Improving Properties of a Novel β-Galactosidase from Lactobacillus plantarum by Covalent Immobilization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Purification of the Enzyme
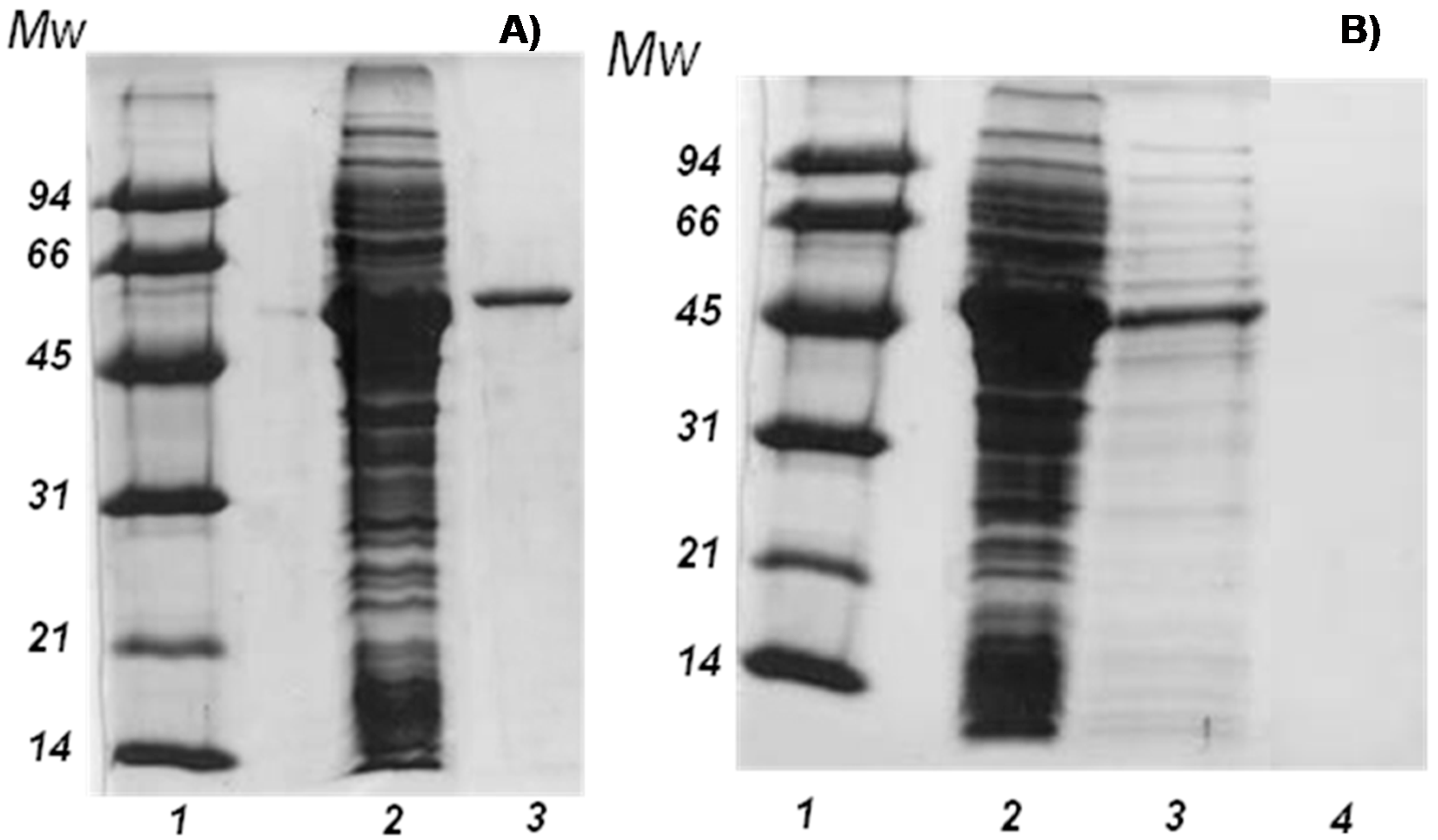
| Step | Activity (UI/mL) | Protein (mg/mL) | Recovered Activity (%) | Specific Activity (UI/mg) | Purification Factor |
|---|---|---|---|---|---|
| Crude extract | 469.7 | 11 | 100 | 42.7 | 1 |
| Ag-IDA-Ni2+ | 365.2 | 0.74 | 84 | 491.2 | 11.5 |
2.2. Biochemical Characterization of the β-Galactosidase from Lactobacillus plantarum (LPG)
| Additives | Relative Activity (%) |
|---|---|
| - | 100 |
| MnSO4 | 20 |
| CaCl2 | 110 |
| MgSO4 | 105 |
| EDTA | 99 |
| NaH2PO4 | 99 |
| KH2PO4 | 99 |
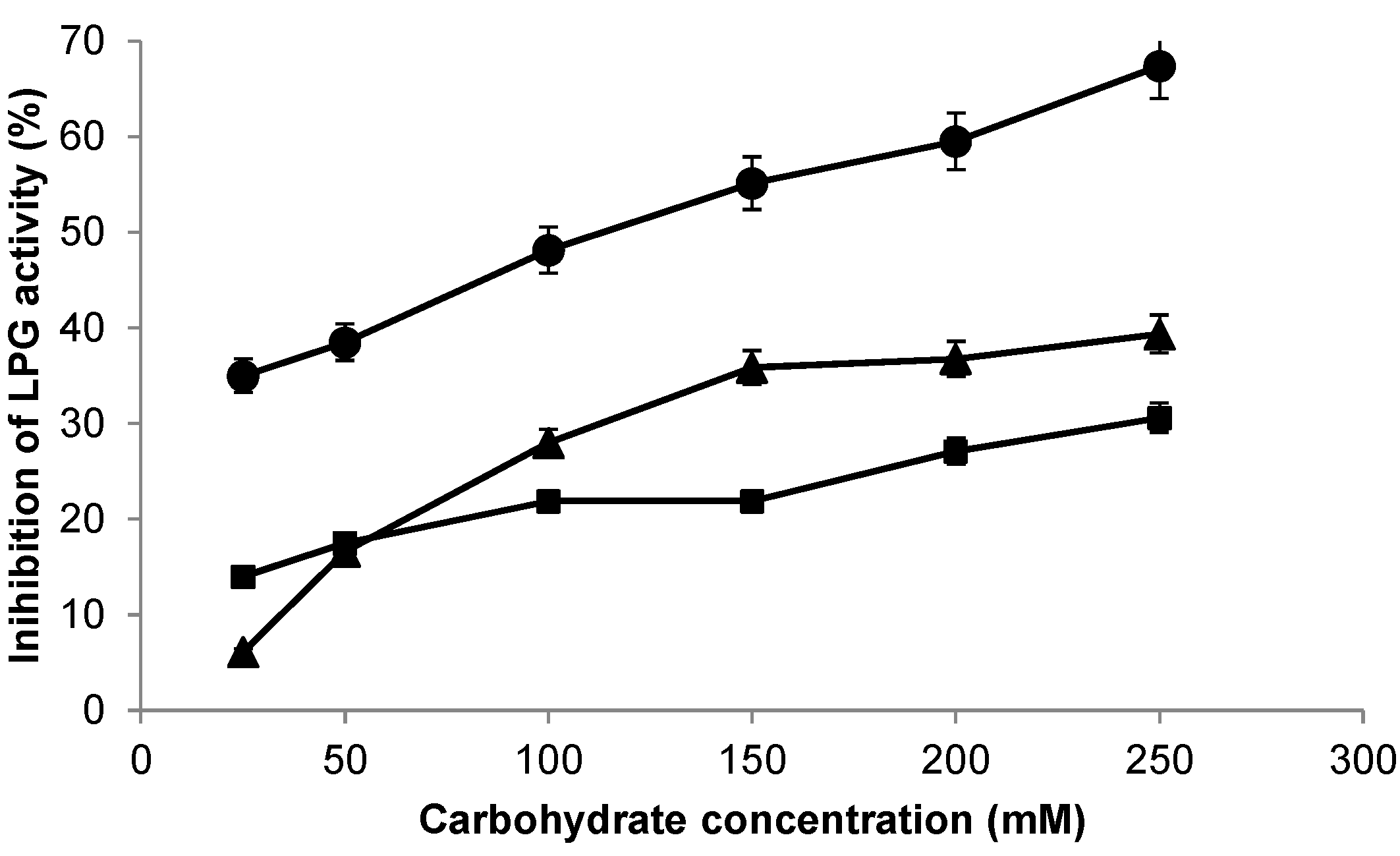
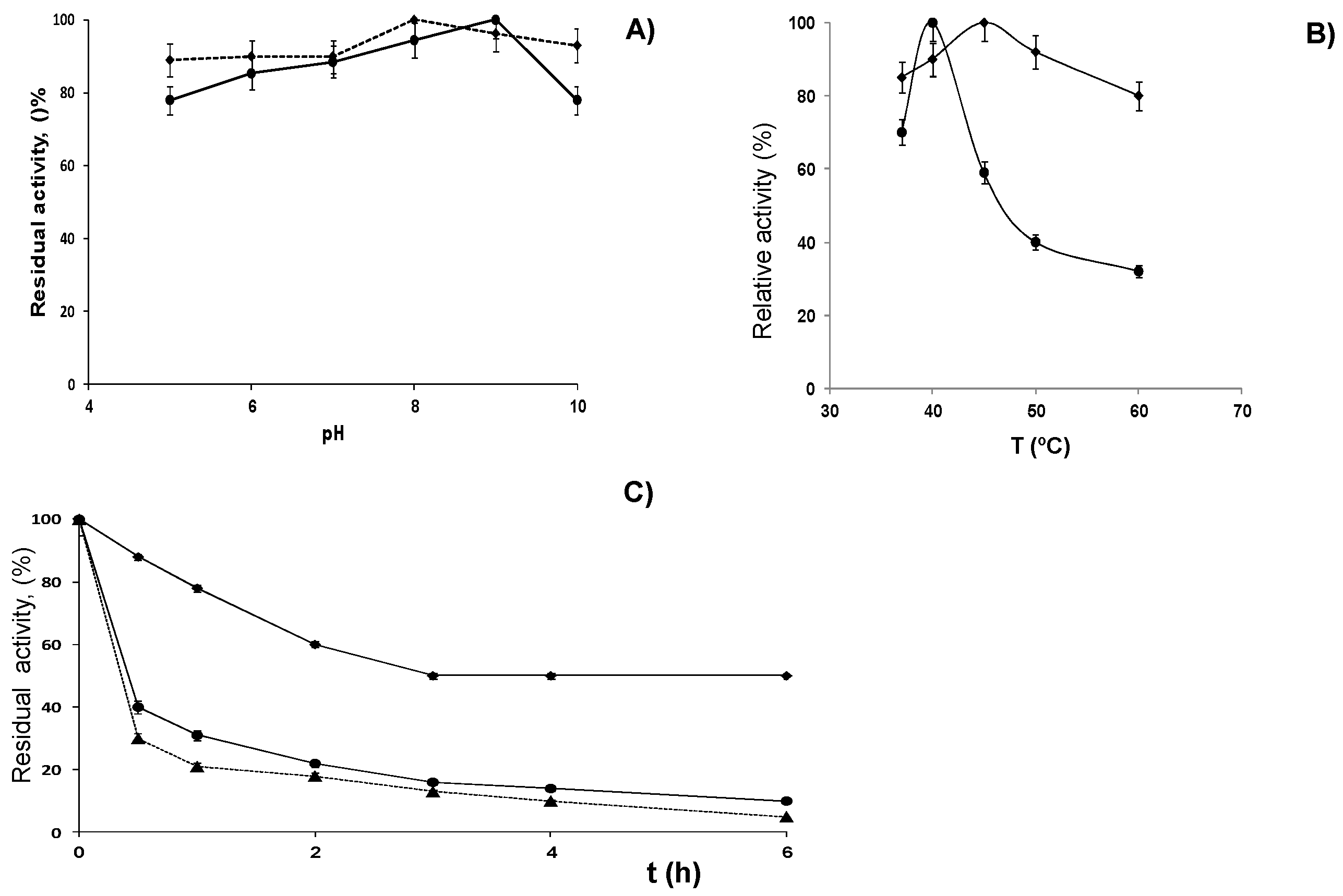
2.3. Stabilization of LPG by Covalent Immobilization
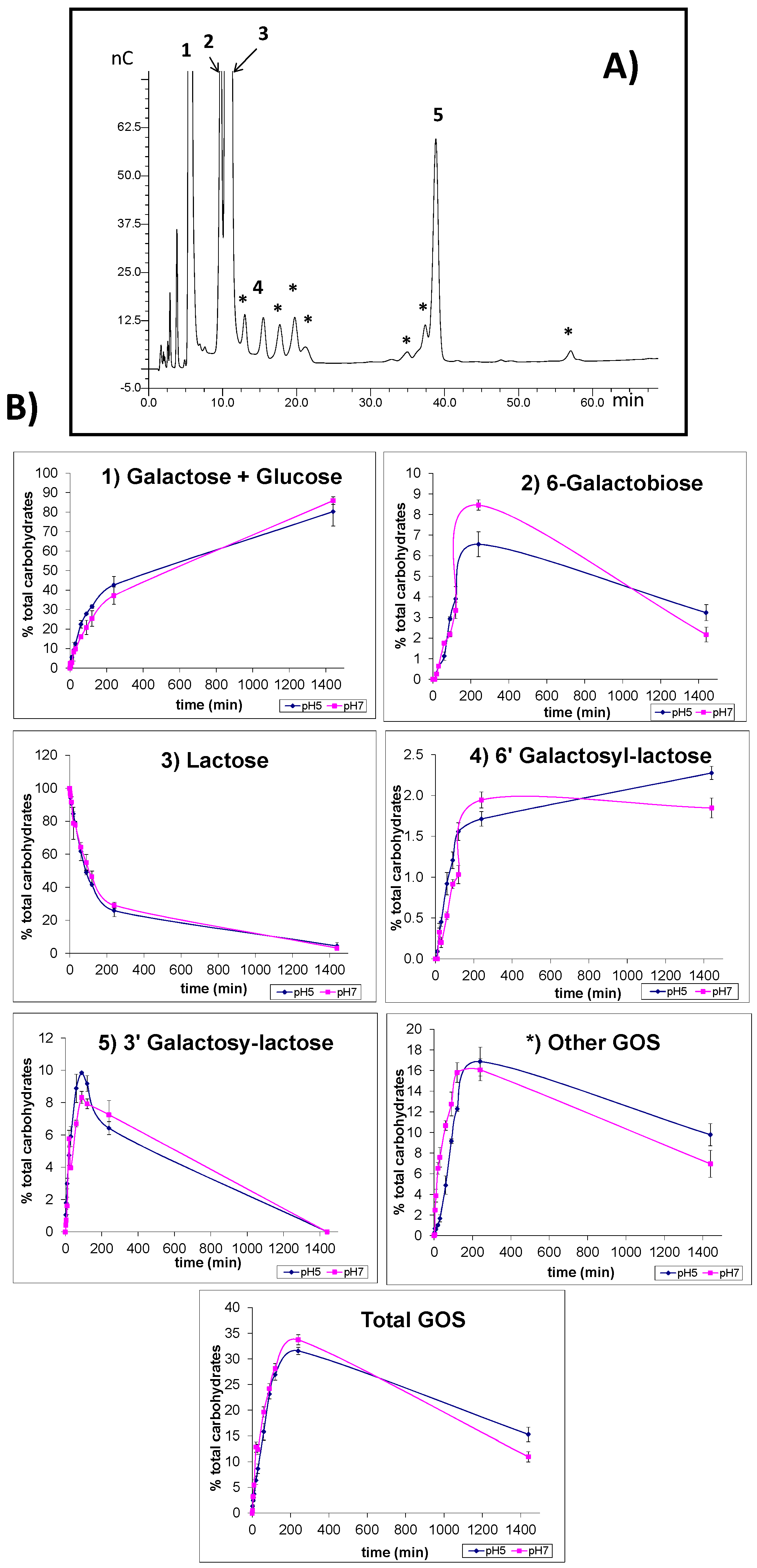
2.4. Synthesis of Oligosaccharides Derived from Lactose (GOS) and Lactulose (OsLu)
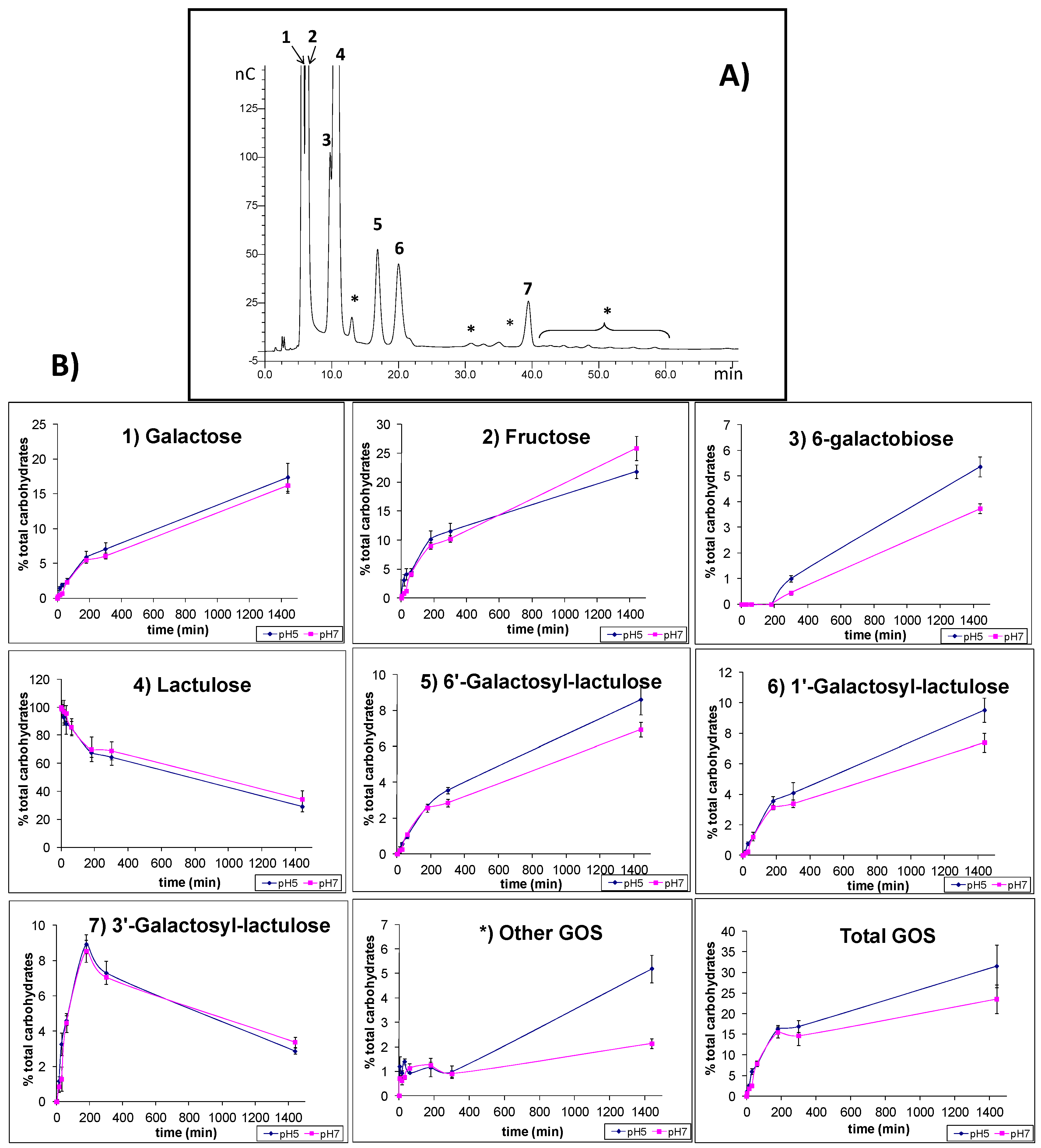
3. Experimental Section
3.1. Chemicals
3.2. Enzymatic Activity Assays
3.3. Purification of β-Galactosidase from Lactobacillus plantarum (LPG)
3.4. LPG Immobilization Process
3.4.1. Immobilization on CNBr-Activated Sepharose
3.4.2. Immobilization on Glyoxyl-Agarose
3.5. Effect of pH and Temperature on Enzyme Activity
3.6. Effect of Sugars Concentrations on LPG Kinetic Parameters
