Isolation of an Angiotensin I-Converting Enzyme Inhibitory Protein with Antihypertensive Effect in Spontaneously Hypertensive Rats from the Edible Wild Mushroom Leucopaxillus tricolor
Abstract
:1. Introduction
2. Results and Discussion
2.1. ACE Inhibitory Activities of Fruit Body Extracts of Different Mushroom Species
| Mushroom Species | ACE Inhibitory Activity (%) of Water Extract |
|---|---|
| Leucopaxillus tricolor | 95.0% ± 0.4 |
| Grifola frondosa | 77.2% ± 0.5 |
| Boletus bicolor | 61.3% ± 0.1 |
| Tuber micheli | 56.5% ± 0.1 |
| Russula aeruginea | 53.1% ± 0.7 |
| Boletus edulis | 47.2% ± 0.2 |
| Morchella vulgaris | 43.3% ± 0.2 |
| Ramaria botrytoides | 37.8% ± 0.0 |
| Oudemansiella radicata | 30.8% ± 0.1 |
| Gloeostereum incarnatum | 29.2% ± 0.9 |
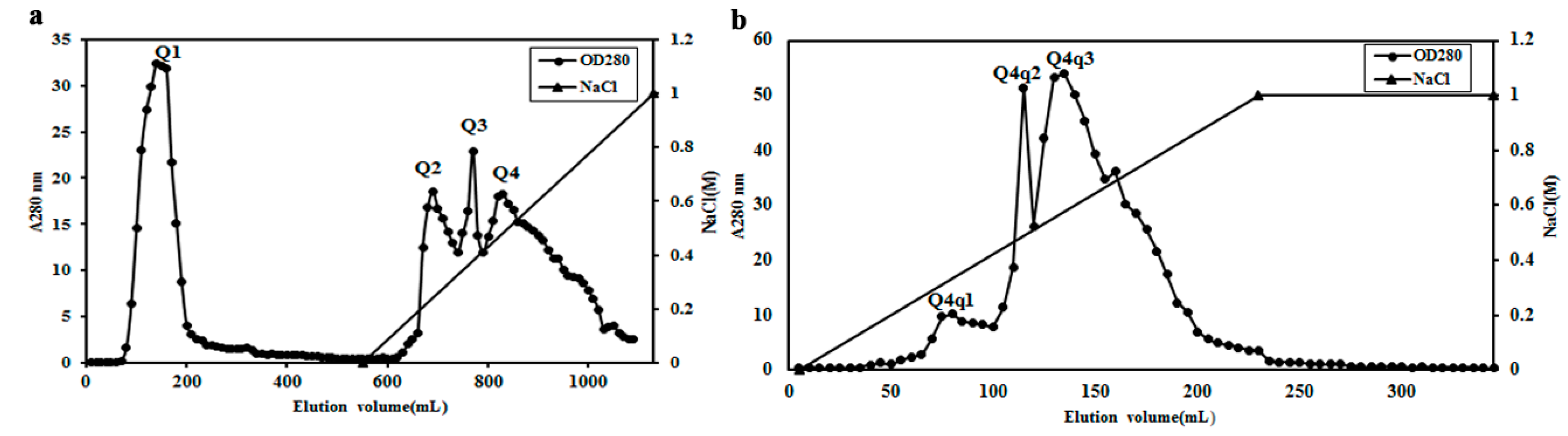
2.2. Purification of Potential ACE Inhibitor

| Mushroom Species | Peptide/Protein | Mode of Inhibition of ACE | Mol Weight (Da) | IC50 |
|---|---|---|---|---|
| Leucopaxillus tricolor | (N-terminal) DGPTMHRQAVADFKQ | competitive | 86,000 | 1.64 mg∙mL−1 |
| Agaricus bisporus [12] | AHEPVK RIGLF PSSNK | competitive competitive non-competitive | 605.3 679.5 532.3 | 63 μM 116 μM 129 μM |
| Tricholoma giganteum [16] | GQP | competitive | 301.1 | 0.04 mg∙mL−1 |
| P.cystidiosus [22] | AHEPVK GPSMR | competitive | 679.5 546.3 | 62.8 μM 277.5 μM |
| Hypsizygus marmoreus [23] | LSMGSASLSP | non-competitive | 567.3 | 0.19 mg∙mL−1 |
| G. lucidum [20] | Cystathionine beta synthase-like protein, | ND | 49,000 | <0.2 mg∙mL−1 |
| DEAD/DEAH box helicase-like protein, | 43,700 | |||
| Paxillin-like protein, | 46,800 | |||
| Alpha/beta hydrolase-like protein | 37,100 | |||
| P.cornucopiae [24] | RLPSEFDLSAFLRA RLSGQTIEVTSEYLFRH | competitive non-competitive | 1622.8 2037.2 | 0.46 mg∙mL−1 1.14 mg∙mL−1 |
| Pholiota adiposa [25] | GQGGP | ND | 414.0 | 0.044 mg∙mL−1 |
| Grifola frondosa [14] | VIQKYP | competitive | ND | 0.097 mg∙mL−1 |
2.3. Identification of the ACE Inhibitor
| Identified Peptide | Percentage of Hydrophobic Amino Acids (%) | Mass | m/z | Expected Value |
|---|---|---|---|---|
| VLITTDLLAR | 60.0 | 1113.6732 | 557.8439 | 0.047 |
| LAVNMVPFPR | 80.0 | 1142.624 | 572.3193 | 0.06 |
| ALLFGISGLR | 60.0 | 1045.6632 | 523.8389 | 0.13 |
| VAPEEHPVLLTEAPLNPK | 61.1 | 1953.0543 | 652.0254 | 5.6 |
| AVGKVLPALAGK | 66.7 | 1122.6681 | 562.3413 | 7.7 |
| FELTGIPPAPPR | 66.7 | 1293.6608 | 432.2275 | 22 |
| AAGGVAALLK | 70.0 | 869.5336 | 435.7741 | 24 |
2.4. Mode of Inhibition of ACE Inhibitor

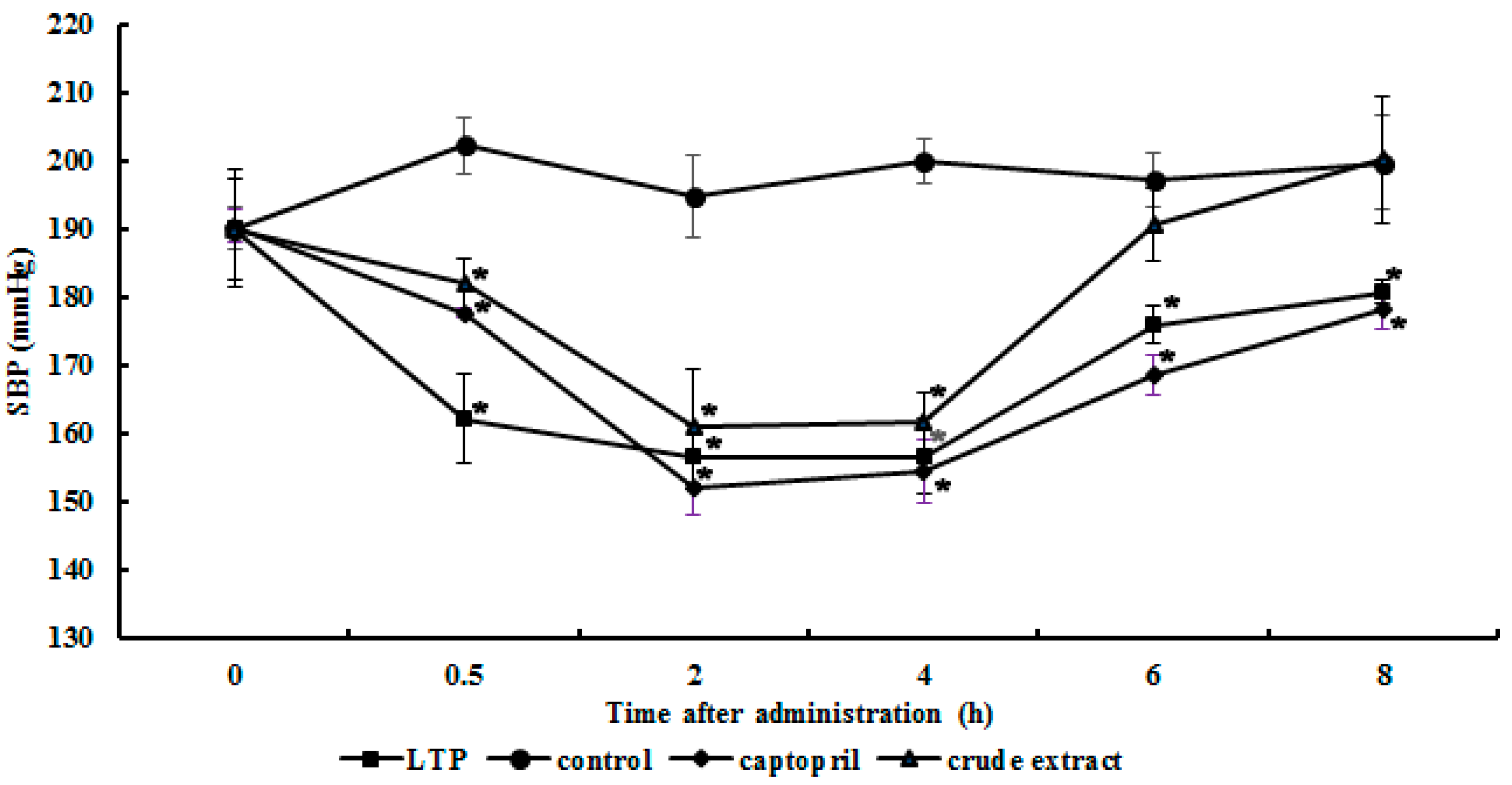
