Optimization of Purification, Identification and Evaluation of the in Vitro Antitumor Activity of Polyphenols from Pinus Koraiensis Pinecones
Abstract
:1. Introduction
2. Results and Discussion
2.1. Adsorption and Desorption on AB-8 Resin
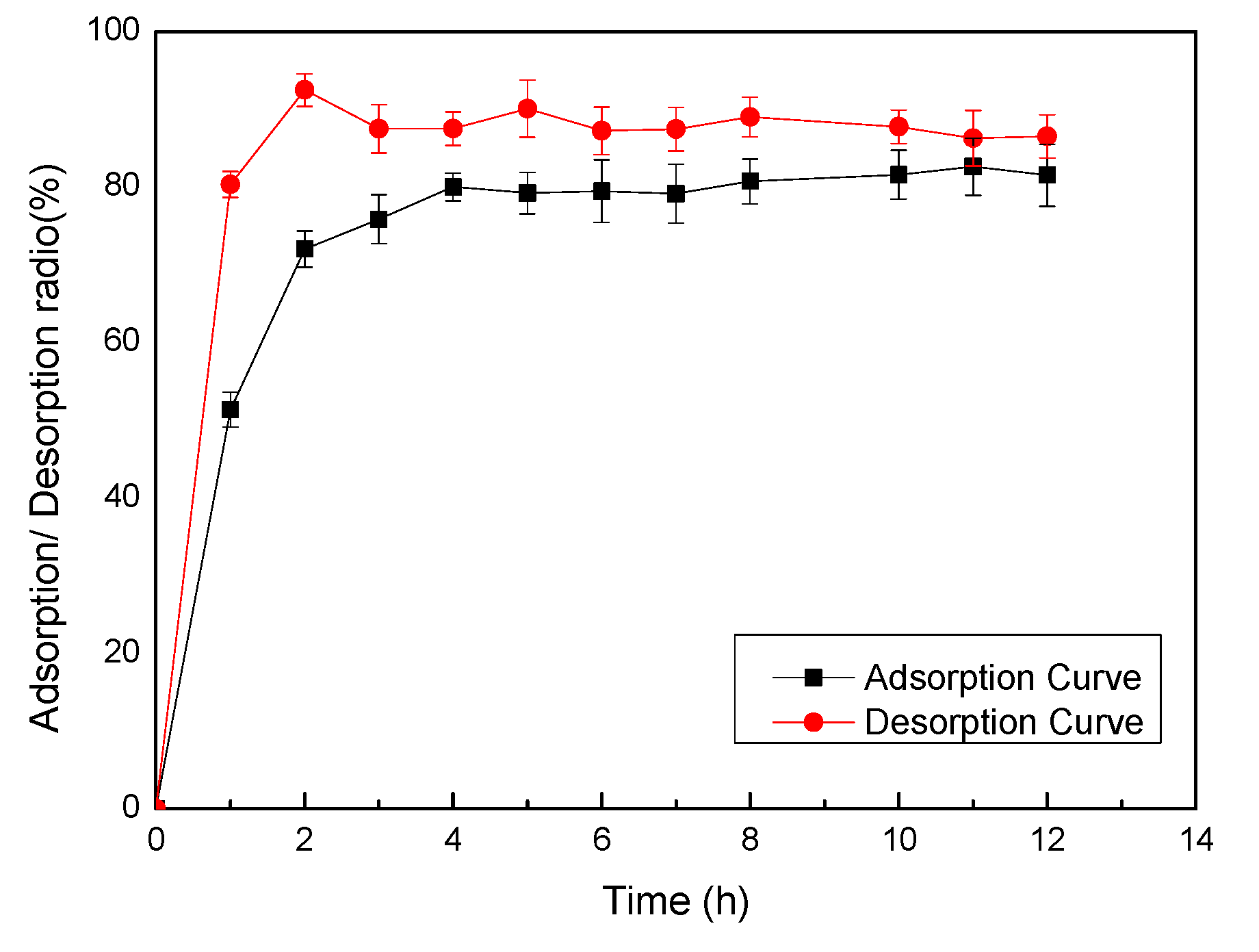
2.2. Effect of Sample Phenolic Concentration on Purity of PPP
2.3. Effect of Sample Volume on Purity of PPP
2.4. Effect of Ethanol Concentration on Purity of PPP
2.5. Optimization of Purification Parameters for PPP
2.5.1. Statistical Analysis and the Model Fitting
| Run | X1 (mg/mL) | X2 (mL) | X3 (%) | Phenolic Purity (%) |
|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 27.45 |
| 2 | −1 | 0 | −1 | 15.46 |
| 3 | 0 | −1 | 1 | 17.62 |
| 4 | 1 | 0 | −1 | 19.21 |
| 5 | −1 | −1 | 0 | 13.59 |
| 6 | −1 | 0 | 1 | 17.11 |
| 7 | 0 | −1 | −1 | 15.60 |
| 8 | 1 | 0 | 1 | 24.90 |
| 9 | 1 | 1 | 0 | 24.41 |
| 10 | 0 | 0 | 0 | 27.14 |
| 11 | 1 | −1 | 0 | 16.61 |
| 12 | −1 | 1 | 0 | 16.35 |
| 13 | 0 | 1 | 1 | 23.36 |
| 14 | 0 | 1 | −1 | 18.16 |
| 15 | 0 | 0 | 0 | 26.48 |
| Source | SS a | DF b | MS c | F-Value | Prob-F |
|---|---|---|---|---|---|
| Modle | 325.17 | 9 | 36.13 | 156.06 | <0.0001 |
| Residual | 1.16 | 5 | 0.23 | ||
| Lack of fit | 0.67 | 3 | 0.22 | 0.91 | 0.5629 |
| Pure error | 0.49 | 2 | 0.25 | ||
| Cor.total | 326.33 | 14 | |||
| R2 = 0.9965; R2adj = 0.9901; CV = 2.38 | |||||
2.5.2. Optimization of the Purification Conditions

2.6. Verification of Predictive Model
| Sample Phenolic Concentration (mg/mL) | Sample Volume (mL) | Ethanol Concentration (%) | Phenolic Purity (%) | |
|---|---|---|---|---|
| Optimum Conditions (predicted) | 1.66 | 21.65 | 63.14 | 28.17 |
| Modified Conditions (actual) | 1.70 | 22.000 | 63.00 | 27.93 ± 0.14 |
2.7. Antiproliferative Activity of PPP

2.8. UPLC-Q-TOF-MS Analysis of Purified PPP

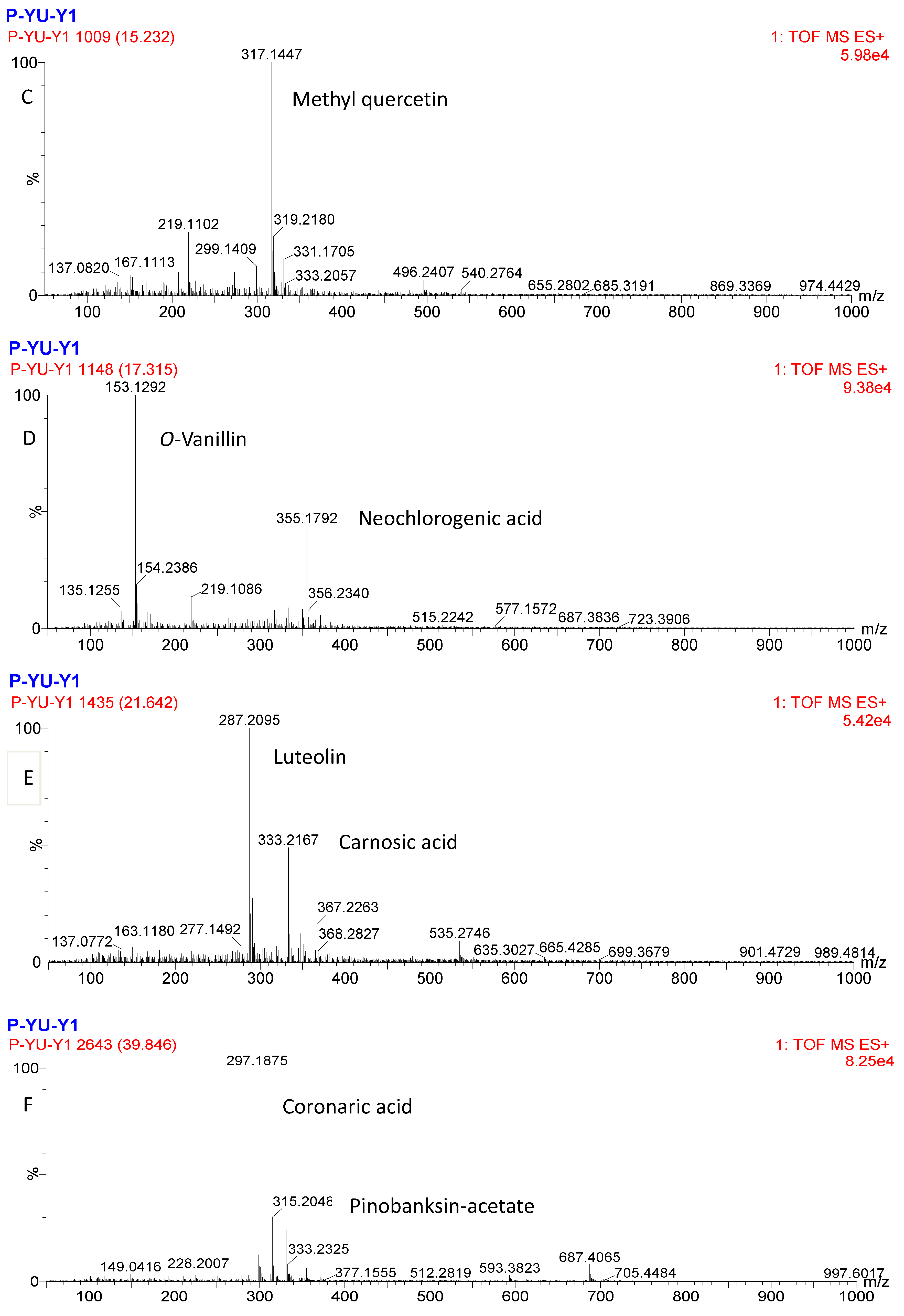
| Peak | tR (min) | MS (m/z) | MW | Molecular Formula | Identification |
|---|---|---|---|---|---|
| 1 | 2.64 | 291 | 290 | C15H14O6 | Catechin |
| 2 | 4.38 | 207 | 206 | C11H10O4 | Scopoletin + CH2 |
| 3 | 6.55 | 167 | 166 | C8H7O4 | Vanillic acid-H |
| 4 | 7.11 | 167 | 166 | C8H7O4 | Vanillic acid-H |
| 6 | 12.72 | 317 | 316 | C16H12O7 | 3-Hydroxy-4-O-β-glucosylbenzoic acid |
| 7 | 14.55 | 317 | 316 | C16H12O7 | Isorhamnetin |
| 8 | 15.23 | 317 | 316 | C16H12O7 | Methyl quercetin |
| 9 | 17.32 | 153 | 152 | C8H8O3 | o-Vanillin |
| 10 | 17.72 | 153 | 152 | C8H8O3 | p-Vanillin |
| 12 | 20.39 | 291 | 290 | C15H14O6 | Epicatechin |