3.7. Synthesis of Oligosaccharides Derived from Lactose (GOS) and Lactulose (OsLu)
3.8. Analytical Techniques
3.8.1. SDS-PAGE Analysis of the Free and Immobilized Enzyme
3.8.2. Chromatographic Determination of Oligosaccharides Derived from Lactose (GOS) and Lactulose (OsLu)
High-Performance Anion Exchange Chromatography with Pulsed Amperometric Detection (HPAEC-PAD) Analysis
Gas Chromatographic-Mass Spectrometric (GC-MS) Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Husain, Q. Beta galactosidases and their potential applications: A review. Crit. Rev. Biotechnol. 2010, 30, 41–62. [Google Scholar] [CrossRef] [PubMed]
- Puri, M.; Gupta, S.; Pahuja, P.; Kaur, A.; Kanwar, J.R.; Kennedy, J.F. Cell disruption optimization and covalent immobilization of β-d-galactosidase from Kluyveromyces marxianus YW-1 for lactose hydrolysis in milk. Appl. Biochem. Biotechnol. 2010, 160, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.P.; Fernandez Leiro, R.; Cerdan, M.E.; Gonzalez, M.I.; Becerra-Fernandez, M. Kluyveromyces lactis β-galactosidase crystallization using full-factorial experimental design. J. Mol. Catal. B.-Enzym. 2008, 52, 178–182. [Google Scholar] [CrossRef]
