An Ultra-High Performance Liquid Chromatographic-Tandem Mass Spectrometric Method for the Determination of Sinomenine in Human Plasma after Transdermal Delivery of the Zhengqing Fengtongning Injection
Abstract
:1. Introduction
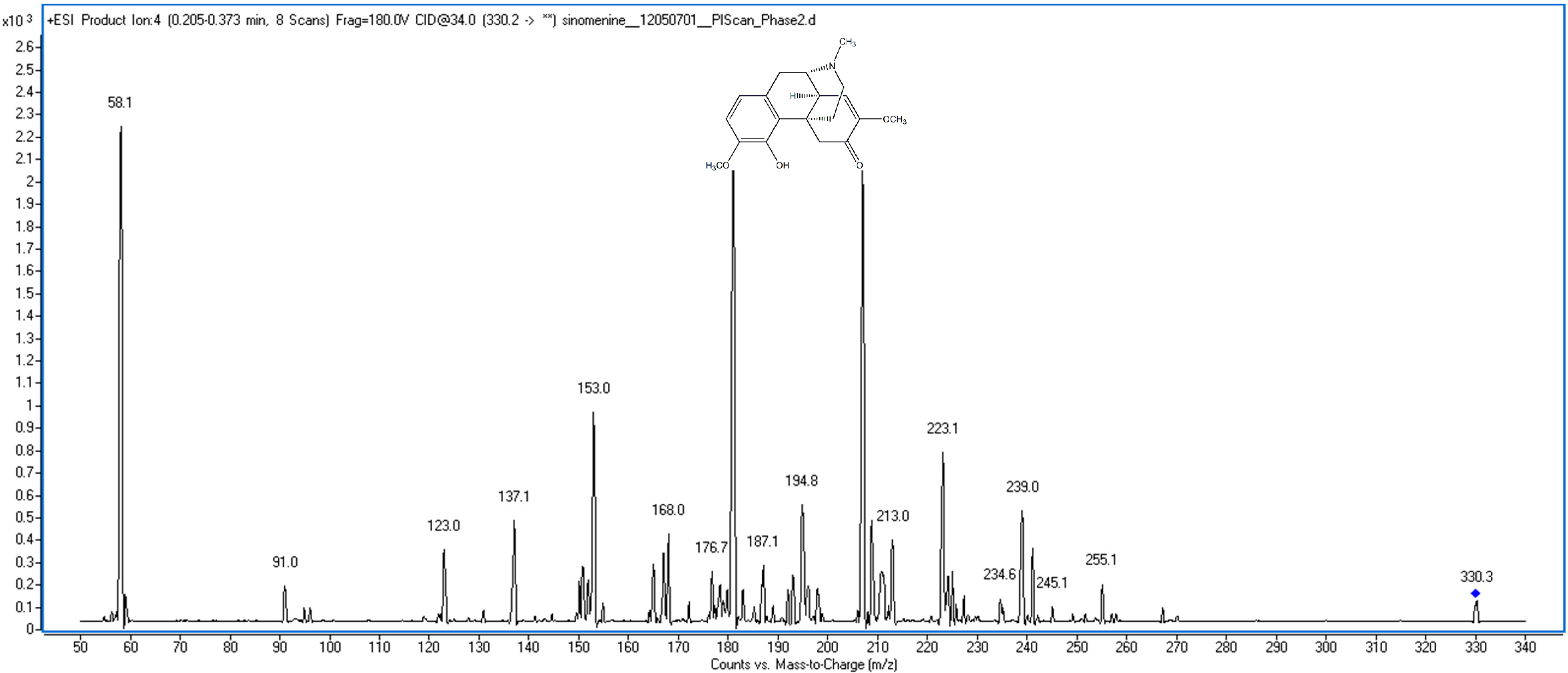
2. Results and Discussion
2.1. UHPLC-MS/MS Method Development
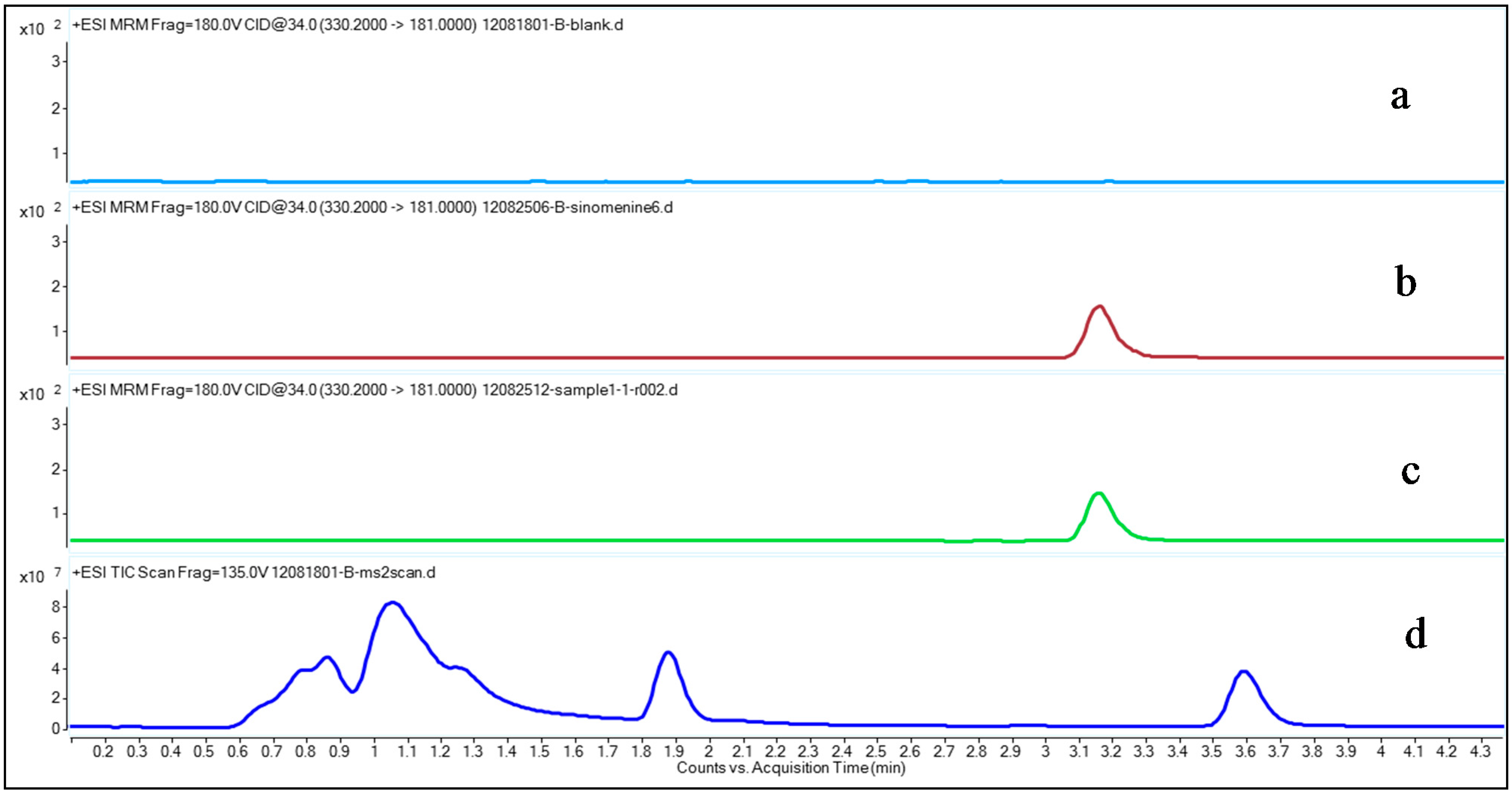
2.2. Method Validation
2.2.1. Specificity
2.2.2. Linearity and Lower Limit of Quantification
2.2.3. Precision and Accuracy
| Condition | Added conc. (ng/mL) | Measured conc. (ng/mL) | Accuracy (%) | Precision (CV, %) |
|---|---|---|---|---|
| Intra-day | 0.2347 | 0.1880 ± 0.0227 | 80.1 | 12.1 |
| 1.878 | 1.861 ± 0.063 | 99.1 | 3.40 | |
| 15.02 | 13.47 ± 0.51 | 89.7 | 3.79 | |
| Inter-day | 0.2347 | 0.1999 ± 0.0266 | 85.2 | 13.3 |
| 1.878 | 1.901 ± 0.074 | 101 | 3.88 | |
| 15.02 | 13.76 ± 0.56 | 91.6 | 4.07 |
2.2.4. Recovery and Matrix Effect
2.2.5. Stability
| Conditions | Added Conc. (ng/mL) | Measured Conc. (ng/mL) | Precision (CV, %) | Relative Error (%) * |
|---|---|---|---|---|
| At 4 °C (12 h, plasma) | 0.2347 | 0.2149 ± 0.0219 | 10.2 | −8.43 |
| 1.878 | 1.965 ± 0.172 | 8.75 | 4.63 | |
| 15.02 | 15.02 ± 0.32 | 2.13 | −0.03 | |
| At −80 °C (30 days, plasma) | 0.2347 | 0.2181 ± 0.0147 | 6.75 | −7.06 |
| 1.878 | 1.946 ± 0.148 | 7.62 | 3.62 | |
| 15.02 | 15.47 ± 0.46 | 3.00 | 2.99 | |
| Freeze-thaw cycles (3 times, plasma) | 0.2347 | 0.2389 ± 0.0198 | 8.27 | 1.79 |
| 1.878 | 2.075 ± 0.129 | 6.22 | 10.5 | |
| 15.02 | 15.10 ± 0.32 | 2.15 | 0.524 | |
| At 4 °C (3 days, solution) | 0.2347 | 0.2502 ± 0.0146 | 5.83 | 6.60 |
| 1.878 | 2.112 ± 0.036 | 1.70 | 12.5 | |
| 15.02 | 15.36 ± 0.09 | 0.59 | 2.24 | |
| At 10 °C (24 h, solution) | 0.2347 | 0.2056 ± 0.0119 | 5.79 | −12.4 |
| 1.878 | 1.931 ± 0.042 | 2.20 | 2.83 | |
| 15.02 | 15.41 ± 0.33 | 2.13 | 2.60 |
2.3. Determination of Sinomenine in Human Plasma
| Subject | No. | Concentration * (ng/mL) | ||
|---|---|---|---|---|