| 13 | 21.61 | 287 | 286 | C15H10O6 | Luteolin |
| 14 | 23.60 | 291 | 290 | C15H14O6 | Epicatechin |
| 15 | 24.38 | 275 | 274 | C15H14O5 | Phloretin |
| 16 | 25.14 | 347 | 346 | C20H26O5 | Rosmanol isomer |
| 17 | 28.87 | 319 | 318 | C15H10O8 | Myricetin |
| 20 | 37.21 | 287 | 286 | C15H10O6 | Kaempferol |
| 21 | 39.59 | 331 | 330 | C20H26O4 | Carnosol isomer |
| 22 | 39.85 | 297 | 296 | C18H32O3 | Coronaric acid |
| 23 | 40.73 | 333 | 332 | C16H12O6 | Monogalloyl glucose |
| 24 | 42.93 | 317 | 316 | C16H12O7 | Tamarixetin |
3. Experimental Section
3.1. Samples and Chemicals
3.2. Extraction Procedure of PPP
3.3. Determination of Total Phenolic Content (TPC)
3.4. Static Adsorption and Desorption of AB-8 Resin Test
3.5. Determination of Total Phenolic Purity (TPP)
3.6. Experimental Design
3.7. Cell Culture
3.8. Measurement of Antiproliferation of PPP on Cancer Cells
3.9. Identification of Purified PPP by UPLC-Q-TOF-MS
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgment
Author Contributions
Conflicts of Interest
Nonstandard Abbreviations
References
- Khonkarn, R.; Okonogi, S.; Ampasavate, C.; Anuchapreeda, S. Investigation of fruit peel extracts as sources for compounds with antioxidant and antiproliferative activities against human cell lines. Food Chem. Toxicol. 2010, 48, 2122–2129. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; He, X.W.; Jiang, J.G.; Xu, X.L. Hydroxytyrosol and Its Potential Therapeutic Effects. J. Agric. Food Chem. 2014, 62, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Happy, M.; Dejoie, J.; Zajac, C.K.; Cortez, B.; Chakraborty, K.; Aderemi, J.; Sauane, M. Sigma 1 Receptor antagonist potentiates the anti-cancer effect of p53 by regulating ER stress, ROS production, Bax levels, and caspase-3 activation. Biochem. Biophys. Res. Commun. 2015, 456, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.R.; Dong, H.W.; Chen, B.Q.; Zhao, P.; Liu, R.H. Fresh apples suppress mammary carcinogenesis and proliferative activity and induce apoptosis in mammary tumors of the Sprague-Dawley rat. J. Agric. Food Chem. 2009, 57, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Luo, Y.; Tian, M.; Zhang, S.Y.; Lu, R.; Wang, J.H.; Kasimu, R.; Li, X. Plant natural products: From traditional compounds to new emerging drugs in cancer therapy. Cell Prolif. 2014, 47, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Jung, H.S.; Eo, W.K.; Lee, S.Y.; Kim, S.H.; Shim, B.S. Rhus verniciflua Stokes extract as a potential option for treatment of metastatic renal cell carcinoma: Report of two cases. Ann. Oncol. 