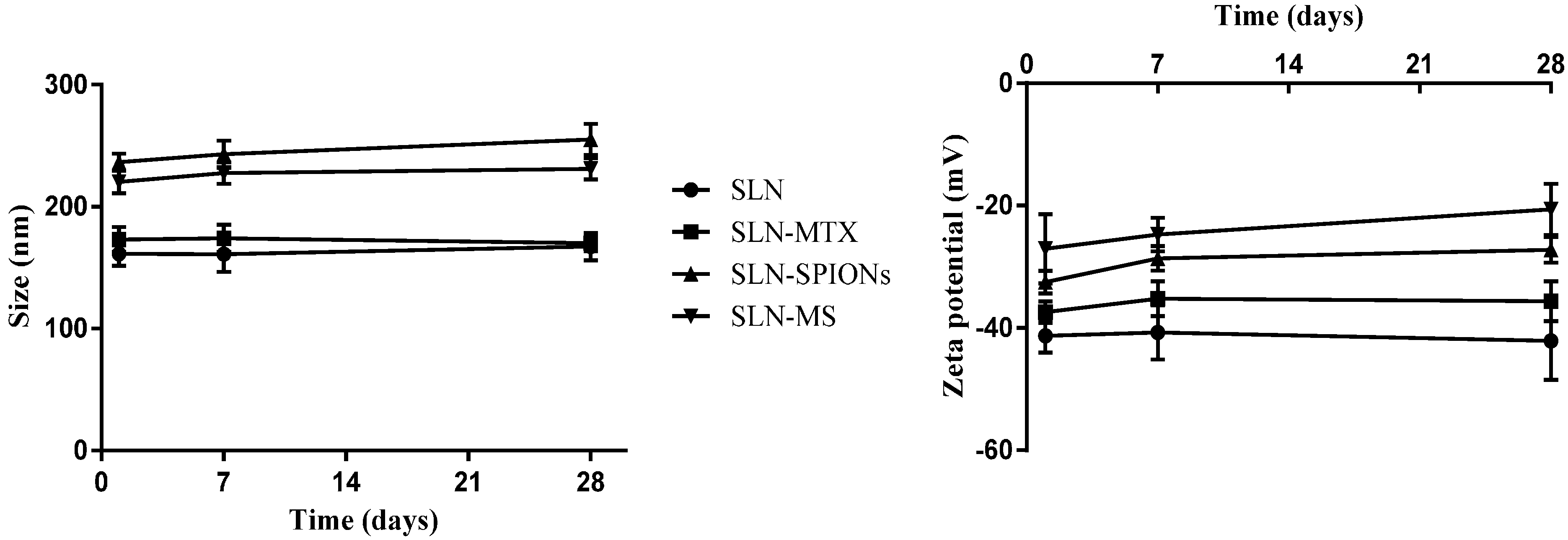
2.1.1. Nanoparticle Size, Polydispersion Index and Zeta Potential
2.1.2. Transmission Electron Microscopy


2.1.3. MTX Association Efficiency
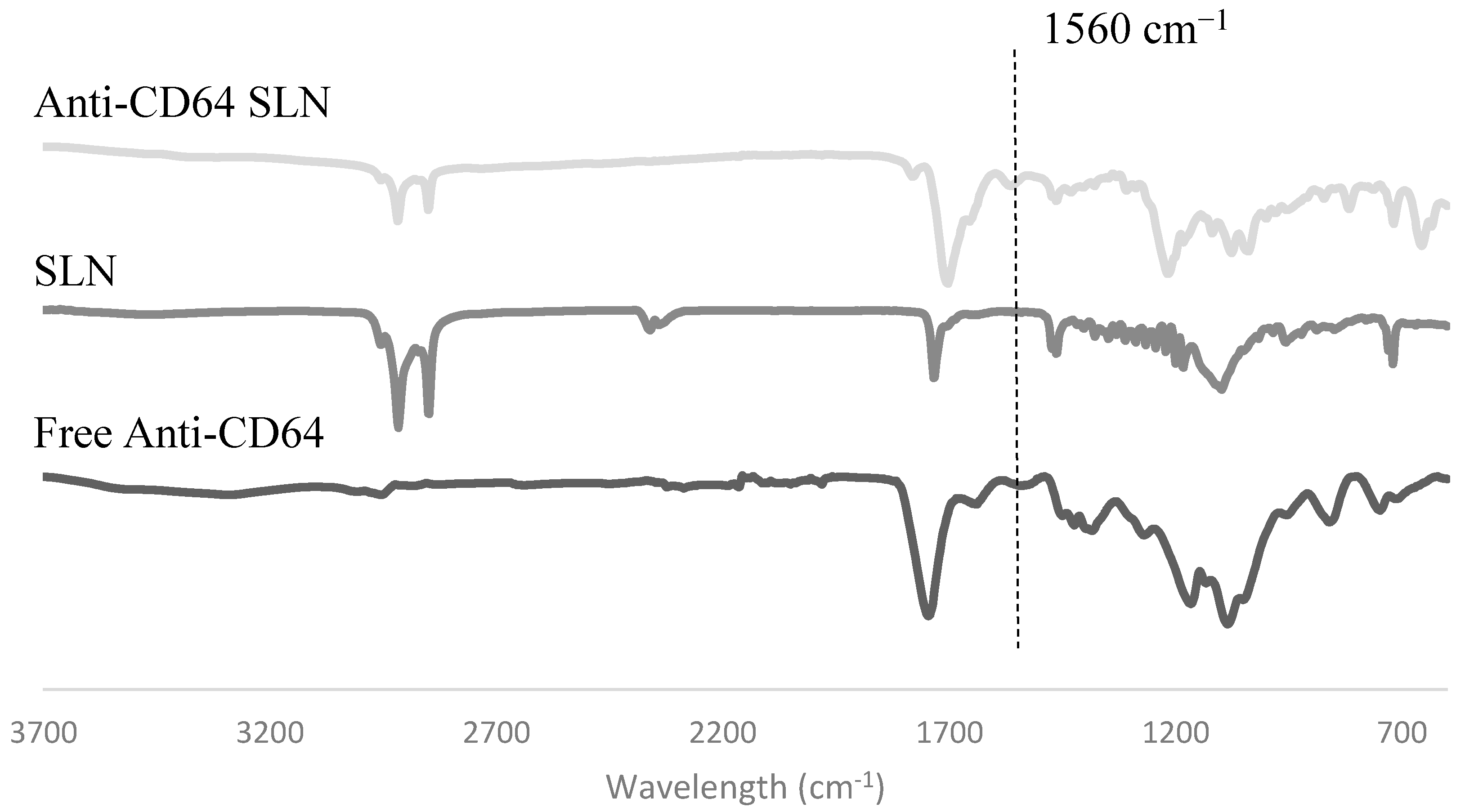
2.1.4. Antibody Conjugation
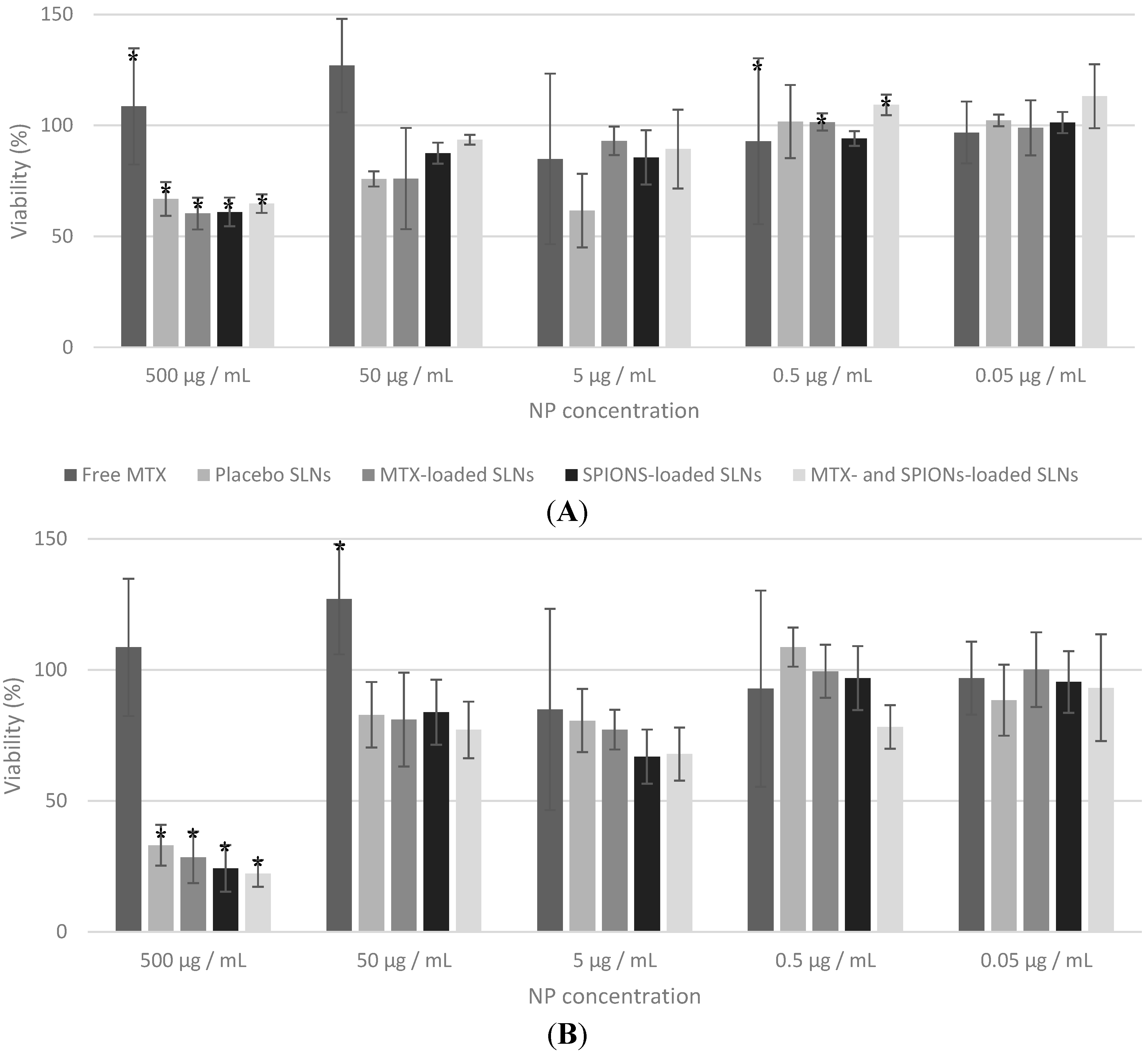
2.2. In Vitro Studies
3. Experimental Section
3.1. Materials
3.2. Methods
3.2.1. Preparation of Multifunctional Nanoparticles
Nanoparticle Production Method

Antibody Conjugation to the Solid Lipid Nanoparticles
3.2.2. Nanoparticle Characterization
Dynamic Light Scattering and Phase Analysis Light Scattering
Transmission Electron Microscopy
MTX Quantification
Fourier Transform Infrared Spectroscopy
3.2.3. In Vitro Studies
MTT Assay
3.2.4. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smolen, J.S.; Aletaha, D.; Bijlsma, J.W.; Breedveld, F.C.; Boumpas, D.; Burmester, G.; Combe, B.; Cutolo, M.; de Wit, M.; Dougados, M.; et al. Treating rheumatoid arthritis to target: Recommendations of an international task force. Ann. Rheum. Dis. 2010, 69, 631–637. [Google Scholar]
- Choy, E.H.; Kavanaugh, A.F.; Jones, S.A. The problem of choice: Current biologic agents and future prospects in RA. Nat. Rev. Rheumatol. 2013, 9, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Majithia, V.; Geraci, S.A. Rheumatoid arthritis: Diagnosis and management. Am. J. Med. 2007, 120, 936–939. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D.; Koeller, M.; Weisman, M.H.; Emery, P. New therapies for treatment of rheumatoid arthritis. Lancet 2007, 370, 1861–1874. [Google Scholar] [CrossRef]
- Narváez, J.A.; Narváez, J.; de Lama, E.; de Albert, M. MR imaging of early rheumatoid arthritis. RadioGraphics 2010, 30, 143–163. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.M.; Kim, D.K.; Haj, A.J.E.; Dobson, J. Development of superparamagnetic iron oxide nanoparticles (SPIONS) for translation to clinical applications. IEEE Trans. Nanobiosci. 2008, 7, 298–305. [Google Scholar]
