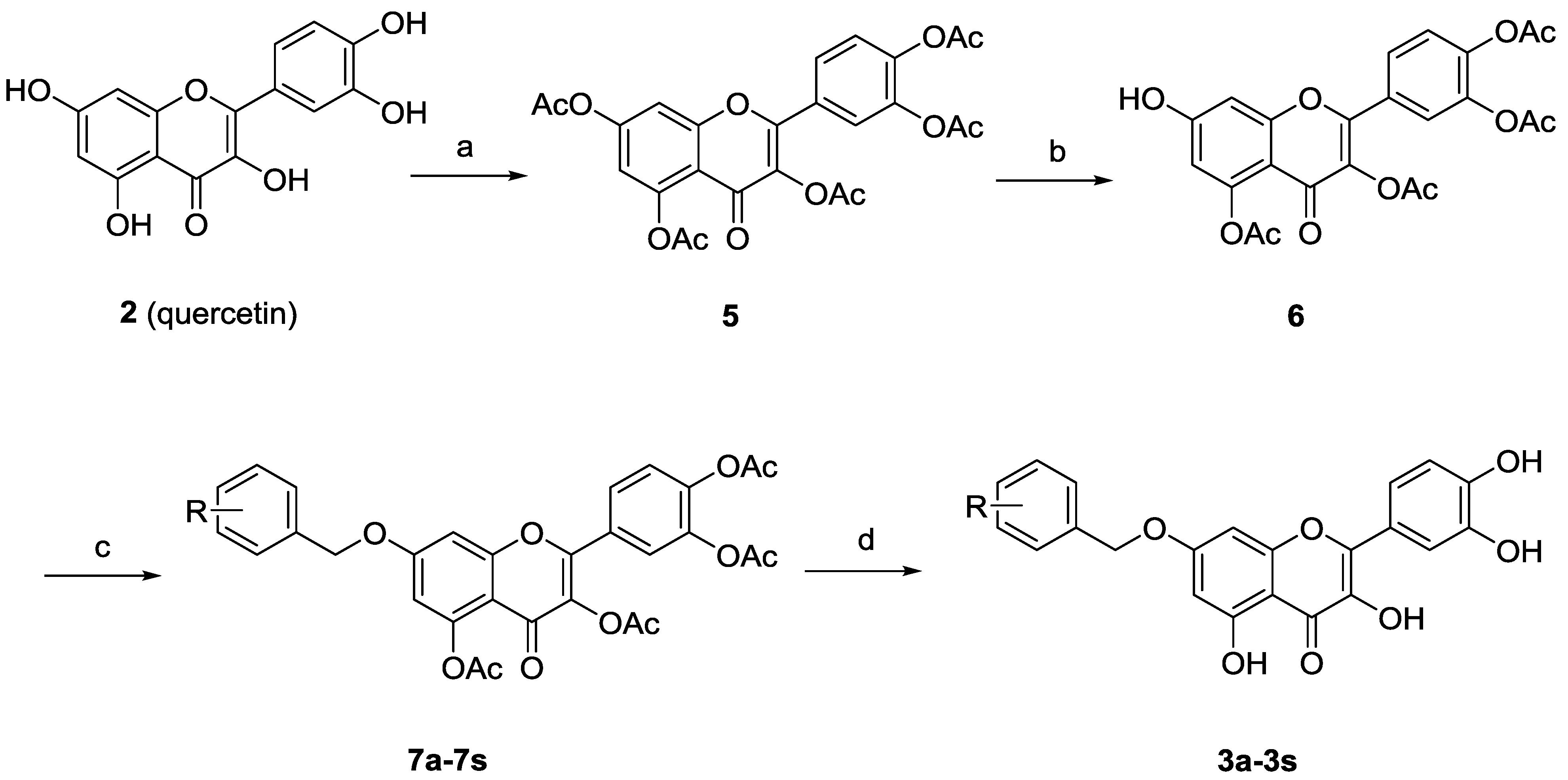
3.5. General Procedure for the Preparation of 3a–3s
The solution of crude compound 7a–7s in NH3/MeOH (10 mL) was stirred for 1 h at 0 °C and concentrated under reduced pressure. This residue was purified by silica gel column chromatography (CH2Cl2/MeOH, 50/1, v/v) to give the desired compound 3a–3s.
7-(Benzyloxy)-2-(3,4-dihydroxyphenyl)-3,5-dihydroxy-4H-chromen-4-one (3a). Yield 50%. m.p. 255–257 °C; 1H-NMR (DMSO-d6) δ 7.69 (d, J = 2.0 Hz, 1H, -ArH), 7.54 (dd, J = 8.6, 2.0 Hz, 1H, -ArH), 7.35–7.46 (m, 5H, -ArH) 6.88 (d, J = 8.6 Hz, 1H, -ArH), 6.78 (d, J = 2.0 Hz, 1H, -ArH), 6.41 (d, J = 2.0 Hz, 1H, -ArH), 5.21 (s, 2H, -CH2); 13C-NMR (DMSO-d6) δ 176.4, 164.3, 160.9, 156.4, 148.3, 147.8, 145.5, 136.7, 136.5, 129.0 (2C), 128.6, 128.3 (2C), 122.3, 120.5, 116.0, 115.7, 104.6, 98.5, 93.2, 70.4; ESI-MS (m/z): 392.8 [M+H]+, 412.9 [M+Na]+.