2010, 21, 1383–1385. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, H.; Zhang, Y.; Ma, Y.; Wang, J. Two new diterpenoid acids from Pinus koraiensis. Fitoterapia 2008, 79, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Jang, M.K.; Lee, D.G.; Yu, K.H.; Jang, H.J.; Kim, M.; Kim, S.G.; Yoo, B.H.; Lee, S.H. Comparison of methods for proanthocyanidin extraction from pine (Pinus densiflora) needles and biological activities of the extracts. Nutr. Res. Pract. 2010, 4, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Zhang, Y.; Wang, Z.Y.; Zu, Y.G. In vivo antioxidant activity of Pinus koraiensis nut oil obtained by optimised supercritical carbon dioxide extraction. Nat. Prod. Res. 2011, 25, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, Q.; Li, J.; Zhang, T.; Han, Z.; Gao, D.; Zheng, F. Antitumor Activity of the Procyanidins from Pinus koraiensis Bark on Mice Bearing U14 Cervical Cancer. Yakugaku Zasshi 2007, 127, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yang, H.Y.; Lee, H.S.; Hong, S.K. Chemical composition and antimicrobial activity of essential oil from cones of Pinus koraiensis. J. Microbiol. Biotechnol. 2008, 18, 497–502. [Google Scholar] [PubMed]
- Su, X.; Wang, J.; Yang, X.; Cao, W. Analysis of volatile oil constituents in pinecone of Pinus koraiensis by gas chromatography-mass spectrometry. Chin. J. Anal. Chem. 2006, 34, S217–S219. [Google Scholar]
- Zou, P.; Yang, X.; Huang, W.W.; Zhao, H.T.; Wang, J.; Xu, R.B.; Hu, X.L.; Shen, S.Y.; Qin, D. Characterization and Bioactivity of Polysaccharides Obtained from Pine Cones of Pinus koraiensis by Graded Ethanol Precipitation. Molecules 2013, 18, 9933–9948. [Google Scholar] [CrossRef] [PubMed]
- Nave, F.; Cabrita, M.J.; Costa, C.T. Use of solid-supported liquid–liquid extraction in the analysis of polyphenols in wine. J. Chromatogr. A 2007, 1169, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Russo, C. A new membrane process for the selective fractionation and total recovery of polyphenols, water and organic substances from vegetation waters (VW). J. Membr. Sci. 2007, 2288, 239–246. [Google Scholar] [CrossRef]
- Farías-Campomanes, A.M.; Rostagno, M.A.; Meireles, M.A. Production of polyphenol extracts from grape bagasse using supercritical fluids: Yield, extract composition and economic evaluation. J. Supercrit. Fluid. 2013, 77, 70–78. [Google Scholar] [CrossRef]
