Inhibitory Effects of Verrucarin A on Tunicamycin-Induced ER Stress in FaO Rat Liver Cells
Abstract
:1. Introduction
2. Results and Discussion
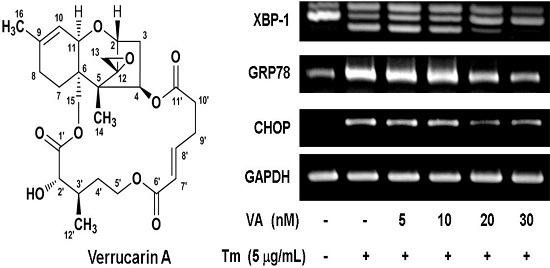
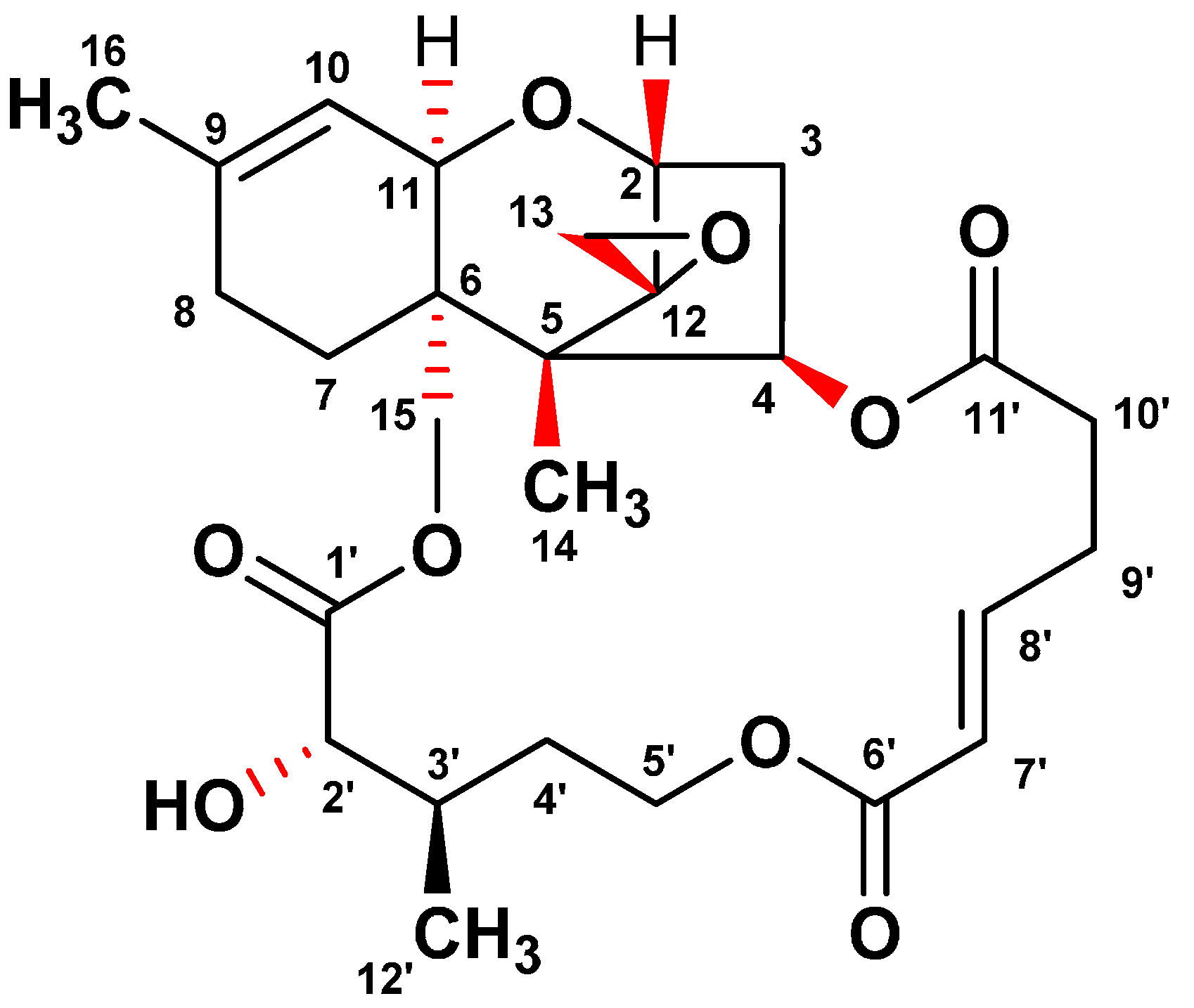
2.1. Isolation and Identification of Verrucarin A
2.2. Verrucarin A Inhibits Tunicamycin-Induced GRP78 Expression

2.3. Effect of Verrucarin A on Cell Viability

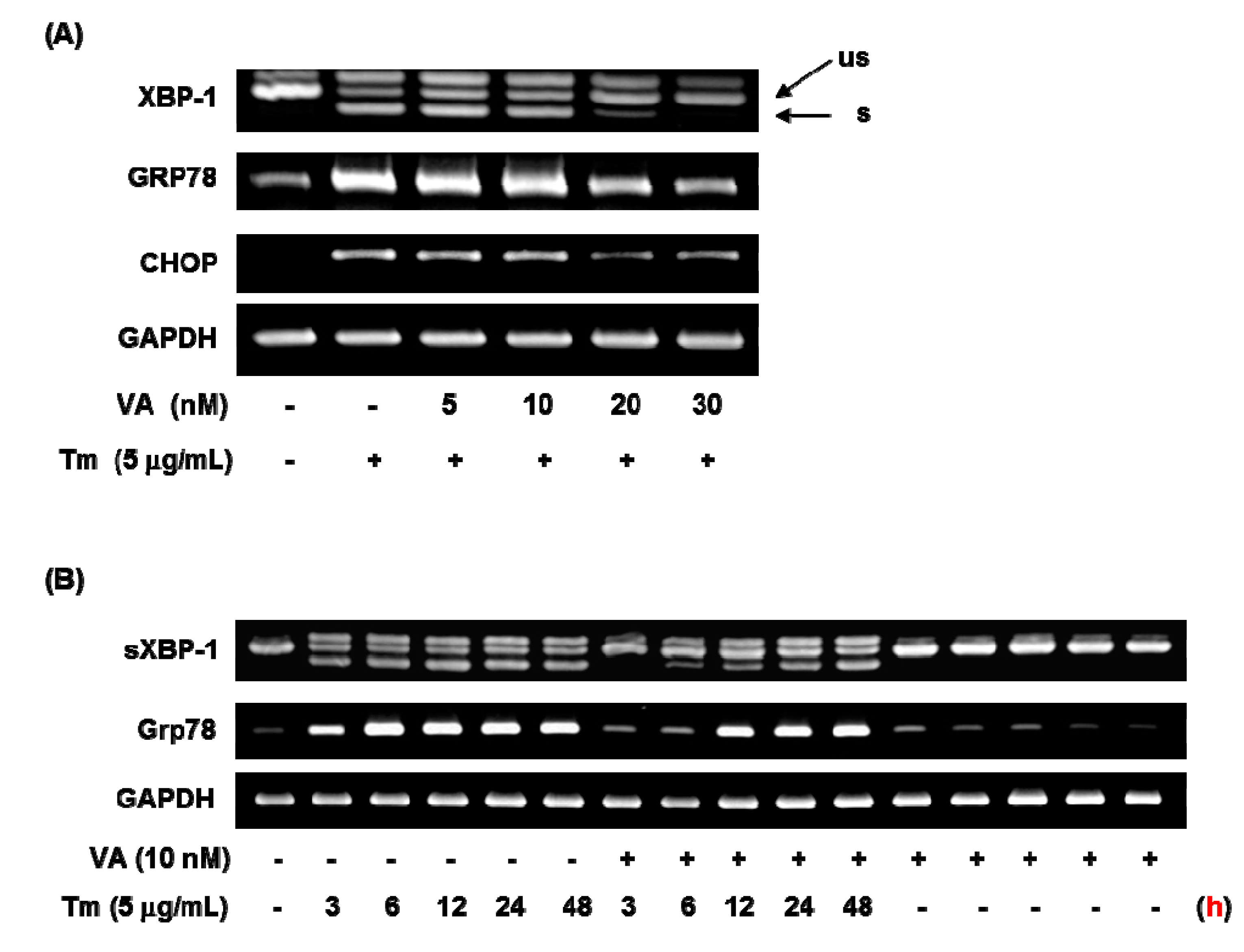
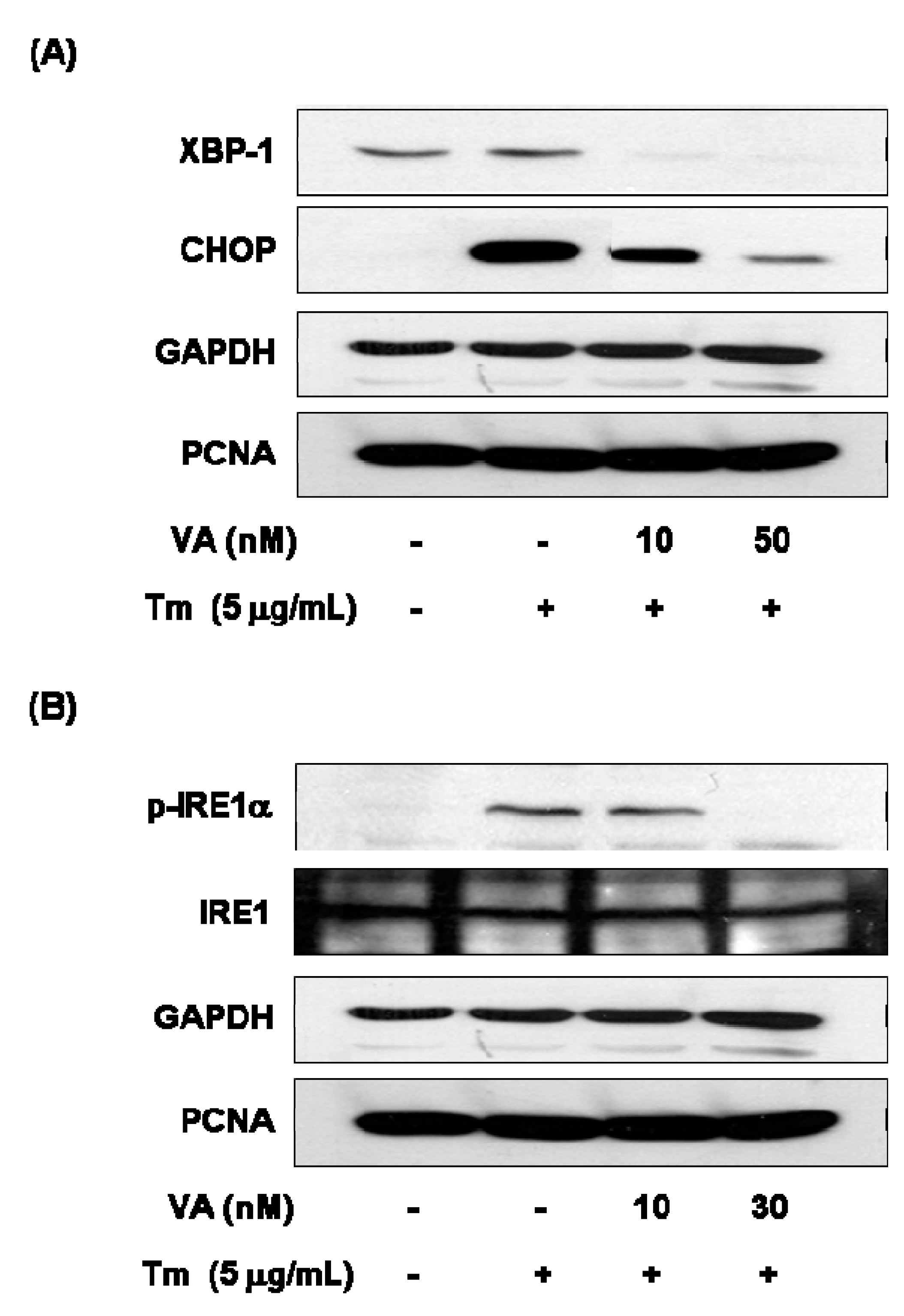
2.4. Effect of Verrucarin A on ER Stress-Related Gene Expression
3. Experimental Section
3.1. Fermentation, Extraction and Isolation of Verrucarin A
3.2. Chemicals
3.3. Cell Culture and Transfection
3.4. Cell Viability Assay
3.5. Semiquantitative RT-PCR Experiments
3.6. Plasmid
3.7. Western Blot Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ellgaard, L.; Molinari, M.; Helenius, A. Setting the standards: Quality control in the secretory pathway. Science 1999, 286, 1882–1888. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Bailly-Maitre, B.; Reed, J.C. Endoplasmic reticulum stress: Cell life and death decisions. J. Clin. Investig. 2005, 115, 2656–2664. [Google Scholar] [CrossRef] [PubMed]