2-(3,4-Dihydroxyphenyl)-7-((2-fluorobenzyl)oxy)-3,5-dihydroxy-4H-chromen-4-one (3b). Yield 51%. m.p. 254–255 °C; 1H-NMR (DMSO-d6) δ 12.52 (s, 1H, -OH), 9.68 (s, 1H, -OH), 9.56 (s, 1H, -OH), 9.34 (s, 1H, -OH), 7.74 (d, J = 2.0 Hz, 1H, -ArH), 7.68–7.58 (m, 1H, -ArH), 7.58 (dd, J = 8.4, 2.0 Hz, 1H, -ArH), 7.44–7.49 (m, 1H, -ArH), 7.25–7.32 (m, 2H, -ArH), 6.90 (d, J = 8.4 Hz, 1H, -ArH), 6.86 (d, J = 2.0 Hz, 1H, -ArH), 6.55 (d, J = 2.0 Hz, 1H, -ArH), 5.27 (s, 2H, -CH2); 13C-NMR (DMSO-d6) δ 176.4, 164.1, 160.9 (d, JC-F = 245.1 Hz), 160.9, 156.4, 148.3, 147.8, 145.5, 136.6, 131.4 (d, JC-F = 3.8 Hz), 131.2 (d, JC-F = 8.3 Hz), 125.1 (d, JC-F = 3.3 Hz), 123.5 (d, JC-F = 14.5 Hz), 122.3, 120.5, 116.0 (d, JC-F = 20.6 Hz), 116.0, 115.7, 104.7, 98.4, 93.1, 64.8 (d, JC-F = 3.6 Hz); ESI-MS (m/z): 411.1 [M+H]+, 433.0 [M+Na]+.
7-((2-Chlorobenzyl)oxy)-2-(3,4-dihydroxyphenyl)-3,5-dihydroxy-4H-chromen-4-one (3c). Yield 49%. m.p. 253–254 °C; 1H-NMR (DMSO-d6) δ 12.52 (s, 1H, -OH), 9.67 (brs, 1H, -OH), 9.54 (brs, 1H, -OH), 9.32 (s, 1H, -OH), 7.74 (d, J = 2.0 Hz, 1H, -ArH), 7.63–7.65 (m, 1H, -ArH), 7.58 (dd, J = 8.4, 2.0 Hz, 1H, -ArH), 7.54–7.56 (m, 1H, -ArH), 7.40–7.46 (m, 2H, -ArH), 6.90 (d, J = 8.4 Hz, 1H, -ArH), 6.86 (d, J = 2.4 Hz, 1H, -ArH), 6.45 (d, J = 2.4 Hz, 1H, -ArH), 5.29 (s, 2H, -CH2); 13C-NMR (DMSO-d6) δ 175.8, 163.6, 160.3, 155.9, 147.8, 147.3, 145.0, 136.0, 133.4, 132.8, 130.4, 130.1, 129.4, 127.4, 121.7, 119.9, 115.5, 115.2, 104.2, 97.8, 92.6, 67.5; ESI-MS (m/z): 427.0 [M+H]+.
7-((2-Bromobenzyl)oxy)-2-(3,4-dihydroxyphenyl)-3,5-dihydroxy-4H-chromen-4-one (3d). Yield 44%. m.p. 248–249 °C; 1H-NMR (DMSO-d6) δ 12.53 (s, 1H, -OH), 9.67 (brs, 1H, -OH), 9.55 (brs, 1H, -OH), 9.33 (s, 1H, -OH), 7.74 (d, J = 2.4 Hz, 1H, -ArH), 7.72 (dd, J = 8.0, 1.2 Hz, 1H, -ArH), 7.63 (dd, J = 7.6, 1.6 Hz, 1H, -ArH), 7.58 (dd, J = 8.4, 2.4 Hz, 1H, -ArH), 7.46 (td, J = 7.6, 1.2 Hz, 1H, -ArH), 7.35 (td, J = 7.6, 1.6 Hz, 1H, -ArH), 6.90 (d, J = 8.4 Hz, 1H, -ArH), 6.86 (d, J = 2.0 Hz, 1H, -ArH), 6.45 (d, J = 2.0 Hz, 1H, -ArH), 5.25 (s, 2H, -CH2); 13C-NMR (DMSO-d6) δ 176.4, 164.1, 160.9, 156.5, 148.3, 147.9, 145.5, 136.6, 135.5, 133.2, 131.1, 130.0, 128.5, 123.7, 122.3, 120.5, 116.0, 115.7, 104.8, 98.4, 93.1, 70.3; ESI-MS (m/z): 471.0, 473.0 [M+H]+.
2-(3,4-Dihydroxyphenyl)-3,5-dihydroxy-7-((2-methylbenzyl)oxy)-4H-chromen-4-one (3e). Yield 47%. m.p. 241–242 °C; 1H-NMR (DMSO-d6) δ 12.52 (s, 1H, -OH), 9.66 (s, 1H, -OH), 9.53 (s, 1H, -OH), 9.33 (s, 1H, -OH) 7.74 (d, J = 2.4 Hz, 1H, -ArH), 7.58 (dd, J = 8.4, 2.4 Hz, 1H, -ArH), 7.44 (d, J = 8.8 Hz, 1H, -ArH), 7.21–7.30 (m, 3H, -ArH), 6.90 (d, J = 8.4 Hz, 1H, -ArH), 6.85 (d, J = 2.0 Hz, 1H, -ArH), 6.46 (d, J = 2.0 Hz, 1H, -ArH), 5.22 (s, 2H, -CH2), 2.34 (s, 3H, -CH3); 13C-NMR (DMSO-d6) δ 175.9, 164.0, 160.4, 156.0, 147.8, 147.3, 145.1, 136.8, 134.1, 130.2, 128.6, 128.3, 125.8, 121.8, 120.0, 115.5, 115.2, 104.1, 97.9, 92.7, 68.7, 18.4; ESI-MS (m/z): 407.0 [M+H]+, 429.1 [M+Na]+.
2-(((2-(3,4-Dihydroxyphenyl)-3,5-dihydroxy-4-oxo-4H-chromen-7-yl)oxy)methyl)benzonitrile (3f). Yield 40%. m.p. 248–249 °C; 1H-NMR (DMSO-d6) δ 12.54 (s, 1H, -OH), 9.68 (s, 1H, -OH), 9.56 (s, 1H, -OH), 9.33 (s, 1H, -OH), 7.95 (d, J = 7.6 Hz, 1H, -ArH), 7.78–7.81 (m, 2H, -ArH), 7.74 (d, J = 2.0 Hz, 1H, -ArH), 7.57–7.63 (m, 2H, -ArH), 6.90 (d, J = 7.6 Hz, 1H, -ArH), 6.89 (d, J = 2.0 Hz, 1H, -ArH), 6.47 (d, J = 2.0 Hz, 1H, -ArH), 5.39 (s, 2H, -CH2); 13C-NMR (DMSO-d6) δ 176.4, 163.9, 160.9, 156.4, 148.4, 147.9, 145.6, 139.6, 136.6, 134.0, 133.9, 129.9, 122.3, 120.5, 117.6, 116.0, 115.7, 111.9, 104.9, 98.4, 93.3, 68.7; ESI-MS (m/z): 417.7 [M+H]+, 856.5 [2M+Na]+.