2.5. Antihypertensive Action of the Purified ACE Inhibitor
3. Experimental Section
3.1. Materials
3.2. Purification of ACE Inhibitor
3.3. Assay of ACE Inhibitory Activity
3.4. Molecular Mass Determination and Amino Acid Sequence Analysis of the Isolated ACE Inhibitor
3.5. Determination of Mode of Inhibition of ACE
3.6. Antihypertensive Action in Spontaneously Hypertensive Rats (SHRs)
Acknowledgements
Author Contributions
Conflicts of Interest
References
- WHO. Mortality and Global Health Estimates: Causes of Death‒Ten Leading Causes of Death. Available online: http://apps.who.int/gho/data/view.wrapper.MGHEMORTCAUSE10-2012?lang=en&menu=hide (accessed on 29 July 2014).
- WHO. A Global Brief on Hypertension. Available online: http://www.who.int/ cardiovascular_diseases/publications/global_brief_hypertension/en/ (accessed on 29 July 2014).
- Lafarga, T.; Hayes, M. Bioactive peptides from meat muscle and by-products: Generation, functionality and application as functional ingredients. Meat. Sci. 2014, 98, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Shearer, F.; Lang, C.C.; Struthers, A.D. Renin-angiotensin-aldosterone system inhibitors in heart failure. Clin. Pharmacol. Ther. 2013, 94, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Quist, E.E.; Phillips, R.D.; Saalia, F.K. Angiotensin converting enzyme inhibitory activity of proteolytic digests of peanut (Arachis hypogaea L.) flour. Lwt-Food Sci. Technol. 2009, 42, 694–699. [Google Scholar] [CrossRef]
- Brown, N.J.; Vaughan, D.E. Angiotensin-converting enzyme inhibitors. Circulation 1998, 97, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.H.; Bartelt, D.C.; Greene, L.J. Isolation of bradykinin-potentiating peptides from Bothrops jararaca venom. Biochemistry 1970, 9, 2583–2593. [Google Scholar] [CrossRef] [PubMed]
- Vercruysse, L.; Van Camp, J.; Smagghe, G. ACE inhibitory peptides derived from enzymatic hydrolysates of animal muscle protein: A review. J. Agric. Food. Chem. 2005, 53, 8106–8115. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.F.; Harrington, R.A. Comparative effectiveness of angiotensin-converting-enzyme inhibitors: Is an ACE always an ace? Can. Med. Assoc. J. 2008, 178, 1316–1319. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Davidge, S.T.; Wu, J. Bioactive natural constituents from food sources-potential use in hypertension prevention and treatment. Crit. Rev. Food Sci. Nutr. 2013, 53, 615–630. [Google Scholar] [CrossRef] [PubMed]