- Osman, A.; Tzortzis, G.; Rastall, R.A.; Charalampopoulos, D. A comprehensive investigation of the synthesis of prebiotic galactooligosaccharides by whole cells of Bifidobacterium bifidum NCIMB 41171. J. Biotechnol. 2010, 150, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Goulas, T.; Goulas, A.; Tzortzis, G.; Gibson, G.R. Expression of four β-galactosidases from Bifidobacterium bifidum NCIMB41171 and their contribution on the hydrolysis and synthesis of galactooligosaccharides. Appl. Microbiol. Biotechnol. 2009, 84, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Nguyen, T.H.; Nguyen, T.T.; Maischberger, T.; Haltrich, D. β-Galactosidase from Lactobacillus plantarum WCFS1: Biochemical characterization and formation of prebiotic galacto-oligosaccharides. Carbohydr. Res. 2010, 345, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Bridiau, N.; Maugard, T. A comparative study of the regioselectivity of the β-galactosidases from Kluyveromyces lactis and Bacillus circulans in the enzymatic synthesis of N-acetyl-lactosamine in aqueous media. Biotechnol. Prog. 2011, 27, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Ladero, M.; Santos, A.; Garcia-Ochoa, F. Kinetic modelling of the thermal inactivation of an industrial β-galactosidase from Kluyveromyces fragilis. Enzym. Microb. Technol. 2006, 38, 1–9. [Google Scholar] [CrossRef]