2-(3,4-Dihydroxyphenyl)-3,5-dihydroxy-7-((2-nitrobenzyl)oxy)-4H-chromen-4-one (3g). Yield 43%. m.p. 260–261 °C; 1H-NMR (DMSO-d6) δ 12.53 (s, 1H, -OH), 9.66 (s, 1H, -OH), 9.54 (brs, 1H, -OH), 9.32 (s, 1H, -OH), 8.17 (d, J = 8.4 Hz, 1H, -ArH), 7.78–7.82 (m, 2H, -ArH), 7.73 (d, J = 2.0 Hz, 1H, -ArH), 7.63–7.67 (m, 1H, -ArH), 7.58 (dd, J = 8.8, 2.0 Hz, 1H, -ArH), 6.89 (d, J = 8.8 Hz, 1H, -ArH), 6.83 (d, J = 2.4 Hz, 1H, -ArH), 6.50 (d, J = 2.4 Hz, 1H, -ArH), 5.61 (s, 2H, -CH2); 13C-NMR (DMSO-d6) δ 176.4, 163.8, 160.9, 154.6, 148.3, 147.9, 145.5, 136.6, 134.6, 132.2, 129.8, 129.6, 125.4, 122.3, 120.5, 116.0, 115.7, 104.9, 98.4, 93.2, 67.5; ESI-MS (m/z): 438.1 [M+H]+.
2-(3,4-Dihydroxyphenyl)-7-((3-fluorobenzyl)oxy)-3,5-dihydroxy-4H-chromen-4-one (3h). Yield 54%. m.p. 243–245 °C; 1H-NMR (DMSO-d6) δ 12.52 (s, 1H, -OH), 9.67 (s, 1H, -OH), 9.54 (s, 1H, -OH), 9.33 (s, 1H, -OH), 7.72 (d, J = 2.4 Hz, 1H, -ArH), 7.56 (dd, J = 8.4, 2.4 Hz, 1H, -ArH), 7.45–7.48 (m, 1H, -ArH), 7.31–7.33 (m, 2H, -ArH), 7.20–7.22 (m, 1H, -ArH), 6.90 (d, J = 8.4 Hz, 1H, -ArH), 6.81 (d, J = 2.0 Hz, 1H, -ArH), 6.45 (d, J = 2.0 Hz, 1H, -ArH), 5.27 (s, 2H, -CH2); 13C-NMR (DMSO-d6) δ 176.4, 164.1, 162.7 (d, JC-F = 242.2Hz), 160.9, 156.4, 148.3, 147.9, 145.6, 139.6 (d, JC-F = 7.5 Hz), 136.5, 131.8 (d, JC-F = 8.3 Hz), 124.1 (d, JC-F = 2.8 Hz), 122.3, 120.5, 116.0, 115.7, 115.3 (d, JC-F = 20.6 Hz), 114.8 (d, JC-F = 21.8 Hz), 104.7, 98.5, 93.3, 69.5; ESI-MS (m/z): 411.1 [M+H]+.
7-((3-Chlorobenzyl)oxy)-2-(3,4-dihydroxyphenyl)-3,5-dihydroxy-4H-chromen-4-one (3i). Yield 40%. m.p. 260–261 °C; 1H-NMR (DMSO-d6) δ 12.52 (s, 1H, -OH), 9.67 (brs, 1H, -OH), 9.54 (brs, 1H, -OH), 9.32 (s, 1H, -OH), 7.73 (d, J = 2.4 Hz, 1H, -ArH), 7.55–7.58 (m, 2H, -ArH), 7.41–7.45 (m, 3H, -ArH), 6.90 (d, J = 8.4 Hz, 1H, -ArH), 6.81 (d, J = 2.0 Hz, 1H, -ArH), 6.46 (d, J = 2.0 Hz, 1H, -ArH), 5.26 (s, 2H, -CH2); ESI-MS (m/z): 427.0 [M+H]+.
7-((3-Bromobenzyl)oxy)-2-(3,4-dihydroxyphenyl)-3,5-dihydroxy-4H-chromen-4-one (3j). Yield 47%. m.p. 234–236 °C; 1H-NMR (DMSO-d6) δ 12.52 (s, 1H, -OH), 9.68 (brs, 1H, -OH), 9.55 (brs, 1H, -OH), 9.33 (s, 1H, -OH), 7.72 (d, J = 2.4 Hz, 1H, -ArH), 7.69 (s, 1H, -ArH), 7.56 (dd, J = 8.4, 2.0 Hz, 2H, -ArH), 7.49 (d, J = 8.0 Hz, 1H, -ArH), 7.39 (t, J = 8.0 Hz, 1H, -ArH), 6.90 (d, J = 8.8 Hz, 1H, -ArH), 6.81 (d, J = 2.0 Hz, 1H, -ArH), 6.46 (d, J = 2.0 Hz, 1H, -ArH), 5.25 (s, 2H, -CH2); 13C-NMR (DMSO-d6) δ 176.4, 164.1, 160.9, 156.4, 148.3, 147.8, 145.5, 139.5, 136.5, 131.4, 131.3, 130.8, 127.2, 122.3, 122.2, 120.5, 116.0, 115.7, 104.7, 98.5, 93.3, 69.4; ESI-MS (m/z): 471.0, 473.0 [M+H]+.