- Ebigwai, J.K.; Edu, E.A.; Itam, E.H.; Mofunanya, A.J. Activity of crude cold-water extract of the culinary-medicinal oyster mushroom, Pleurotus ostreatus (Jacq.: Fr.) P. Kumm. (higher Basidiomycetes), and timolol maleate on induced ocular hypertension. Int. J. Med. Mushrooms 2012, 14, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.C.; Abdullah, N.; Shuib, A.S.; Aminudin, N. Novel angiotensin I-converting enzyme inhibitory peptides derived from edible mushroom Agaricus bisporus (J.E. Lange) Imbach identified by LC-MS/MS. Food Chem. 2014, 148, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Hai Bang, T.; Suhara, H.; Doi, K.; Ishikawa, H.; Fukami, K.; Parajuli, G.P.; Katakura, Y.; Yamashita, S.; Watanabe, K.; Adhikari, M.K.; et al. Wild mushrooms in nepal: Some potential candidates as antioxidant and ACE-inhibition sources. Evid. Based Complement. Alternat. Med. 2014, 2014. [Google Scholar] [CrossRef]
- Choi, H.S.; Cho, H.Y.; Yang, H.C.; Ra, K.S.; Suh, H.J. Angiotensin I-converting enzyme inhibitor from Grifola frondosa. Food Res. Int. 2001, 34, 177–182. [Google Scholar] [CrossRef]
- Lau, C.C.; Abdullah, N.; Shuib, A.S.; Aminudin, N. Proteomic analysis of antihypertensive proteins in edible mushrooms. J. Agric. Food. Chem. 2012, 60, 12341–12348. [Google Scholar] [CrossRef] [PubMed]
- Hyoung Lee, D.; Ho Kim, J.; Sik Park, J.; Jun Choi, Y.; Soo Lee, J. Isolation and characterization of a novel angiotensin I-converting enzyme inhibitory peptide derived from the edible mushroom Tricholoma giganteum. Peptides 2004, 25, 621–627. [Google Scholar]
- Hsu, F.L.; Lin, Y.H.; Lee, M.H.; Lin, C.L.; Hou, W.C. Both dioscorin, the tuber storage protein of yam (Dioscorea alata cv. Tainong No. 1), and its peptic hydrolysates exhibited angiotensin converting enzyme inhibitory activities. J. Agric. Food. Chem. 2002, 50, 6109–6113. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.J.; Chen, H.J.; Susumu, K.; Wu, J.B.; Hou, W.C.; Wu, C.H.; Sheu, M.J.; Huang, S.S.; Lin, Y.H. Sweet potato storage root thioredoxin h2 and their peptic hydrolysates exhibited angiotensin converting enzyme inhibitory activity in vitro. Bot. Stud. 2011, 52, 15–22. [Google Scholar]
- Huang, G.J.; Ho, Y.L.; Chen, H.J.; Chang, Y.S.; Huang, S.S.; Hung, H.J.; Lin, Y.H. Sweet potato storage root trypsin inhibitor and their peptic hydrolysates exhibited angiotensin converting enzyme inhibitory activity in vitro. Bot. Stud. 2008, 49, 101–108. [Google Scholar]
- Mohamad Ansor, N.; Abdullah, N.; Aminudin, N. Anti-angiotensin converting enzyme (ACE) proteins from mycelia of Ganoderma lucidum (Curtis) P. Karst. BMC Complement. Altern. Med. 2013, 13. [Google Scholar] [CrossRef]