- Arriagada-Carrazana, J.P.; Sáez-Navarrete, C.; Bordeu, E. Membrane filtration effects on aromatic and phenolic quality of Cabernet Sauvignon wines. J. Food Eng. 2005, 68, 363–368. [Google Scholar] [CrossRef]
- Conidi, C.; Cassano, A.; Drioli, E. A membrane-based study for the recovery of polyphenols from bergamot juice. J. Membr. Sci. 2011, 375, 182–190. [Google Scholar] [CrossRef]
- Garcia-Castello, E.; Cassano, A.; Criscuoli, A.; Conidi, C.; Drioli, E. Recovery and concentration of polyphenols from olive mill wastewaters by integrated membrane system. Water Res. 2010, 44, 3883–3892. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, J.; Kammerer, D.R.; Carle, R. Impact of saccharides and amino acids on the interaction of apple polyphenols with ion exchange and adsorbent resins. J. Food Eng. 2010, 98, 230–239. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; Ma, R.; Zhang, X. Separation of tea polyphenol from Green Tea Leaves by a combined CATUFM-adsorption resin process. J. Food Eng. 2005, 67, 253–260. [Google Scholar] [CrossRef]
- Gao, Z.P.; Yu, Z.F.; Yue, T.L.; Quek, S.Y. Adsorption isotherm, thermodynamics and kinetics studies of polyphenols separation from kiwifruit juice using adsorbent resin. J. Food Eng. 2013, 116, 195–201. [Google Scholar] [CrossRef]
- Toledo, A.L.; Severo, J.B.; Souza, R.R.; Campos, E.S.; Santana, J.C.C.; Tambourgi, E.B. Purification by expanded bed adsorption and characterization of an α-amylases FORILASE NTL® from A. niger. J. Chromatogr. B 2007, 846, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Guo, Y.; Fu, C.; Li, J.; Li, Z. Simultaneous separation and purification of total polyphenols, chlorogenic acid and phlorizin from thinned young apples. Food Chem. 2013, 136, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.; Sheng, Z.; Han, Q.; Zhao, Y.; Cheng, G.; Li, Y. Enrichment and purification of total flavonoids from Flos populi extracts with macroporous resins and evaluation of antioxidant activities in vitro. J. Chromatogr. B 2014, 945, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.M.; Pompeu, D.R.; Larondelle, Y.; Rogez, H. Optimisation of the adsorption of polyphenols from Inga edulis leaves on macroporous resins using an experimental design methodology. Sep. Purif. Technol. 2007, 53, 274–280. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, J.; Chen, X.; Liu, X.; Li, J.; Zhang, W. Separation of tungsten and molybdenum using macroporous resin: Equilibrium adsorption for single and binary systems. Hydrometallurgy 2013, 140, 120–127. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Li, Y.; Chi, R. Optimization of ionic liquid-based microwave-assisted extraction of isoflavones from Radix puerariae by response surface methodology. Sep. Purif. Technol. 2014, 129, 71–79. [Google Scholar] [CrossRef]