2-(3,4-Dihydroxyphenyl)-3,5-dihydroxy-7-((3-methylbenzyl)oxy)-4H-chromen-4-one (3k). Yield 43%. m.p. 224–226 °C; 1H-NMR (DMSO-d6) δ 12.51 (s, 1H, -OH), 9.63 (brs, 1H, -OH), 9.53 (brs, 1H, -OH), 9.33 (brs, 1H, -OH), 7.73 (d, J = 2.0 Hz, 1H, -ArH), 7.57 (d, J = 8.8, 2.0 Hz, 1H, -ArH), 7.16–7.32 (m, 4H, -ArH), 6.90 (d, J = 8.8 Hz, 1H, -ArH), 6.79 (d, J = 2.0 Hz, 1H, -ArH), 6.43 (d, J = 2.0 Hz, 1H, -ArH), 5.19 (s, 2H, -CH2), 2.33 (s, 3H, -CH3); 13C-NMR (DMSO-d6) δ 176.4, 164.4, 160.9, 156.4, 148.3, 147.8, 145.5, 138.2, 136.6, 136.5, 129.2, 128.9, 128.9, 125.4, 122.3, 120.5, 116.0, 115.7, 104.6, 98.5, 93.2, 70.5, 21.5; ESI-MS (m/z): 407.0 [M+H]+.
3-(((2-(3,4-Dihydroxyphenyl)-3,5-dihydroxy-4-oxo-4H-chromen-7-yl)oxy)methyl)benzonitrile (3l). Yield 42%. m.p. 246–247 °C; 1H-NMR (DMSO-d6) δ 12.52 (s, 1H, -OH), 9.66 (s, 1H, -OH), 9.53 (s, 1H, -OH), 9.31 (s, 1H, -OH), 7.96 (brs, 1H, -ArH), 7.82–7.86 (m, 2H, -ArH), 7.72 (d, J = 2.0 Hz, 1H, -ArH), 7.63–7.67 (m, 1H, -ArH), 7.56 (dd, J = 8.4, 2.0 Hz, 1H, -ArH), 6.90 (d, J = 8.4 Hz, 1H, -ArH), 6.83 (d, J = 1.6 Hz, 1H, -ArH), 6.47 (d, J = 1.6 Hz, 1H, -ArH), 5.31 (s, 2H, -CH2); ESI-MS (m/z): 418.1 [M+H]+, 440.0 [M+Na]+.
2-(3,4-Dihydroxyphenyl)-3,5-dihydroxy-7-((3-nitrobenzyl)oxy)-4H-chromen-4-one (3m). Yield 46%. m.p. 264–266 °C; 1H-NMR (DMSO-d6) δ 12.53 (s, 1H, -OH), 9.67 (s, 1H, -OH), 9.54 (brs, 1H, -OH), 9.31 (s, 1H, -OH), 8.35 (s, 1H, -ArH), 8.23 (dd, J = 8.4, 1.6 Hz, 1H, -ArH), 7.94 (d, J = 8.0 Hz, 1H, -ArH), 7.72–7.76 (m, 2H, -ArH), 7.56 (dd, J = 8.4, 2.0 Hz, 1H, -ArH), 6.90 (d, J = 8.4 Hz, 1H, -ArH), 6.85 (d, J = 2.4 Hz, 1H, -ArH), 6.49 (d, J = 2.4 Hz, 1H, -ArH), 5.41 (s, 2H, -CH2); 13C-NMR (DMSO-d6) δ 175.9, 163.4, 160.5, 155.9, 147.8, 147.4, 145.1, 138.6, 136.1, 134.2, 130.2, 123.0, 122.2, 121.8, 120.0, 115.5, 115.2, 104.3, 98.0, 92.9, 68.5; ESI-MS (m/z): 438.1 [M+H]+.
2-(3,4-Dihydroxyphenyl)-7-((4-fluorobenzyl)oxy)-3,5-dihydroxy-4H-chromen-4-one (3n). Yield 50%. m.p.254–255 °C; 1H-NMR (DMSO-d6) δ 12.51 (s, 1H, -OH), 9.68 (s, 1H, -OH), 9.53 (brs, 1H, -OH), 9.33 (s, 1H, -OH) 7.72 (d, J = 2.0 Hz, 1H, -ArH), 7.52–7.58 (m, 3H, -ArH), 7.27 (d, J = 8.8 Hz, 1H, -ArH), 7.24 (d, J = 8.8 Hz, 1H, -ArH), 6.90 (d, J = 8.4 Hz, 1H, -ArH), 6.81 (d, J = 2.4 Hz, 1H, -ArH), 6.43 (d, J = 2.4 Hz, 1H, -ArH), 5.22 (s, 2H, -CH2); 13C-NMR (DMSO-d6) δ 175.9, 163.7, 161.8 (d, JC-F = 244.4 Hz), 160.0, 155.9, 147.8, 147.3, 145.0, 136.0, 132.4 (d, JC-F = 2.9 Hz), 130.2 (d, JC-F = 8.3 Hz, 2C), 121.8, 120.0, 115.5, 115.4(d, J = 21.4 Hz, 2C), 115.2, 104.1, 98.0, 92.7, 69.2; ESI-MS (m/z): 411.1 [M+H]+.
7-((4-Chlorobenzyl)oxy)-2-(3,4-dihydroxyphenyl)-3,5-dihydroxy-4H-chromen-4-one (3o). Yield 41%. m.p. 266–267 °C; 1H-NMR (DMSO-d6) δ 12.51 (s, 1H, -OH), 9.67 (s, 1H, -OH), 9.53 (s, 1H, -OH), 9.32 (s, 1H, -OH), 7.71 (d, J = 2.0 Hz, 1H, -ArH), 7.56 (dd, J = 8.4, 2.0 Hz, 1H, -ArH), 7.47–7.52 (m, 4H, -ArH), 6.90 (d, J = 8.4 Hz, 1H, -ArH), 6.80 (d, J = 2.0 Hz, 1H, -ArH), 6.44 (d, J = 2.0 Hz, 1H, -ArH), 5.24 (s, 2H, -CH2); ESI-MS (m/z): 427.0 [M+H]+.
