Simultaneous Determination of Five Components in Rat Plasma by UPLC–MS/MS and Its Application to a Comparative Pharmacokinetic Study in Baihe Zhimu Tang and Zhimu Extract
Abstract
:1. Introduction

2. Results and Discussion
2.1. Optimization of the Chromatographic Conditions
2.2. Sample Preparation
2.3. Method Validation
2.3.1. Selectivity
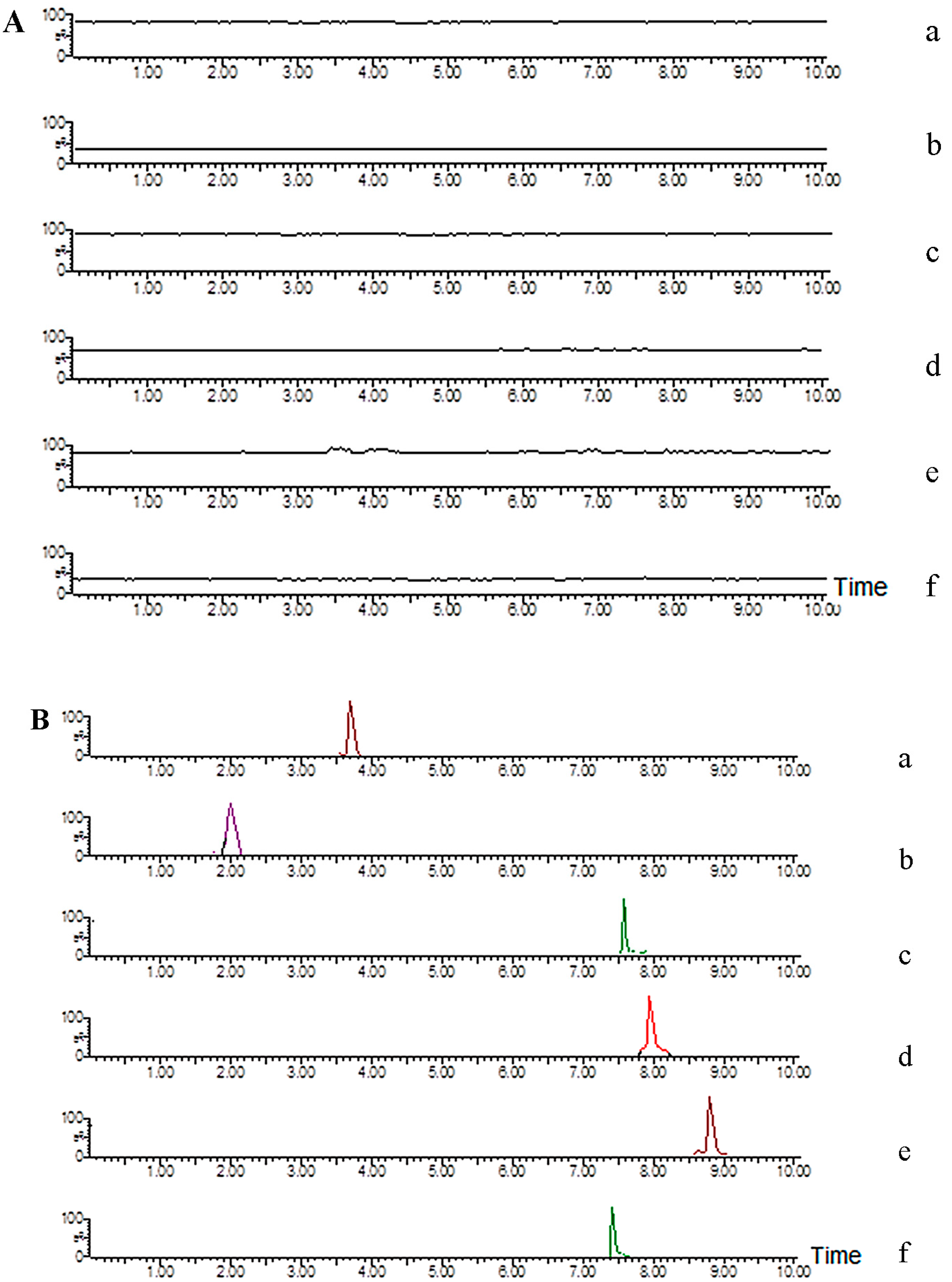
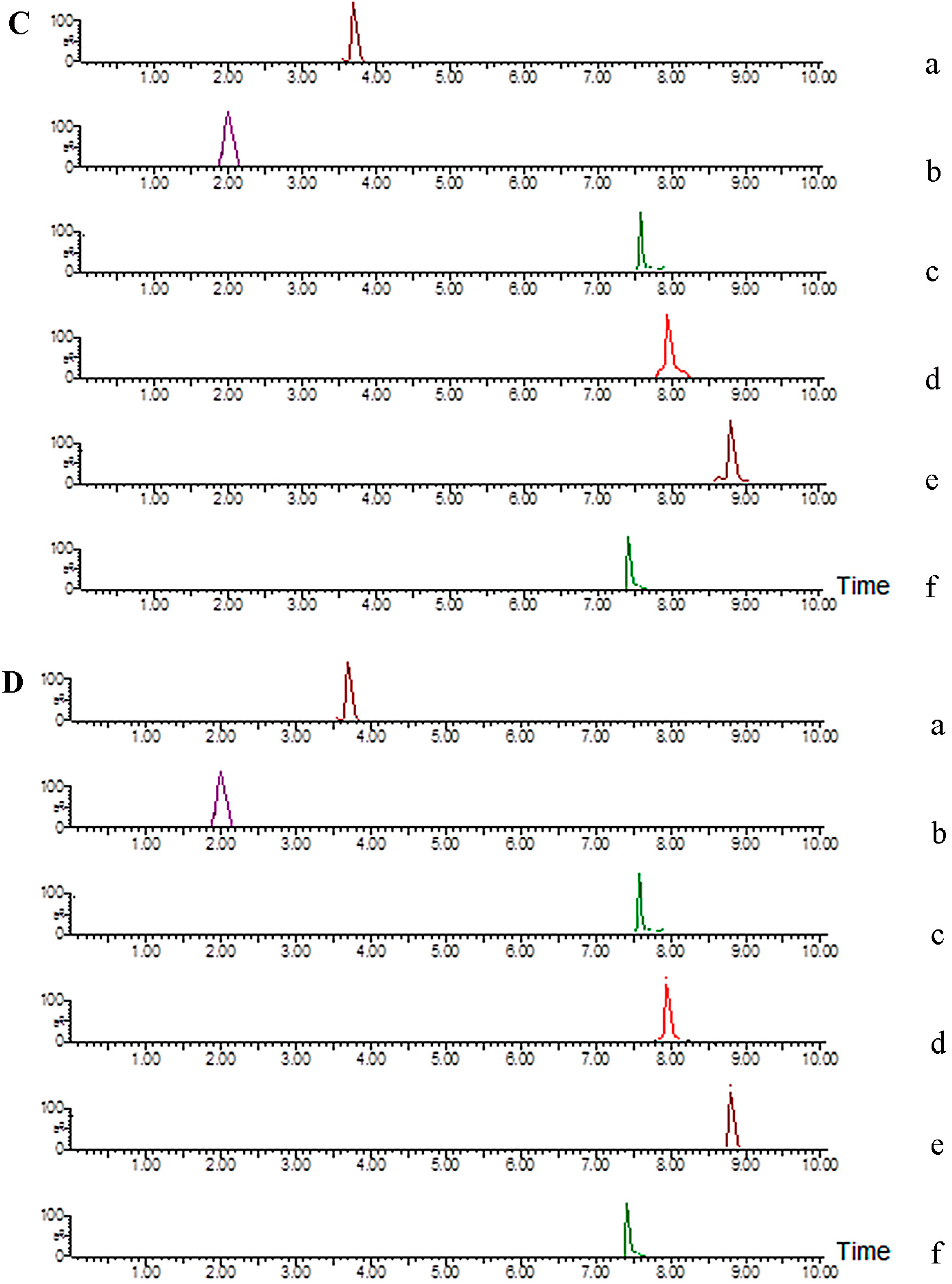
2.3.2. Linearity and LLOQ
2.3.3. Accuracy and Precision
| Analytes | Concentration ng/mL | Intra-Day | Inter-Day | ||
|---|---|---|---|---|---|
| Accuracy (%) | Precision (RSD, %) | Accuracy (%) | Precision (RSD, %) | ||
| Mangiferin | 10.32 | 92.6 | 9.1 | 102.0 | 10.9 |
| 103.2 | 91.3 | 10.4 | 95.9 | 9.0 | |
| 1032 | 93.1 | 8.3 | 95.4 | 8.1 | |
| 8.06 | 99.2 | 7.4 | 104.1 | 8.6 | |
| Neomangiferin | 13.2 | 91.5 | 7.2 | 99.6 | 11.8 |
| 132.8 | 99.4 | 6.1 | 102.4 | 10.9 | |
| 1328 | 101.5 | 6.8 | 99.8 | 10.6 | |
| 10.3 | 99.5 | 5.9 | 103.8 | 8.6 | |
| Timosaponin BII | 11.6 | 87.8 | 7.1 | 94.9 | 11.7 |
| 116 | 87.5 | 6.7 | 91.1 | 9.8 | |
| 1160 | 103.9 | 8.4 | 102.6 | 11.1 | |
| 9.05 | 109.8 | 5.2 | 108.0 | 7.4 | |
| Anemarsaponin BIII | 9.2 | 100.4 | 5.7 | 101.7 | 8.7 |
| 92 | 105.8 | 3.6 | 103.6 | 8.8 | |
| 920 | 101.5 | 5.6 | 99.1 | 8.4 | |
| 6.3 | 103.4 | 5.0 | 102.2 | 6.8 | |
| Timosaponin AIII | 8.8 | 92.0 | 6.9 | 99.7 | 10.8 |
| 88.8 | 93.4 | 4.2 | 104.3 | 9.5 | |
| 888 | 97.2 | 5.4 | 103.8 | 10.1 | |
| 6.25 | 103.5 | 5.9 | 103.8 | 4.9 | |
2.3.4. Extraction Recovery and Matrix Effect
| Analytes | Concentration (ng/mL) | Recovery | Matrix Effects | ||
|---|---|---|---|---|---|
| Accuracy (%) | Precision (%) | Accuracy (%) | Precision (RSD, %) | ||