- Morelli, L.L.L.; Prado, M.A. Extraction optimization for antioxidant phenolic compounds in red grape jam using ultrasound with a response surface methodolog. Ultrason. Sonochem. 2012, 19, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xu, W.; Zhang, W.; Hu, Q.; Zeng, X. Optimizing the extraction of phenolic antioxidants from kudingcha made from Ilex kudingcha C.J. Tseng by using response surface methodology. Sep. Purif. Technol. 2011, 78, 311–320. [Google Scholar] [CrossRef]
- Kim, H.K.; Do, J.R.; Lim, T.S.; Akram, K.; Yoon, S.R.; Kwon, J.H. Optimisation of microwave-assisted extraction for functional properties of Vitis coignetiae extract by response surface methodology. J. Sci. Food Agric. 2012, 92, 1780–1785. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, D. A parametric study of supercritical carbon dioxide extraction of oil from Moringa oleifera seeds using a response surface methodology. Sep. Purif. Technol. 2013, 113, 9–17. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Chen, X.; Liu, Y.; Di, D. Preparative separation and purification of lycopene from tomato skins extracts by macroporous adsorption resins. Food Chem. 2010, 123, 1027–1034. [Google Scholar] [CrossRef]
- He, Z.; Xia, W. Preparative separation and purification of phenolic compounds from Canarium album L. by macroporous resins. J. Sci. Food. Agric. 2008, 88, 493–498. [Google Scholar] [CrossRef]
- Nath, A.; Chattopadhyay, P.K. Optimization of oven toasting for improving crispness and other quality attributes of ready to eat potatosoy snack using response surface methodology. J. Food Eng. 2007, 80, 1282–1292. [Google Scholar] [CrossRef]
- Rezaei, P.F.; Fouladdel, S.; Hassani, S.; Yousefbeyk, F.; Ghaffari, S.M.; Amin, G.; Azizi, E. Induction of apoptosis and cell cycle arrest by pericarp polyphenol-rich extract of Baneh in human colon carcinoma HT29 cells. Food Chem. Toxicol. 2012, 50, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Nigam, N.; George, J.; Srivastava, S.; Shukla, Y. Induction of apoptosis by tea polyphenols mediated through mitochondrial cell death pathway in mouse skin tumors. Cancer Biol. Ther. 2009, 8, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhang, Y.; Feng, Y.; Zhang, L.; Li, J.; Xie, Y.; Luo, X. Epigallocatechin-3-gallate inhibits cell growth, induces apoptosis and causes S phase arrest in hepatocellular carcinoma by suppressing the AKT pathway. Int. J. Oncol. 2014, 44, 791–796. [Google Scholar] [PubMed]