| Before Dosing ^ | After Dosing ^ | 1.5 h after Dosing ^ | ||
| RA patients | 1 | - † | 4.407 ± 0.017 | - |
| 2 | - | - | 1.148 ± 0.074 | |
| 3 | - | 2.245 ± 0.594 | - | |
| 4 | - | 0.6849 ± 0.1007 | - | |
| Mean | - | 2.446 ± 1.526 | ||
| Health volunteer | 1 | - | 0.4131 ± 0.0302 | - |
| 2 | - | - | - | |
| 3 | - | 1.514 ± 0.050 | - | |
| 4 | - | 0.5842 ± 0.0000 | - | |
| Mean | - | 0.8371 ± 0.4837 | - | |
2.4. Discussion
3. Experimental Section
3.1. Materials and Reagents
3.2. Instrumentation and Analytical Conditions
3.3. Transdermal Delivery Study in Humans
3.4. Sample Preparation
3.5. Preparation of Standard Solutions and QC Samples
3.6. Method Validation
3.6.1. Specificity
3.6.2. Linearity and Lower Limit of Quantification (LLOQ)
3.6.3. Precision and Accuracy
3.6.4. Recovery and Matrix Effect
3.6.5. Stability
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, L.; Riese, J.; Resch, K.; Kaever, V. Impairment of macrophage eicosanoid and nitric oxide production by an alkaloid from Sinomenium acutum. Arzneimittelforschung 1994, 44, 1223–1226. [Google Scholar] [PubMed]
- Tail, Z.; Hopkin, S.J. Sinomenine: Antiarthritic antinflammatory. Drug Future 1998, 23, 45–49. [Google Scholar] [CrossRef]
- Wang, Q.; Li, X.K. Immunosuppressive and anti-inflammatory activities of sinomenine. Int. Immunopharmacol. 2011, 11, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Buchner, E.; Beitze, D.; Schmidt-Weber, C.B.; Kaever, V.; Emmrich, F.; Kinne, R.W. Amelioration of rat experimental arthritides by treatment with the alkaloid sinomenine. Int. J. Immunopharmacol. 1996, 18, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Umezawa, Y.; Ichikawa, M.; Hirata, M.; Oh-i, T.; Koga, M. A case of drug eruption caused by the crude drug Boi (Sinomenium stem/Sinomeni caulis et Rhizoma). J. Dermatol. 