7-((4-Bromobenzyl)oxy)-2-(3,4-dihydroxyphenyl)-3,5-dihydroxy-4H-chromen-4-one (3p). Yield 44%. m.p.256–257 °C; 1H-NMR (DMSO-d6) δ 12.51 (s, 1H, -OH), 9.68 (brs, 1H, -OH), 9.54 (brs, 1H, -OH), 9.34 (brs, 1H, -OH), 7.72 (d, J = 2.0 Hz, 1H, -ArH), 7.62 (d, J = 8.4 Hz, 2H, -ArH), 7.56 (dd, J = 8.8, 2.0 Hz, 1H, -ArH), 7.44 (d, J = 8.4 Hz, 2H, -ArH), 6.90 (d, J = 8.8 Hz, 1H, -ArH), 6.80 (d, J = 2.4 Hz, 1H, -ArH), 6.43 (d, J = 2.4 Hz, 1H, -ArH), 5.23 (s, 2H, -CH2); 13C-NMR (DMSO-d6) δ 176.4, 164.1, 160.9, 156.4, 148.3, 147.8, 145.5, 136.5, 136.2, 131.9 (2C), 130.4 (2C), 122.3, 121.7, 120.5, 116.0, 115.7, 104.7, 98.5, 93.3, 69.5; ESI-MS (m/z): 471.0, 473.0 [M+H]+.
2-(3,4-Dihydroxyphenyl)-3,5-dihydroxy-7-((4-methylbenzyl)oxy)-4H-chromen-4-one (3q). Yield 56%. m.p. 261–264 °C; 1H-NMR (DMSO-d6) δ 12.51 (s, 1H, -OH), 9.51–9.43 (brs, 3H, -OH), 7.73 (brs, 1H, -ArH), 7.57 (d, J = 8.4 Hz, 1H, -ArH), 7.36 (d, J = 8.0 Hz, 2H, -ArH), 7.22 (d, J = 8.0 Hz, 2H, -ArH), 6.90 (d, J = 8.4 Hz, 1H, -ArH), 6.78 (d, J = 1.6 Hz, 1H, -ArH), 6.41 (d, J = 1.6 Hz, 1H, -ArH), 5.18 (s, 2H, -CH2), 2.31 (s, 3H, -CH3); 13C-NMR (DMSO-d6) δ 175.9, 163.9, 160.4, 155.9, 147.9, 147.3, 145.1, 137.4, 136.0, 133.1, 129.0 (2C), 127.9 (2C), 121.8, 120.0, 115.5, 115.2, 104.1, 98.0, 92.7, 69.8, 20.8; ESI-MS (m/z): 407.0 [M+H]+.
4-(((2-(3,4-Dihydroxyphenyl)-3,5-dihydroxy-4-oxo-4H-chromen-7-yl)oxy)methyl)benzonitrile (3r). Yield 45%. m.p. 252–254 °C; 1H-NMR (DMSO-d6) δ 12.52 (s, 1H, -OH), 9.68 (s, 1H, -OH), 9.55 (s, 1H, -OH), 9.32 (s, 1H, -OH), 7.90 (d, J = 8.0 Hz, 2H, -ArH), 7.72 (d, J = 1.6 Hz, 1H, -ArH), 7.67 (d, J = 8.0 Hz, 2H, -ArH), 7.56 (dd, J = 8.4, 1.6 Hz, 1H, -ArH), 6.90 (d, J = 8.4 Hz, 1H, -ArH), 6.81 (d, J = 1.6 Hz, 1H, -ArH), 6.46 (d, J = 1.6 Hz, 1H, -ArH), 5.37 (s, 2H, -CH2); 13C-NMR (DMSO-d6) δ 176.4, 163.9, 160.9, 156.4, 148.4, 147.9, 145.6, 142.5, 136.6, 133.0(2C), 128.6(2C), 122.3, 120.5, 119.2, 116.0, 115.7, 111.2, 104.8, 98.5, 93.3, 69.3; ESI-MS (m/z): 417.9 [M+H]+.
2-(3,4-Dihydroxyphenyl)-3,5-dihydroxy-7-((4-nitrobenzyl)oxy)-4H-chromen-4-one (3s). Yield 47%. m.p. 253.5–254.5 °C; 1H-NMR (DMSO-d6) δ 12.53 (s, 1H, -OH), 9.68 (s, 1H, -OH), 9.55 (brs, 1H, -OH), 9.32 (s, 1H, -OH), 8.29 (d, J = 8.8 Hz, 2H, -ArH), 7.75 (d, J = 8.8 Hz, 2H, -ArH), 7.72 (d, J = 2.0 Hz, 1H, -ArH), 7.56 (dd, J = 8.4, 2.0 Hz, 1H, -ArH), 6.90 (d, J = 8.4 Hz, 1H, -ArH), 6.83 (d, J = 2.4 Hz, 1H, -ArH), 6.47 (d, J = 2.4 Hz, 1H, -ArH), 5.43 (s, 2H, -CH2); 13C-NMR (DMSO-d6) δ 176.4, 163.9, 161.0, 156.4, 148.4, 147.9, 147.6, 145.6, 144.6, 136.6, 128.8 (2C), 124.2 (2C), 122.3, 120.5, 116.0, 115.7, 104.8, 98.5, 93.4, 69.1, 23.0; ESI-MS (m/z): 438.1 [M+H]+.