- Fan, Z.L.; Wang, Z.Y.; Liu, J.R. Cold-field extracts exert different antioxidant and antiproliferative activities in vitro. Food Chem. 2011, 129, 402–407. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Regueiro, J.; Martínez-Huélamo, M.; Alvarenga, J.F.R.; Leal, L.N.; Lamuela-Raventos, R.M. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014, 154, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Paepe, D.D.; Servaes, K.; Noten, B.; Diels, L.; Loose, M.D.; Droogenbroeck, B.V.; Voorspoels, S. An improved mass spectrometric method for identification and quantification of phenolic compounds in apple fruits. Food Chem. 2013, 136, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.; Halabi, E.A.; Karar, M.G.E.; Kuhnert, N. Identification and characterisation of the phenolics of Ilex glabra L. Gray (Aquifoliaceae) leaves by liquid chromatography tandem mass spectrometry. Phytochemistry 2014, 106, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Mahfoudhia, A.; Prencipe, F.P.; Mighri, Z.; Pellati, F. Metabolite profiling of polyphenols in the Tunisian plant Tamarixaphylla (L.) Karst. J. Pharm. Biomed. 2014, 99, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, G.M.; Al Sammarrae, K.W.; Ad’hiah, A.H.; Zucchetti, M.; Frapolli, R.; Bello, Z.; Erba, E.; D’Incalci, M.; Bagnati, R. Chemical characterization of Iraqi propolis samples and assessing their antioxidant potentials. Food Chem. Toxicol. 2011, 49, 2415–2421. [Google Scholar] [CrossRef] [PubMed]
- Hilbert, G.; Temsamani, H.; Bordenave, L.; Pedrot, E.; Chaher, N.; Cluzet, S.; Delaunay, J.C.; Ollat, N.; Delrot, S.; Mérillon, J.M.; et al. Flavonol profiles in berries of wild Vitis accessions using liquid chromatography coupled to mass spectrometry and nuclear magnetic resonance spectrometry. Food Chem. 2015, 169, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Regalado, E.L.; Tolle, S.; Pinoc, J.A.; Winterhalter, P.; Menendez, R.; Moralesd, A.R.; Rodríguez, J.L. Isolation and identification of phenolic compounds from rum aged in oak barrels by high-speed countercurrent chromatography/high-performance liquid chromatography-diode array detection-electrospray ionization mass spectrometry and screening for antioxidant activity. J. Chromatogr. A 2011, 1218, 7358–7364. [Google Scholar] [PubMed]