1995, 22, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Liu, L.; Qi, C.; Deng, B.; Cai, X. Sinomenine versus NSAIDs for the treatment of rheumatoid arthritis: A systematic review and meta-analysis. Planta Med. 2008, 74, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Jiang, Z.H.; Zhou, H.; Ma, W.Z.; Wong, Y.F.; Liu, Z.Q.; Liu, L. The pharmacokinetic study of sinomenine, paeoniflorin and paeonol in rats after oral administration of a herbal product Qingfu Guanjiesu capsule by HPLC. Biomed. Chromatogr. 2014, 28, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, X.; Zhou, Y.; Liu, Y.; Guo, M.; Zhu, Q.; Xie, Y.; Fan, Z. Development of patch and spray formulations for enhancing topical delivery of sinomenine hydrochloride. J. Pharm. Sci. 2010, 99, 1790–1799. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Jiang, Z.H.; Chan, K.; Zhou, H.; Wong, Y.F.; Bian, Z.X.; Xu, H.X.; Liu, L. Pharmacokinetic interaction of paeoniflorin and sinomenine: Pharmacokinetic parameters and tissue distribution characteristics in rats and protein binding ability in vitro. J. Pharmacol. Sci. 2005, 99, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Chan, K.; Zhou, H.; Jiang, Z.H.; Wong, Y.F.; Xu, H.X.; Liu, L. The pharmacokinetics and tissue distribution of sinomenine in rats and its protein binding ability in vitro. Life Sci. 2005, 77, 3197–3209. [Google Scholar] [CrossRef] [PubMed]
- Long, L.H.; Wu, P.F.; Chen, X.L.; Zhang, Z.; Chen, Y.; Li, Y.Y.; Jin, Y.; Chen, J.G.; Wang, F. HPLC and LC-MS analysis of sinomenine and its application in pharmacokinetic studies in rats. Acta Pharmacol. Sin. 2010, 31, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.S.; Li, G.; Liu, Z.H.; Song, S.C.; Zhu, L.C. Study on Clinical Pharmacokinetics and Bioequivalance of Sinomenine Hydrochloride Liposome Sustained-release Tablets in Healthy Volunteers. China Pharmacol. J. 2009, 44, 213–216. [Google Scholar]