- Cutolo, M.; Sulli, A.; Pizzorni, C. Anti-inflammatory mechanisms of methotrexate in rheumatoid arthritis. Ann. Rheum. Dis. 2001, 60, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Moura, C.C.; Segundo, M.A.; Neves, J.; Reis, S.; Sarmento, B. Co-association of methotrexate and SPIONs into anti-CD64 antibody-conjugated PLGA nanoparticles for theranostic application. Int. J. Nanomed. 2014, 9, 4911–4922. [Google Scholar]
- Mashaghi, S.; Jadidi, T.; Koenderink, G.; Mashaghi, A. Lipid nanotechnology. Int. J Mol. Sci. 2013, 14, 4242–4282. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, R. Nanotechnology and its applications in drug delivery: A review. Int. J. Med. Mol. Med. 2013, 3, WMC002867. [Google Scholar]
- Coco, R.; Plapied, L.; Pourcelle, V.; Jerome, C.; Brayden, D.J.; Schneider, Y.J.; Preat, V. Drug delivery to inflamed colon by nanoparticles: Comparison of different strategies. Int. J. Pharm. 2013, 440, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Couvreur, P. Nanoparticles in drug delivery: Past, present and future. Adv. Drug Deliv. Rev. 2013, 65, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Mussi, S.V.; Sawant, R.; Perche, F.; Oliveira, M.C.; Azevedo, R.B.; Ferreira, L.A.; Torchilin, V.P. Novel nanostructured lipid carrier co-loaded with doxorubicin and docosahexaenoic acid demonstrates enhanced in vitro activity and overcomes drug resistance in MCF-7/Adr cells. Pharm. Res. 2014, 31, 1882–1892. [Google Scholar] [CrossRef] [PubMed]