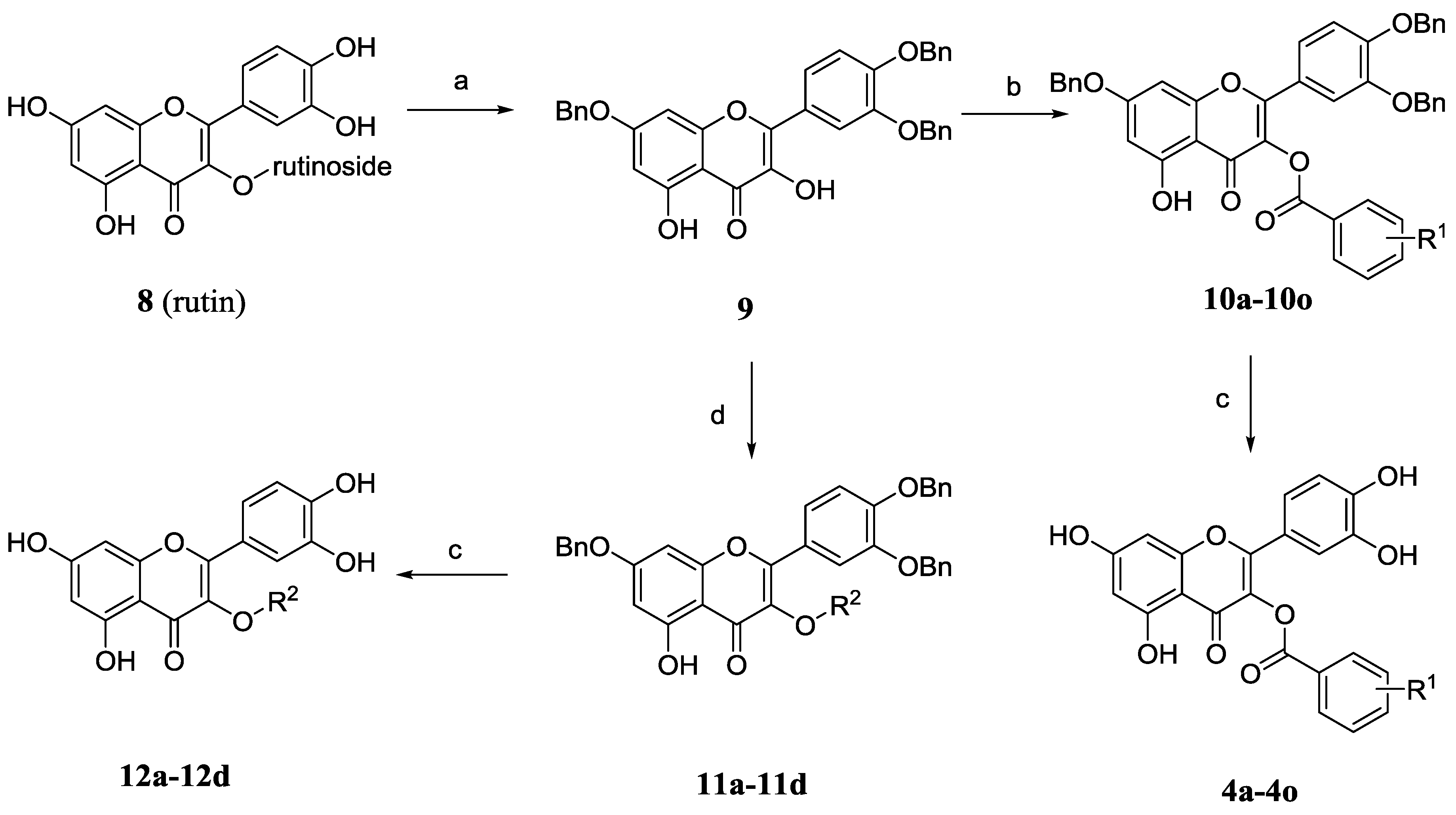
3.8. General Procedure for the Preparation of 4a–4o
To a solution of the appropriate benzyl-protected quercetin-3-O-ester derivatives 10a–10o in ethanol and 1,4-dioxane (20 mL, 3:1) was added 10% palladium on carbon (20 mg). The mixture was stirred under hydrogen atmosphere at room temperature for 6 h. The resulting mixture was filtered, washed with EtOH and purified by silica gel column chromatography (CH2Cl2/MeOH, 20/1, v/v) to give the desired compounds 4a–4o.
2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl benzoate (4a). Yield 54%. m.p. 173–174 °C; 1H-NMR (DMSO-d6): δ 12.16 (s, 1H, -OH), 11.05 (brs, 1H, -OH), 9.90 (brs, 1H, -OH), 9.47 (brs, 1H, -OH), 8.15 (d, J = 7.9 Hz, 2H, -ArH), 7.80 (t, J = 7.9 Hz, 1H, -ArH), 7.64 (dd, J = 7.9, 7.9 Hz, 2H, -ArH), 7.36 (d, J = 2.4 Hz, 1H, -ArH), 7.31 (dd, J = 8.4, 2.4 Hz, 1H, -ArH), 6.86 (d, J = 8.4 Hz, 1H, -ArH), 6.52 (d, J = 2.0 Hz, 1H, -ArH), 6.28 (d, J = 2.0 Hz, 1H, -ArH); ESI-MS (m/z) 407.0 [M+H]+.
2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl 2-fluorobenzoate (4b). Yield 54%. m.p. 186–187 °C; 1H-NMR (DMSO-d6): δ 12.13 (s, 1H, -OH), 11.14 (brs, 1H, -OH), 10.00 (brs, 1H, -OH), 9.54 (brs, 1H, -OH), 8.14 (ddd, J = 7.6, 7.6, 2.0 Hz, 1H, -ArH), 7.86–7.82 (m, 1H, -ArH), 7.51–7.46 (m, 2H, -ArH), 7.38 (d, J = 2.4 Hz, 1H, -ArH), 7.34 (dd, J = 8.4, 2.4 Hz, 1H, -ArH), 6.91 (d, J = 8.4 Hz, 1H, -ArH), 6.52 (d, J = 2.0 Hz, 1H, -ArH), 6.28 (d, J = 2.0 Hz, 1H, -ArH); ESI-MS (m/z) 425.0 [M+H]+.
2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl 2-aminobenzoate (4c). Yield 58%. m.p. 198–199 °C; 1H-NMR (DMSO-d6): δ 12.24 (s, 1H, -OH), 11.02 (brs, 1H, -OH), 9.91 (brs, 1H, -OH), 9.45 (brs, 1H, -OH), 7.95 (dd, J = 8.0, 2.0 Hz, 1H, -ArH), 7.38–7.34 (m, 2H, -ArH), 7.30 (dd, J = 8.4, 2.4 Hz, 1H, -ArH), 6.85 (d, J = 8.4 Hz, 1H, -ArH), 6.84 (d, J = 7.6 Hz, 1H, -ArH), 6.74 (brs, 2H, -ArH), 6.62 (dd, J = 7.6, 7.6 Hz, 1H, -ArH), 6.51 (d, J = 2.0 Hz, 1H, -ArH), 6.26 (d, J = 2.0 Hz, 1H, -ArH); ESI-MS (m/z) 422.0 [M+H]+.