- Curiel, J.A.; Betancor, L.; de las Rivas, B.; Muñoz, R.; Guisan, J.M.; Fernández-Lorente, G. Hydrolysis of tannic acid catalyzed by immobilized-stabilized derivatives of tannase from Lactobacillus platarum. J. Agric. Food Chem. 2010, 58, 6403–6409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battestin, V.; Macedo, G.A.; de Freitas, V.A.P. Hydrolysis of epigallocatechin gallate using a tannase from Paecilomyces variotii. Food Chem. 2008, 108, 228–233. [Google Scholar] [CrossRef]
- Mayo, B.; González, B.; Arca, P.; Suarez, J.E. Cloning and expression of the plasmad-encoded beta-d-galactosidase gene from a Lactobacillus plantarum strain of dairy origin. FEMS Microbiol. Lett. 1994, 122, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Schwab, C.; Sorensen, K.I.; Ganzle, M.G. Heterologous expression of glycoside hydrolase family 2 and 42 beta-galactosidases of lactic acid bacteria in Lactococcus lactis. Syst. Appl. Microbiol. 2010, 33, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Acebrón, I.; Curiel, J.A.; de las Rivas, B.; Muñoz, R.; Mancheño, J.M. Cloning, production, purification and preliminary crystallographic analysis of a glycosidase from the food lactic acid bacterium Lactobacillus plantarum CECT 748T. Protein Expr. Purif. 2009, 168, 77–182. [Google Scholar]
- Shivam, K.; Mishra, S.K. Purification and characterization of a thermostable α-galactosidase with transglycosylation activity from Aspergillus parasiticus MTCC-2796. Process. Biochem. 2010, 45, 1088–1093. [Google Scholar] [CrossRef]