- Jaiswal, R.; Müller, H.; Müller, A.; Karar, M.G.E.; Kuhnert, N. Identification and characterization of chlorogenic acids, chlorogenic acid glycosides and flavonoids from Lonicera henryi L. (Caprifoliaceae) leaves by LC-MSn. Phytochemistry 2014, 108, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Borrás-Linares, I.; Stojanović, Z.; Quirantes-Piné, R.; Arráez-Román, D.; Švarc-Gajić, J.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Rosmarinus Officinalis Leaves as a Natural Source of Bioactive Compounds. Int. J. Mol. Sci. 2014, 15, 20585–20606. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.F.; Li, S.; Li, S.J.; Song, G.H.; Yu, L.J.; Zhang, H. Extraction optimiza tion approach to improveaccessibility of functional fraction based on combination of total polyphenol, chroma tographic profiling and antioxidant activity evaluation: Pyracantha fortuneana fruit as an example. J. Funct. Foods 2013, 5, 715–728. [Google Scholar] [CrossRef]
- Iswaldi, I.; Arráez-Román, D.; Gómez-Caravaca, A.M.; Contreras, M.M.; Uberos, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Identification of polyphenols and their metabolites in human urine after cranberry-syrup consumption. Food Chem. Toxicol. 2013, 55, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Mari, A.; Tedesco, I.; Nappo, A.; Russo, G.L.; Malorni, A.; Carbone, V. Phenolic compound characterisation and antiproliferative activity of ‘‘Annurca” apple, a southern Italian cultivar. Food Chem. 2010, 123, 157–164. [Google Scholar] [CrossRef]
- Roseiroa, L.B.; Duartea, L.C; Oliveira, D.L.; Roque, R.; Bernardo-Gil, M.G.; Martins, A.I.; Sepúlveda, C.; Almeida, J.; Meireles, M.; Gírioa, F.M.; et al. Supercritical, ultrasound and conventional extracts from carob (Ceratonia siliqua L.) biomass: Effect on the phenolic profile and antiproliferative activity. Ind. Crop. Prod. 2013, 47, 132–138. [Google Scholar] [CrossRef]
- Corsi, L.; Avallone, R.; Cosenza, F.; Farina, F.; Baraldi, C.; Baraldi, M. Antiproliferative effects of Ceratonia siliqua L. on mouse hepatocellular carcinoma cell line. Fitoterapia 2002, 73, 674–684. [Google Scholar] [CrossRef]
- Bishayee, A.; Haznagy-Radnai, E.; Mbimba, T.; Sipos, P.; Morazzoni, P.; Darvesh, A.S.; Bhatia, D.; Hohmann, J. Anthocyanin-rich black currant extract suppresses the growth of human hepatocellular carcinoma cells. Nat. Prod. Commun. 2010, 5, 1613–1618. [Google Scholar] [PubMed]