| Mangiferin | 10.32 | 76.9 | 7.6 | 96.3 | 4.1 |
| 103.2 | 72.9 | 5.8 | 109.8 | 10.9 | |
| 1032 | 78.8 | 9.6 | 96.2 | 7.0 | |
| Neomangiferin | 13.2 | 78.8 | 7.4 | 91.1 | 8.4 |
| 132.8 | 74.5 | 1.2 | 98.6 | 9.9 | |
| 1328 | 75.3 | 7.3 | 100.6 | 9.4 | |
| Timosaponin BII | 11.6 | 96.4 | 11.9 | 99.0 | 10.4 |
| 116 | 88.8 | 5.5 | 88.8 | 8.3 | |
| 1160 | 89.4 | 7.3 | 87.7 | 8.1 | |
| Anemarsaponin BIII | 9.2 | 89.3 | 11.5 | 88.9 | 9.1 |
| 92 | 101.8 | 12.3 | 93.9 | 5.9 | |
| 920 | 91.4 | 10.1 | 96.2 | 7.7 | |
| Timosaponin AIII | 8.8 | 92.0 | 7.9 | 94.5 | 7.9 |
| 88.8 | 82.1 | 9.9 | 87.8 | 6.5 | |
| 888 | 98.6 | 7.9 | 95.7 | 9.6 | |
2.3.5. Sample Stability
| Analytes | Concentration (ng/mL) | Auto-sampler for 24 h | At −80 °C for 1 month | Freeze-Thaw Cycles | |||
|---|---|---|---|---|---|---|---|
| Mean (%) | RSD (%) | Mean (%) | RSD (%) | Mean (%) | RSD (%) | ||
| Mangiferin | 10.32 | 95.2 | 8.7 | 90.3 | 4.7 | 97.1 | 11.7 |
| 103.2 | 99.7 | 10.8 | 104.9 | 10.5 | 101.6 | 8.6 | |
| 1032 | 98.1 | 9.9 | 84.3 | 12.7 | 97.7 | 10.1 | |
| Neomangiferin | 13.2 | 84.5 | 9.2 | 84.7 | 4.8 | 91.7 | 12.5 |
| 132.8 | 89.0 | 4.3 | 84.0 | 12.3 | 97.5 | 9.2 | |
| 1328 | 95.5 | 8.6 | 81.3 | 13.1 | 92.5 | 9.5 | |
| Timosaponin BII | 11.6 | 98.6 | 9.2 | 100.2 | 10.4 | 91.4 | 9.2 |
| 116 | 83.2 | 4.1 | 88.8 | 5.5 | 84.6 | 9.5 | |
| 1160 | 90.5 | 9.9 | 95.6 | 5.6 | 93.8 | 12.7 | |
| Anemarsaponin BIII | 9.2 | 92.6 | 12.0 | 96.3 | 10.3 | 102.2 | 11.3 |
| 92 | 101.9 | 5.4 | 101.8 | 7.0 | 97.1 | 8.2 | |
| 920 | 98.4 | 5.3 | 103.7 | 5.4 | 100.3 | 3.8 | |
| Timosaponin AIII | 8.8 | 102.7 | 6.2 | 96.6 | 6.3 | 97.3 | 11.7 |
| 88.8 | 88.7 | 4.6 | 93.6 | 12.1 | 87.6 | 8.0 | |