- Mateo, C.; Fernández-Lorente, G.; Abian, O.; Fernández-Lafuente, R.; Guisán, J.M. Multifunctional epoxy supports: A new tool to improve the covalent immobilization of proteins. The promotion of physical adsorptions of proteins on the supports before their covalent linkage. Biomocromolecules 2000, 1, 739–745. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Armisén, P.; Mateo, C.; Cortés, E.; Barredo, J.L.; Salto, F.; Díez, B.; Rodés, L.; García, J.L.; Fernadez-Lafuente, R.; Guisan, J.M. Selective adsorption of poly-His tagged glutaryl acylase on tailor-made metal chelate supports. J. Chromatgr. A. 1999, 848, 61–70. [Google Scholar] [CrossRef]
- Mateo, C.; Monti, R.; Pessela, B.C.; Fuentes, M.; Torres, R.; Guisán., J.M.; Fernández-Lafuente, R. Immobilization of Lactase from Kluyveromyces lactis Greatly Reduces the Inhibition Promoted by Glucose. Full Hydrolysis of Lactose in Milk. Biotechnol. Prog. 2004, 20, 1259–1262. [Google Scholar] [CrossRef] [PubMed]
- Pessela, B.C.; Mateo, C.; Filho, M.; Carrascosa, A.V.; Fernandez-Lafuente, R.; Guisán, J.M. Stabilization of the quaternary structure of a hexameric alpha-galactosidase from Thermus sp. T2 by immobilization and post-immobilization techniques. Process. Biochem. 2008, 43, 193–198. [Google Scholar] [CrossRef]