- Wu, L.C.; Hsu, H.W.; Chen, Y.C.; Chiu, C.C.; Lin, Y.I.; Ho, J.A.A. Antioxidant and antiproliferative activities of red pitaya. Food Chem. 2006, 95, 319–327. [Google Scholar] [CrossRef]
- Serra, A.T.; Matias, A.A.; Frade, R.F.M.; Duarte, R.O.; Feliciano, R.P.; Bronze, M.R.; Figueira, M.E.; de Carvalho, A.; Duarte, C.M.M. Characterization of traditional and exotic apple varieties from Portugal. Part 2-Antioxidant and antiproliferative activities. J. Funct. Foods 2010, 2, 46–53. [Google Scholar] [CrossRef]
- Fattahi, S.; Zabihi, E.; Abedian, Z.; Pourbagher, R.; Motevalizadeh, A.A.; Mostafazadeh, A.; Akhavan-Niaki, H. Total Phenolic and Flavonoid Contents of Aqueous Extract of Stinging Nettle and In Vitro Antiproliferative Effect on Hela and BT-474 Cell Lines. Int. J. Mol. Cell. Med. 2014, 3, 102–107. [Google Scholar] [PubMed]
- Ghali, W.; Vaudry, D.; Jouenne, T.; Marzouki, M.N. Assessment of cyto-protective, antiproliferative and antioxidant potential of a medicinal plant Jatropha podagrica. Ind. Crop. Prod. 2013, 44, 111–118. [Google Scholar] [CrossRef]
- Faezizadeh, Z.; Mesbah-Namin, S.A.R.; Allameh, A. The effect of silymarin on telomerase activity in the human leukemia cell line K562. Planta Med. 2012, 78, 899–902. [Google Scholar] [CrossRef] [PubMed]
- Samples Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, J.; Wang, Z.; Bai, H.; Yu, X.; Jing, J.; Zuo, L. Optimization of Purification, Identification and Evaluation of the in Vitro Antitumor Activity of Polyphenols from Pinus Koraiensis Pinecones. Molecules 2015, 20, 10450-10467. https://doi.org/10.3390/molecules200610450
Yi J, Wang Z, Bai H, Yu X, Jing J, Zuo L. Optimization of Purification, Identification and Evaluation of the in Vitro Antitumor Activity of Polyphenols from Pinus Koraiensis Pinecones. Molecules. 2015; 20(6):10450-10467. https://doi.org/10.3390/molecules200610450
Chicago/Turabian StyleYi, Juanjuan, Zhenyu Wang, Haina Bai, Xiaojin Yu, Jing Jing, and Lili Zuo. 2015. "Optimization of Purification, Identification and Evaluation of the in Vitro Antitumor Activity of Polyphenols from Pinus Koraiensis Pinecones" Molecules 20, no. 6: 10450-10467. https://doi.org/10.3390/molecules200610450
APA StyleYi, J., Wang, Z., Bai, H., Yu, X., Jing, J., & Zuo, L. (2015). Optimization of Purification, Identification and Evaluation of the in Vitro Antitumor Activity of Polyphenols from Pinus Koraiensis Pinecones. Molecules, 20(6), 10450-10467. https://doi.org/10.3390/molecules200610450