| 888 | 101.2 | 11.0 | 98.0 | 7.8 | 100.1 | 12.1 | |
2.4. Pharmacokinetic Study
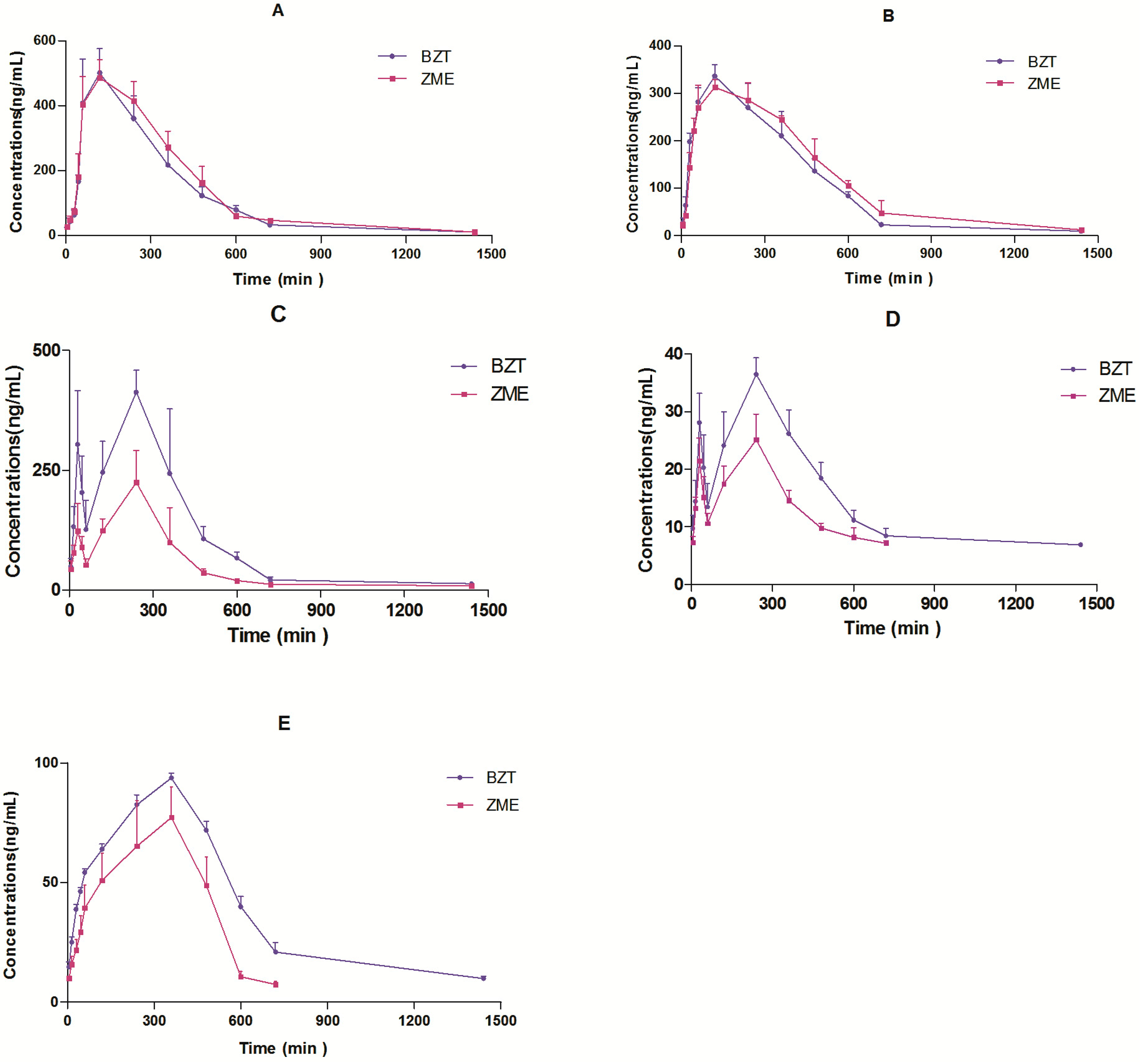
| Parameters | Mangiferin | Neomangiferin | ||
|---|---|---|---|---|
| BZT | ZME | BZT | ZME | |
| Cmax (ng/mL) | 522.83 ± 53.3 | 486.7 ± 20.1 | 336.7 ± 24.5 | 318.0 ± 10.5 |
| Tmax (min) | 96.0 ± 32.8 | 132.0 ± 65.7 | 120 | 144 ± 53.7 |
| T1/2 (min) | 285.92 ± 13.6 | 269.5 ± 165.4 | 258.2 ± 24.4 | 261.9 ± 34.4 |
| AUC0-t (ng/L min) | 175,960.7 ± 21,321.91 | 190,276.9 ± 12,608.0 | 145,297 ± 8204.4 | 164,643 ± 13,198.7 |
| MRT0-t (min) | 331.8 ± 14.5 | 312.1 ± 4.6 | 324.4 ± 11.5 | 365.4 ± 34.7 |
| Parameters | Timosaponin BII | Anemarsaponin BIII | ||
| BZT | ZME | BZT | ZME | |
| Cmax (ng/mL) | 434.6 ± 33.0 * | 239.5 ± 47.6 | 36.4 ± 2.8 * | 25.0 ± 4.5 |
| Tmax (min) | 264 ± 53.6 | 240 ± 84.8 | 240 | 264 ± 53.6 |
| T1/2 (min) | 304.6 ± 136.3 * | 161.7 ± 44.3 | 299.8 ± 128.4 * | 197.7 ± 60.4 |
| AUC0-t (ng/L min) | 150,045.5 ± 20,160.6 * | 70,668.9 ± 11,834.0 | 17,531.5 ± 3348.7 * | 9766.9 ± 1345.5 |
| MRT0-t (min) | 333.6 ± 13.9 | 331.9 ± 39.3 | 289.0 ± 22.2 | 389.7 ± 106.5 |
| Parameters | Timosaponin AIII | |||
| BZT | ZME | |||
| Cmax (ng/mL) | 94.4 ± 3.8 | 84.3 ± 8.6 | ||
| Tmax (min) | 336.0 ± 53.7 | 312 ± 65.7 | ||
| T1/2 (min) | 211.8 ± 98.8 * | 104.2 ± 10.3 | ||
| AUC0-t (ng/L min) | 56,464.5 ± 7189.6 * | 31,753.7 ± 3134.9 | ||
| MRT0-t (min) | 454.9 ± 23.2 * | 308.1 ± 16.8 | ||
3. Experimental
3.1. Chemicals and Reagents
3.2. UPLC–MS/MS Instrumentation and Conditions
3.3. Preparation of Zhimu Baihe Tang and Zhimu Extract
3.4. Preparation of Calibration Standards, Quality Control and Internal Standard
3.5. Sample Preparation
3.6. Method Validation