- Venishetty, V.K.; Chede, R.; Komuravelli, R.; Adepu, L.; Sistla, R.; Diwan, P.V. Design and evaluation of polymer coated carvedilol loaded solid lipid nanoparticles to improve the oral bioavailability: A novel strategy to avoid intraduodenal administration. Colloids Surf. B Biointerfaces 2012, 95, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.A.; Md, S.; Sahni, J.K.; Baboota, S.; Dang, S.; Ali, J. Nanostructured lipid carriers system: Recent advances in drug delivery. J. Drug Target. 2012, 20, 813–830. [Google Scholar] [CrossRef] [PubMed]
- Sibeko, B.; Choonara, Y.E.; du Toit, L.C.; Modi, G.; Naidoo, D.; Khan, R.A.; Kumar, P.; Ndesendo, V.M.; Iyuke, S.E.; Pillay, V. Composite polylactic-methacrylic Acid copolymer nanoparticles for the delivery of methotrexate. J. Drug Deliv. 2012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Zhang, Y.L.; Zhou, J.P.; Lv, H.X. Solid lipid nanoparticles modified with stearic acid-octaarginine for oral administration of insulin. Int. J. Nanomed. 2012, 7, 3333–3339. [Google Scholar]
- Patil, S.M.; Hemant, P.; Joshi, H.P. Lipid shell modified with combination of lipid and phospholipids in solid lipid nanoparticles for engineered specificity of paclitaxel in tumor bearing mice. Int. J. Drug Deliv. 2013, 5, 196–205. [Google Scholar]
- Sapsford, K.E.; Algar, W.R.; Berti, L.; Gemmill, K.B.; Casey, B.J.; Oh, E.; Stewart, M.H.; Medintz, I.L. Functionalizing nanoparticles with biological molecules: Developing chemistries that facilitate nanotechnology. Chem. Rev. 2013, 113, 1904–2074. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Myerson, J.W.; Hu, L.; Marsh, J.N.; Hou, K.; Scott, M.J.; Allen, J.S.; Hu, G.; San Roman, S.; Lanza, G.M.; et al. Programmable nanoparticle functionalization for in vivo targeting. FASEB J. 2013, 27, 255–264. [Google Scholar]