- Yao, Y.M.; Tan, Z.R.; Hu, Z.Y.; Guo, X.; Cheng, Z.N.; Wang, L.S.; Zhou, H.H. Determination of sinomenine in human plasma by HPLC/ESI/ion trap mass spectrum. Clin. Chim. Acta 2005, 356, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Su, M.X.; Song, M.; Sun, D.Z.; Zhao, H.; Gu, X.; Zhu, L.; Zhan, X.L.; Xu, Z.N.; Wen, A.D.; Hang, T.J. Determination of sinomenine sustained-release capsules in healthy Chinese volunteers by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2012, 889–890, 39–43. [Google Scholar]
- Chinese Pharmacopoeia commission. In Chinese Pharmacopeia; China Medical Science Press: Beijing, China, 2010; Volume II, pp. 195–199.
- Zhang, W.; Zhang, H.; Sun, S.; Sun, F.F.; Chen, J.; Zhao, L.; Zhang, G.Q. Comparative Pharmacokinetics of Hypaconitine after Oral Administration of Pure Hypaconitine, Aconitum carmichaelii Extract and Sini Decoction to Rats. Molecules 2015, 20, 1560–1570. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the sinomenine and FTN injection are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Xiang, Z.; Dong, G.; Luo, P.; Qiu, P.; Wang, S.; Huang, B.; Wen, Y.; Wu, F.; Liu, L.; et al. An Ultra-High Performance Liquid Chromatographic-Tandem Mass Spectrometric Method for the Determination of Sinomenine in Human Plasma after Transdermal Delivery of the Zhengqing Fengtongning Injection. Molecules 2015, 20, 6454-6465. https://doi.org/10.3390/molecules20046454
Chen T, Xiang Z, Dong G, Luo P, Qiu P, Wang S, Huang B, Wen Y, Wu F, Liu L, et al. An Ultra-High Performance Liquid Chromatographic-Tandem Mass Spectrometric Method for the Determination of Sinomenine in Human Plasma after Transdermal Delivery of the Zhengqing Fengtongning Injection. Molecules. 2015; 20(4):6454-6465. https://doi.org/10.3390/molecules20046454
Chicago/Turabian StyleChen, Tingbo, Zheng Xiang, Gengting Dong, Pei Luo, Ping Qiu, Shenzhi Wang, Baoming Huang, Yingyi Wen, Feichi Wu, Liang Liu, and et al. 2015. "An Ultra-High Performance Liquid Chromatographic-Tandem Mass Spectrometric Method for the Determination of Sinomenine in Human Plasma after Transdermal Delivery of the Zhengqing Fengtongning Injection" Molecules 20, no. 4: 6454-6465. https://doi.org/10.3390/molecules20046454