- Breckenridge, D.G.; Germain, M.; Mathai, J.P.; Nguyen, M.; Shore, G.C. Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene 2003, 22, 8608–8618. [Google Scholar] [CrossRef] [PubMed]
- Moenner, M.; Pluquet, O.; Bouchecareilh, M.; Chevet, E. Integrated endoplasmic reticulum stress responses in cancer. Cancer Res. 2007, 67, 10631–10634. [Google Scholar] [CrossRef] [PubMed]
- Verfaillie, T.; Garg, A.D.; Agostinis, P. Targeting ER stress induced apoptosis and inflammation in cancer. Cancer Lett. 2012, 332, 249–264. [Google Scholar] [CrossRef]
- Calfon, M.; Zeng, H.; Urano, F.; Till, J.H.; Hubbard, S.R.; Harding, H.P.; Clark, S.G.; Ron, D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 2002, 415, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Matsui, T.; Yamamoto, A.; Okada, T.; Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 2001, 107, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Tirasophon, W.; Shen, X.; Michalak, M.; Prywes, R.; Okada, T.; Yoshida, H.; Mori, K.; Kaufman, R.J. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Gene. Dev. 2002, 16, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.H.; Iwakoshi, N.N.; Glimcher, L.H. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell. Biol. 2003, 23, 7448–7459. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Lu, J.; Zahed, M.; Kita, K.; Suzuki, N. Reduction of GRP78 expression with siRNA activates unfolded protein response leading to apoptosis in HeLa cells. Arch. Biochem. Biophys. 2007, 468, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, U.; Cao, Q.; Yilmaz, E.; Lee, A.H.; Iwakoshi, N.N.; Ozdelen, E.; Tuncman, G.; Görgün, C.; Glimcher, L.H.; Hotamisligil, G.S. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004, 306, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, B.B.; Stahly, G.P.; Pavanasasivam, G.; Mazzola, E. Antileukemic compounds derived from the chemical modification of macrocyclic trichothecenes. 1. Derivatives of verrucarin A. J. Med. Chem. 1980, 23, 1054–1058. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-Y.; Li, Z.-L.; Guan, L.-P.; Wu, X.; Pan, H.-Q.; Bai, J.; Hua, H.