- Duangjit, S.; Pamornpathomkul, B.; Opanasopit, P.; Rojanarata, T.; Obata, Y.; Takayama, K.; Ngawhirunpat, T. Role of the charge, carbon chain length, and content of surfactant on the skin penetration of meloxicam-loaded liposomes. Int. J. Nanomed. 2014, 9, 2005–2017. [Google Scholar] [CrossRef] [PubMed]
- Sussman, E.M.; Jayagopal, A.; Haselton, F.R.; Shastri, V.P. Engineering of solid lipid nanoparticles for biomedical applications. In New Delivery Systems for Controlled Drug Release from Naturally Occurring Materials; Parris, N., Liu, L., Song, C., Shastri, V.P., Eds.; American Chemical Society: Washington, DC, USA, 2008; Volume 992, pp. 139–152. [Google Scholar]
- Sperling, R.A.; Parak, W.J. Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Philos. Trans. Ser. A 2010, 368, 1333–1383. [Google Scholar] [CrossRef] [PubMed]
- Scholer, N.; Hahn, H.; Muller, R.H.; Liesenfeld, O. Effect of lipid matrix and size of solid lipid nanoparticles (SLN) on the viability and cytokine production of macrophages. Int. J. Pharm. 2002, 231, 167–176. [Google Scholar] [CrossRef]
- Martins, S.; Tho, I.; Reimold, I.; Fricker, G.; Souto, E.; Ferreira, D.; Brandl, M. Brain delivery of camptothecin by means of solid lipid nanoparticles: Formulation design, in vitro and in vivo studies. Int. J. Pharm. 2012, 439, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Block, I.A. Particle Sizing by 3D Cross-correlation DLS in Highly Scattering Samples; LS Instruments: Fribourg, Switzerland, 2010. [Google Scholar]
- Ekambaram, P.; Satahali, A.A.H.; Priyanka, K. Solid lipid nanoparticles: Review. Sci. Rev. Chem. Commun. 2012, 2, 80–102. [Google Scholar]
- Raamsdonk, M.; Mei, H.C.; Soet, J.J.; Busscher, H.J.; Graaff, J. Effect of Polyclonal and Monoclonal antibodies on Surface properties of Streptococcus Sobrinus. Infect. Immun. 1995, 63, 1698–1702. [Google Scholar] [PubMed]