- Mateo, C.; Palo, J.M.; Fuentes, M.; Betancor, L.M.; Grazu, V.; López-Gallego, F.; Pessela, B.C.; Hidalgo, A.; Fernández-Lorente, G.; Lafuente, R.; et al. Glyoxyl agsrose: A full inert and hydrophilic support for immobilization and high stabilization o proteins. Enzym. Microb. Technol. 2006, 39, 274–280. [Google Scholar] [CrossRef]
- Splechtna, B.; Nguyen, T.H.; Steinböck, M.; Kulbe, K.D.; Lorenz, W.; Haltrich, D. Production of prebiotic galactooligosaccharides from lactose using β-galactosidase from Lactobacillus reuteri. J. Agric. Food Chem. 2006, 54, 4999–5006. [Google Scholar] [CrossRef] [PubMed]
- Cardelle-Cobas, A.; Corzo, N.; Olano, A.; Pélaez, C.; Requena, T.; Ávila, M. Galactooligosaccharides derived from lactose and lactulose: Influence of linkage and composition on Streptococcus, and Bifidobacterium strains growth. Int. J. Food Microbiol. 2011, 149, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Cardelle-Cobas, A.; Olano, A.; Corzo, N.; Villamiel, M.; Collins, M.; Kolida, S.; Rastall, R.A. In vitro fermentation of lactulose derived oligosaccharides by mixed faecal microbiota. J. Agric. Food Chem. 2012, 60, 2024–2032. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Villaluenga, C.; Cardelle-Cobas, A.; Olano, A.; Corzo, N.; Villamiel, M.; Jimeno, M.L. Enzymatic synthesis and identification of two trisaccharides produced from lactulose by transgalactosylation. J. Agric. Food Chem. 2008, 56, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Brobst, K.M.; Lott, C.E. Determination of some components in corn syrup by gas-liquid chromatography of trimethylsilyl derivatives. Cereal Chem. 1966, 43, 35–43. [Google Scholar]