-M. Structure determination of two new trichothecenes from a halotolerant fungus Myrothecium sp. GS-17 by NMR spectroscopy. Magn. Reson. Chem. 2012, 50, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Katschinski, D.M.; Jacobson, E.L.; Wiedemann, G.J.; Robins, H.I. Modulation of VP-16 cytotoxicity by carboplatin and 41.8 degrees C hyperthermia. J. Cancer Res. Clin. Oncol. 2001, 127, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, E.Y.; Lee, S.W.; Seong, S.; Cho, W.; Ahn, J.S.; Cho, H.-S. Inhibitory Effects of Verrucarin A on Tunicamycin-Induced ER Stress in FaO Rat Liver Cells. Molecules 2015, 20, 8988-8996. https://doi.org/10.3390/molecules20058988
Bae EY, Lee SW, Seong S, Cho W, Ahn JS, Cho H-S. Inhibitory Effects of Verrucarin A on Tunicamycin-Induced ER Stress in FaO Rat Liver Cells. Molecules. 2015; 20(5):8988-8996. https://doi.org/10.3390/molecules20058988
Chicago/Turabian StyleBae, Eun Young, Seung Woong Lee, Sin Seong, Wonjun Cho, Jong Seog Ahn, and Hyun-Sug Cho. 2015. "Inhibitory Effects of Verrucarin A on Tunicamycin-Induced ER Stress in FaO Rat Liver Cells" Molecules 20, no. 5: 8988-8996. https://doi.org/10.3390/molecules20058988