- Shukla, N.; Liu, C.; Jones, P.M.; Weller, D. FTIR study of surfactant bonding to FePt nanoparticles. J. Magn. Magn. Mater. 2003, 266, 178–184. [Google Scholar] [CrossRef]
- Kato, Y.; Yamazaki, H.; Tomozawa, M. Detection of phase separation by ftir in a liquid-crystal-display substrate aluminoborosilicate glass. J. Am. Ceram. Soc. 2001, 84, 2111–2116. [Google Scholar] [CrossRef]
- Weyhers, H.; Ehlers, S.; Hahn, H.; Souto, E.B.; Muller, R.H. Solid Lipid Nanoparticles (SLN)—Effects of lipid composition on in vitro degradation and in vivo toxicity. Pharmazie 2006, 61, 539–544. [Google Scholar] [PubMed]
- Widemann, B.C.; Adamson, P.C. Understanding and managing methotrexate nephrotoxicity. Oncologist 2006, 11, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Houngbossa, K.; Guenzet, J.; Bourin, M. Effets de la température et du pH sur la cinétique de dissolution du méthotrexato en milieu aqueux. Ann. Pharm. Fr. 1996, 54, 77–84. [Google Scholar] [PubMed]
- Sample Availability: Samples of the any of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albuquerque, J.; Moura, C.C.; Sarmento, B.; Reis, S. Solid Lipid Nanoparticles: A Potential Multifunctional Approach towards Rheumatoid Arthritis Theranostics. Molecules 2015, 20, 11103-11118. https://doi.org/10.3390/molecules200611103
Albuquerque J, Moura CC, Sarmento B, Reis S. Solid Lipid Nanoparticles: A Potential Multifunctional Approach towards Rheumatoid Arthritis Theranostics. Molecules. 2015; 20(6):11103-11118. https://doi.org/10.3390/molecules200611103
Chicago/Turabian StyleAlbuquerque, João, Catarina Costa Moura, Bruno Sarmento, and Salette Reis. 2015. "Solid Lipid Nanoparticles: A Potential Multifunctional Approach towards Rheumatoid Arthritis Theranostics" Molecules 20, no. 6: 11103-11118. https://doi.org/10.3390/molecules200611103