- Sample Availability: Samples of the different GOS (3' and 6' galactosyl-lactose) are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benavente, R.; Pessela, B.C.; Curiel, J.A.; De las Rivas, B.; Muñoz, R.; Guisán, J.M.; Mancheño, J.M.; Cardelle-Cobas, A.; Ruiz-Matute, A.I.; Corzo, N. Improving Properties of a Novel β-Galactosidase from Lactobacillus plantarum by Covalent Immobilization. Molecules 2015, 20, 7874-7889. https://doi.org/10.3390/molecules20057874
Benavente R, Pessela BC, Curiel JA, De las Rivas B, Muñoz R, Guisán JM, Mancheño JM, Cardelle-Cobas A, Ruiz-Matute AI, Corzo N. Improving Properties of a Novel β-Galactosidase from Lactobacillus plantarum by Covalent Immobilization. Molecules. 2015; 20(5):7874-7889. https://doi.org/10.3390/molecules20057874
Chicago/Turabian StyleBenavente, Rocio, Benevides C. Pessela, Jose Antonio Curiel, Blanca De las Rivas, Rosario Muñoz, Jose Manuel Guisán, Jose M. Mancheño, Alejandra Cardelle-Cobas, Ana I. Ruiz-Matute, and Nieves Corzo. 2015. "Improving Properties of a Novel β-Galactosidase from Lactobacillus plantarum by Covalent Immobilization" Molecules 20, no. 5: 7874-7889. https://doi.org/10.3390/molecules20057874