3.6.1. Selectivity
3.6.2. Linearity and LLOQ
3.6.3. Accuracy and Precision
3.6.4. Recovery and Matrix Effect
3.6.5. Stability
3.7. Pharmacokinetic Study
3.8. Pharmacokinetic and Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sze, S.C.W.; Ip, C.W.; Ng, T.B.; Zhang, K.Y.; Zhang, Z.J.; Cheung, H.P.; Cheng, C.L.Y.; Tong, Y. Compatibility of multiple herbal components in Erxian Decoction, a Chinese medicinal formula, for treating osteoporosis. Eur. J. Integr. Med. 2012, 4, e187–e196. [Google Scholar] [CrossRef]
- Yang, S.S.; Zhang, K.R.; Lin, X.; Miao, Y.Q.; Meng, L.K.; Chen, W.; Tang, X. Pharmacokinetic comparisons of single herb extract of Fufang Danshen preparation with different combinations of its constituent herbs in rats. J. Pharm. Biomed. Anal. 2012, 67–68, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Yue, P.F.; Wu, B.; Hu, P.Y.; Wu, Z.F.; Yang, M. Pharmacokinetics comparative study of a novel Chinese traditional herbal formula and its compatibility. J. Ethnopharmacol. 2011, 137, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J. Jin Kui Yao Lue; Publishing House of Ancient Chinese Medical Books: Beijing, China, 1997. [Google Scholar]
- Ma, C.H.; Wang, L.X.; Tang, Y.H.; Fan, M.S.; Xiao, H.B.; Huang, C.G. Identification of major xanthones and steroidal saponins in rat urine by liquid chromatography-atmospheric pressure chemical ionization mass spectrometry technology following oral administration of Rhizoma Anemarrhenae decoction. Biomed. Chromatogr. 2008, 22, 1066–1083. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.H.; Fan, M.S.; Tang, Y.H.; Li, Z.X.; Sun, Z.L.; Ye, G.; Huang, C.G. Identification of major alkaloids and steroidal saponins in rat serum by HPLC-diode array detection-MS/MS following oral administration of Huangbai-Zhimu herb-pair Extract. Biomed. Chromatogr. 