- Fagyas, M.; Uri, K.; Siket, I.M.; Darago, A.; Boczan, J.; Banyai, E.; Edes, I.; Papp, Z.; Toth, A. New perspectives in the renin-angiotensin-aldosterone system (RAAS) I: Endogenous angiotensin converting enzyme (ACE) inhibition. PLoS ONE 2014, 9, e87843. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.C.; Abdullah, N.; Shuib, A.S. Novel angiotensin I-converting enzyme inhibitory peptides derived from an edible mushroom, Pleurotus cystidiosus O.K. Miller identified by LC-MS/MS. BMC Complement. Altern. Med. 2013, 13. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.G.; Kim, Y.H.; Bolormaa, Z.; Kim, M.K.; Seo, G.S.; Lee, J.S. Characterization of an antihypertensive angiotensin I-converting enzyme inhibitory peptide from the edible mushroom Hypsizygus marmoreus. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Jeong, S.C.; Kim, J.H.; Lee, Y.H.; Ju, Y.C.; Lee, J.S. Characterisation of a new antihypertensive angiotensin I-converting enzyme inhibitory peptide from Pleurotus cornucopiae. Food Chem. 2011, 127, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Koo, K.C.; Lee, D.H.; Kim, J.H.; Yu, H.E.; Park, J.S.; Lee, J.S. Production and characterization of antihypertensive angiotensin I-converting enzyme inhibitor from Pholiota adiposa. J. Microbiol. Biotechnol. 2006, 16, 757–763. [Google Scholar]
- Duan, X.; Wu, F.; Li, M.; Yang, N.; Wu, C.; Jin, Y.; Yang, J.; Jin, Z.; Xu, X. Naturally occurring angiotensin I-converting enzyme inhibitory peptide from a fertilized egg and its inhibitory mechanism. J. Agric. Food. Chem. 2014, 62, 5500–5506. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Du, M.; Zhang, Y.; Xu, W.; Wang, C.; Wang, K.; Zhang, L. Purification and identification of an ACE inhibitory peptide from walnut protein. J. Agric. Food. Chem. 2013, 61, 4097–4100. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, T. Sample preparation and reversed phase-high performance liquid chromatography analysis of food-derived peptides. Anal. Chim. Acta 1997, 352, 119–139. [Google Scholar] [CrossRef]
- Puchalska, P.; Alegre, M.L.; Lopez, M.C. Isolation and characterization of peptides with antihypertensive activity in foodstuffs. Crit. Rev. Food Sci. Nutr. 2013, 55, 521–551. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Aluko, R.E.; Nakai, S. Structural requirements of angiotensin I-converting enzyme inhibitory peptides: Quantitative structure-activity relationship study of di- and tripeptides. J. Agric. Food. Chem. 2006, 54, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Sun, J.; Liu, Y.; Zeng, H.; Su, Y.; Yang, Y. ACE inhibitory peptides and antioxidant peptides derived from in vitro digestion hydrolysate of hen egg white lysozyme. Food Chem. 2012, 135, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Hai-Lun, H.; Xiu-Lan, C.; Cai-Yun, S.; Yu-Zhong, Z.; Bai-Cheng, Z. Analysis of novel angiotensin-I-converting enzyme inhibitory peptides from protease-hydrolyzed marine shrimp Acetes chinensis. J. Pept. Sci. 2006, 12, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Li, L.; Liu, G.; Hu, S.Q. Inhibition mechanism and model of an angiotensin I-converting enzyme (ACE)-inhibitory hexapeptide from yeast (Saccharomyces cerevisiae). PLoS ONE 2012, 7, e37077. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, N.; Okazaki, M.; Ohga, S. Antihypertensive effect of Pleurotus nebrodensis in spontaneously hypertensive rats. J. Oleo Sci. 2008, 57, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Hayakari, M.; Kondo, Y.; Izumi, H. A rapid and simple spectrophotometric assay of angiotensin-converting enzyme. Anal. Biochem. 1978, 84, 361–369. [Google Scholar] [CrossRef]
- Cushman, D.W.; Cheung, H.S.; Sabo, E.F.; Ondetti, M.A. Design of potent competitive inhibitors of angiotensin-converting enzyme. Carboxyalkanoyl and mercaptoalkanoyl amino acids. Biochemistry 1977, 16, 5484–5491. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K.; Favre, M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J. Mol. Biol. 1973, 80, 575–599. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, X.; Tian, G.; Zhang, W.; Zhao, Y.; Zhao, L.; Ryu, M.; Wang, H.; Ng, T.B. Isolation of an Angiotensin I-Converting Enzyme Inhibitory Protein with Antihypertensive Effect in Spontaneously Hypertensive Rats from the Edible Wild Mushroom Leucopaxillus tricolor. Molecules 2015, 20, 10141-10153. https://doi.org/10.3390/molecules200610141
Geng X, Tian G, Zhang W, Zhao Y, Zhao L, Ryu M, Wang H, Ng TB. Isolation of an Angiotensin I-Converting Enzyme Inhibitory Protein with Antihypertensive Effect in Spontaneously Hypertensive Rats from the Edible Wild Mushroom Leucopaxillus tricolor. Molecules. 2015; 20(6):10141-10153. https://doi.org/10.3390/molecules200610141
Chicago/Turabian StyleGeng, Xueran, Guoting Tian, Weiwei Zhang, Yongchang Zhao, Liyan Zhao, Mansok Ryu, Hexiang Wang, and Tzi Bun Ng. 2015. "Isolation of an Angiotensin I-Converting Enzyme Inhibitory Protein with Antihypertensive Effect in Spontaneously Hypertensive Rats from the Edible Wild Mushroom Leucopaxillus tricolor" Molecules 20, no. 6: 10141-10153. https://doi.org/10.3390/molecules200610141