2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl 2-methoxybenzoate (4d). Yield 52%. m.p. 183–184 °C; 1H-NMR (DMSO-d6): δ 12.23 (s, 1H, -OH), 11.11 (brs, 1H, -OH), 10.00 (brs, 1H, -OH), 9.48 (brs, 1H, -OH), 7.98 (dd, J = 7.6, 2.0 Hz, 1H, -ArH), 7.69 (ddd, J = 8.8, 7.6, 2.0 Hz, 1H, -ArH), 7.41 (d, J = 2.4 Hz, 1H, -ArH), 7.38 (dd, J = 8.4, 2.4 Hz, 1H, -ArH), 7.26 (d, J = 8.8 Hz, 1H, -ArH), 7.13 (dd, J = 7.6, 7.6 Hz, 1H, -ArH), 6.90 (d, J = 8.4 Hz, 1H, -ArH), 6.53 (d, J = 2.0 Hz, 1H, -ArH), 6.29 (d, J = 2.0 Hz, 1H, -ArH), 3.88 (s, 3H, -CH3); ESI-MS (m/z) 437.0 [M+H]+.
2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl 2-cyanobenzoate (4e). Yield 57%. m.p. 196–197 °C; 1H-NMR (DMSO-d6): δ 12.24 (s, 1H, -OH), 11.02 (brs, 1H, -OH), 9.91 (brs, 1H, -OH), 9.45 (brs, 1H, -OH), 8.45–8.43 (m, 1H, -ArH), 8.17–8.14 (m, 1H, -ArH), 8.00–7.97 (m, 2H, -ArH), 7.38 (d, J = 2.4 Hz, 1H, -ArH), 7.35 (dd, J = 8.4, 2.4 Hz, 1H, -ArH), 6.89 (d, J = 8.4 Hz, 1H, -ArH), 6.56 (d, J = 2.0 Hz, 1H, -ArH), 6.31 (d, J = 2.0 Hz, 1H, -ArH); ESI-MS (m/z) 432.0 [M+H]+, 454.0 [M+Na]+.
2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl 3-fluorobenzoate (4f). Yield 52%. m.p. 211–213 °C; 1H-NMR (DMSO-d6) δ 12.11 (s, 1H, -OH), 11.07 (brs, 1H, -OH), 9.92 (brs, 1H, -OH), 9.50 (brs, 1H, -OH), 8.01 (ddd, J = 6.8, 1.6, 2.0 Hz, 1H, -ArH), 7.92 (dd, J = 9.2, 2.0 Hz, 1H, -ArH), 7.74–7.65 (m, 2H, -ArH), 7.36 (d, J = 2.0 Hz, 1H, -ArH), 7.32 (dd, J = 8.4, 2.0 Hz, 1H, -ArH), 6.87 (d, J = 8.4 Hz, 1H, -ArH), 6.53 (d, J = 2.0 Hz, 1H, -ArH), 6.28 (d, J = 2.0 Hz, 1H, -ArH); 13C-NMR (DMSO-d6) δ 174.51, 164.73, 162.30 (d, JC-F = 3.0 Hz), 162.05 (d, JC-F = 244.3 Hz), 161.03, 156.66, 156.39, 149.37, 145.47, 131.50 (d, JC-F = 7.9 Hz), 129.98 (d, JC-F = 7.5 Hz), 129.69, 126.32 (d, JC-F = 2.5 Hz), 121.66 (d, JC-F = 21.1 Hz), 120.56, 119.41, 116.55 (d, JC-F = 23.1 Hz), 115.91, 115.09, 103.40, 99.18, 94.20; ESI-MS (m/z) 425.0 [M+H]+, 871.2 [2M+Na]+.
2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl 3-aminobenzoate (4g). Yield 50%. m.p. 216–217 °C; 1H-NMR (DMSO-d6) δ 12.20 (s, 1H, -OH), 11.03 (brs, 1H, -OH), 9.92 (brs, 1H, -OH), 9.45 (brs, 1H, -OH), 7.34 (d, J = 2.4 Hz, 1H, -ArH), 7.32 (d, J = 2.0 Hz, 1H, -ArH), 7.28 (dd, J = 8.4, 2.4 Hz, 1H, -ArH), 7.25–7.21 (m, 2H, -ArH), 6.91–6.89 (m, 1H, -ArH), 6.86 (d, J = 8.4 Hz, 1H, -ArH), 6.51 (d, J = 2.0 Hz, 1H, -ArH), 6.27 (d, J = 2.0 Hz, 1H, -ArH); ESI-MS (m/z) 422.0 [M+H]+.
2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl 3-methoxybenzoate (4h). Yield 60%. m.p. 173–174 °C; 1H-NMR (DMSO-d6) δ 12.16 (s, 1H, -OH), 11.06 (brs, 1H, -OH), 9.92 (brs, 1H, -OH), 9.47 (brs, 1H, -OH), 7.74 (d, J = 7.6 Hz, 1H, -ArH), 7.59 (dd, J = 3.0, 2.0Hz, 1H, -ArH), 7.55 (dd, J = 7.6, 7.6 Hz, 1H, -ArH), 7.37–7.35 (m, 2H, -ArH), 7.30 (dd, J = 8.4, 2.4 Hz, 1H, -ArH), 6.86 (d, J = 8.4 Hz, 1H, -ArH), 6.52 (d, J = 2.0 Hz, 1H, -ArH), 6.27 (d, J = 2.0 Hz, 1H, -ArH); ESI-MS (m/z) 437.0 [M+H]+, 459.1 [M+Na]+, 895.2 [2M+Na]+.
2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl 3-cyanobenzoate (4i). Yield 49%. m.p. 142–143 °C; 1H-NMR (DMSO-d6) δ 12.08 (s, 1H, -OH), 11.08 (brs, 1H, -OH), 9.92 (brs, 1H, -OH), 9.50 (s, 1H, -OH), 8.58 (dd, J = 2.0, 2.0 Hz, 1H, -ArH), 8.45 (ddd, J = 8.0, 2.0, 2.0 Hz, 1H, -ArH), 8.27 (ddd, J = 8.0, 2.0, 2.0 Hz, 1H, -ArH), 7.86 (d, J = 8.0 Hz, 1H, -ArH), 7.37 (d, J = 2.4 Hz, 1H, -ArH), 6.83 (dd, J = 8.4, 2.4 Hz, 1H, -ArH), 6.87 (d, J = 8.4 Hz, 1H, -ArH), 6.53 (d, J = 2.0 Hz, 1H, -ArH), 6.28 (d, J = 2.0 Hz, 1H, -ArH); ESI-MS (m/z) 432.1 [M+H]+, 454.0 [M+Na]+.