2008, 22, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Pharmacopoeia of the People’s Republic of China (English Edition); People’s Medical Publishing House: Beijing, China, 2010/2015; Volume I.
- He, K.; Li, X.G.; Chen, X.; Ye, X.L.; Huang, J.; Jin, Y.A.; Li, P.P.; Deng, Y.F.; Jin, Q.; Shi, Q.; et al. Evaluation of antidiabetic potential of selected traditional Chinese medicines in STZ-induced diabetic mice. J. Ethnopharmacol. 2011, 137, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Zhang, M.Y.; Sugahara, K.; Sagara, Y.; Meng, Z.Z; Xu, S.X.; Kodama, H. Effect of steroidal saponins of Anemarrhenae Rhizoma on superoxide generation in human neutrophils. Biochem. Biophys. Res. Commun. 1999, 259, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.Q.; Qiu, Y.; Li, T.J.; Tao, X.; Sun, L.N.; Chen, W.S. Timosaponin B-II inhibits pro-inflammatory cytokine induction by lipopolysaccharide in BV2 cells. Arch. Pharm. Res. 2009, 32, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.Q.; Qiu, Y.; Li, T.J.; Tao, X.; Sun, L.N.; Chen, W.S. Antiplatelet and antithrombotic activities of timosaponin B-II, an extract of Anemarrhena asphodeloides. Clin. Exp. Pharmacol. Physiol. 2011, 38, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.G.; Du, Y.F.; Liu, Y.; Chang, L.; Ren, Y.P.; Cao, L.; Sun, Q.; Shi, X.W.; Wang, Q.; Zhang, L.T. Simultaneous determination of 9 components in Anemarrhena asphodeloides by liquid chromatography–tandem mass spectrometry combined with chemometric techniques. J. Sep. Sci. 2012, 35, 1796–1807. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.R.; Dong, X.; Ding, X.; Chen, X.F.; Lv, L.; Li, Y.Y.; Chai, Y.F. Comparative pharmacokinetics of timosaponin B-II and timosaponin A-III after oral administration of Zhimu–Baihe herb-pair, Zhimu extract, free timosaponin B-II and free timosaponin A-III to rats. J. Chromatogr. B. 2013, 926, 28–35. [Google Scholar] [CrossRef]
- Sun, Y.G.; Du, Y.F.; Yang, K.; Chang, L.; Cao, L.; Ren, Y.P.; Sun, Q.; Wang, Q.; Zhang, L.T.; Lv, P.T. A comparative study on the pharmacokinetics of a traditional Chinese herbal preparation with the single herb extracts in rats by LC–MS/MS method. J. Pharm. Biomed. Anal. 2013, 34, 81–82. [Google Scholar]
- Feng, Y.; Chen, B.; Lin, A.H.; Liu, Y.M. Simultaneous determination of timosaponin B-II and A-III in rat plasma by LC–MS/MS and its application to pharmacokinetic study. J. Chromatogr. B 2014, 965, 119–126. [Google Scholar] [CrossRef]
- Kite, G.C.; Porter, E.A.; Simmonds, M.S.J. Chromatographic behaviour of steroidal saponins studied by high-performance liquid chromatography–mass spectrometry. J. Chromatogr. A 2007, 1148, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Sun, L.N.; Gao, S.H.; Yang, Y.; Yang, Q.; Chen, W.S. A rapid and sensitive liquid chromatography–tandem mass spectrometric method for the determination of timosaponin B-II in blood plasma and a study of the pharmacokinetics of saponin in the rat. J. Pharm. Biomed. Anal. 2008, 48, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Xu, W.; Wei, H.; Sun, L.N.; Gao, S.H.; Yang, Q.; Feng, J.; Zhang, F.; Chen, W.S. Simultaneous determination of active xanthone glycosides, timosaponins and alkaloids in rat plasma after oral administration of Zi-Shen Pill extract for the pharmacokinetic study by liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2010, 878, 1845–1854. [Google Scholar] [CrossRef]
- Guide for the Care and Use of Laboratory Animals; US National Research Council: Washington, DC, USA, 1996.
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Tang, Z.; Yang, J.; Duan, J.; Qian, D.; Guo, J.; Zhu, Z.; Liu, H. Simultaneous Determination of Five Components in Rat Plasma by UPLC–MS/MS and Its Application to a Comparative Pharmacokinetic Study in Baihe Zhimu Tang and Zhimu Extract. Molecules 2015, 20, 6700-6714. https://doi.org/10.3390/molecules20046700
Li G, Tang Z, Yang J, Duan J, Qian D, Guo J, Zhu Z, Liu H. Simultaneous Determination of Five Components in Rat Plasma by UPLC–MS/MS and Its Application to a Comparative Pharmacokinetic Study in Baihe Zhimu Tang and Zhimu Extract. Molecules. 2015; 20(4):6700-6714. https://doi.org/10.3390/molecules20046700
Chicago/Turabian StyleLi, Guolong, Zhishu Tang, Jie Yang, Jinao Duan, Dawei Qian, Jianming Guo, Zhenhua Zhu, and Hongbo Liu. 2015. "Simultaneous Determination of Five Components in Rat Plasma by UPLC–MS/MS and Its Application to a Comparative Pharmacokinetic Study in Baihe Zhimu Tang and Zhimu Extract" Molecules 20, no. 4: 6700-6714. https://doi.org/10.3390/molecules20046700
APA StyleLi, G., Tang, Z., Yang, J., Duan, J., Qian, D., Guo, J., Zhu, Z., & Liu, H. (2015). Simultaneous Determination of Five Components in Rat Plasma by UPLC–MS/MS and Its Application to a Comparative Pharmacokinetic Study in Baihe Zhimu Tang and Zhimu Extract. Molecules, 20(4), 6700-6714. https://doi.org/10.3390/molecules20046700