2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl 3-chlorobenzoate (4j). Purified by prepared HPLC. Yield 35%. m.p. > 300 °C; 1H-NMR (DMSO-d6) δ 8.13 (brs, 1H, -ArH), 8.09 (d, J = 8.0 Hz, 1H, -ArH), 7.71 (d, J = 8.0 Hz, 1H, -ArH), 7.56 (dd, J = 8.0, 8.0 Hz, 1H, -ArH), 7.33 (brs, 1H, -ArH), 7.29 (d, J = 8.0 Hz, 1H, -ArH), 6.81 (d, J = 8.0 Hz, 1H, -ArH), 6.42 (brs, 1H, -ArH), 6.21 (brs, 1H, -ArH); ESI-MS (m/z) 440.8 [M+H]+.
2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl 4-fluorobenzoate (4k). Yield 56%. m.p. 203–205 °C; 1H-NMR (DMSO-d6) δ 12.14 (s, 1H, -OH), 11.06 (brs, 1H, -OH), 9.91 (brs, 1H, -OH), 9.48 (brs, 1H, -OH), 8.24 (d, J = 8.8 Hz, 1H, -ArH), 8.23(d, J = 8.8 Hz, 1H, -ArH) 7.48 (d, J = 8.8 Hz, 1H, -ArH), 7.46 (d, J = 8.8 Hz, 1H, -ArH), 7.36 (d, J = 2.4 Hz, 1H, -ArH), 7.31 (dd, J = 8.4, 2.4 Hz, 1H, -ArH), 6.87 (d, J = 8.4 Hz, 1H, -ArH), 6.52 (d, J = 2.0 Hz, 1H, -ArH), 6.28 (d, J = 2.0 Hz, 1H, -ArH); 13C-NMR (DMSO-d6) 174.59, 165.72 (d, JC-F = 251.8 Hz) 164.64, 162.34, 160.98, 156.59, 156.28, 149.26, 145.40, 133.06 (d, JC-F = 9.8 Hz, 2C), 129.67, 124.36 (d, JC-F = 2.6 Hz), 120.45, 119.43, 116.33 (d, JC-F = 22.1 Hz, 2C), 115.81, 115.02, 103.35, 99.08, 94.10; ESI-MS (m/z) 425.0 [M+H]+, 871.2 [2M+Na]+.
2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl 4-aminobenzoate (4l). The product was prepared according to procedure described above starting from 9 and 4-nitrobenzoic acid. Yield 55%. m.p. 233–234 °C; 1H-NMR (DMSO-d6) δ 12.30 (s, 1H, -OH), 11.00 (brs, 1H, -OH), 9.88 (brs, 1H, -OH), 9.42 (s, 1H, -OH), 7.78 (d, J = 8.8 Hz, 2H, -ArH), 7.35 (d, J = 2.0 Hz, 1H, -ArH), 7.28 (dd, J = 8.4, 2.0 Hz, 1H, -ArH), 6.84 (d, J = 8.4 Hz, 1H, -ArH), 6.63 (d, J = 8.8 Hz, 2H, -ArH), 6.49 (d, J = 2.4 Hz, 1H, -ArH), 6.26 (brs, 2H, -ArH), 6.25 (d, J = 2.4 Hz, 1H, -ArH); ESI-MS (m/z) 422.0 [M+H]+.
2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl 4-methoxybenzoate (4m). Yield 50%. m.p. 194–195 °C; 1H-NMR (DMSO-d6) δ 12.20 (s, 1H, -OH), 11.03 (brs, 1H, -OH), 9.90 (brs, 1H, -OH), 9.44 (s, 1H, -OH), 8.10 (d, J = 8.8 Hz, 2H, -ArH), 7.35 (d, J = 2.0 Hz, 1H, -ArH), 7.29 (dd, J = 8.4, 2.0 Hz, 1H, -ArH), 7.14 (d, J = 8.8 Hz, 2H, -ArH), 6.84 (d, J = 8.4 Hz, 1H, -ArH), 6.51 (d, J = 2.0 Hz, 1H, -ArH), 6.27 (d, J = 2.0 Hz, 1H, -ArH), 3.89(s, 3H, -CH3); ESI-MS (m/z) 437.0 [M+H]+, 895.2 [2M+Na]+.
2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl 4-cyanobenzoate (4n). Yield 58%. m.p. 196–197 °C; 1H-NMR (DMSO-d6) δ 12.07 (s, 1H, -OH), 11.08 (brs, 1H, -OH), 9.92 (brs, 1H, -OH), 9.50 (brs, 1H, -OH), 8.31 (d, J = 8.4 Hz, 2H, -ArH), 8.12 (d, J = 8.4 Hz, 2H, -ArH),7.35 (d, J = 2.0 Hz, 1H, -ArH), 7.31 (dd, J = 8.4, 2.0 Hz, 1H, -ArH), 6.86 (d, J = 8.4 Hz, 1H, -ArH), 6.53 (d, J = 2.0 Hz, 1H, -ArH), 6.28 (d, J = 2.0 Hz, 1H, -ArH); ESI-MS (m/z) 432.1 [M+H]+, 885.1 [2M+Na]+.
2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl 4-chlorobenzoate (4o). Purified by preparative HPLC. Yield 40%. m.p. 194–195 °C; 1H-NMR (400 MHz, d6-DMSO) δ 8.14 (d, J = 8.0 Hz, 2H, -ArH), 7.57 (d, J = 8.0 Hz, 2H, -ArH),7.34 (brs, 1H, -ArH), 7.28 (d, J = 8.0 Hz, 1H, -ArH), 6.81 (d, J = 8.0 Hz, 1H, -ArH), 6.45 (brs, 1H, -ArH), 6.24 (brs, 1H, -ArH); ESI-MS (m/z) 440.9 [M+H]+.