Nitric Oxide Donors and Selective Carbonic Anhydrase Inhibitors: A Dual Pharmacological Approach for the Treatment of Glaucoma, Cancer and Osteoporosis
Abstract
:1. Introduction
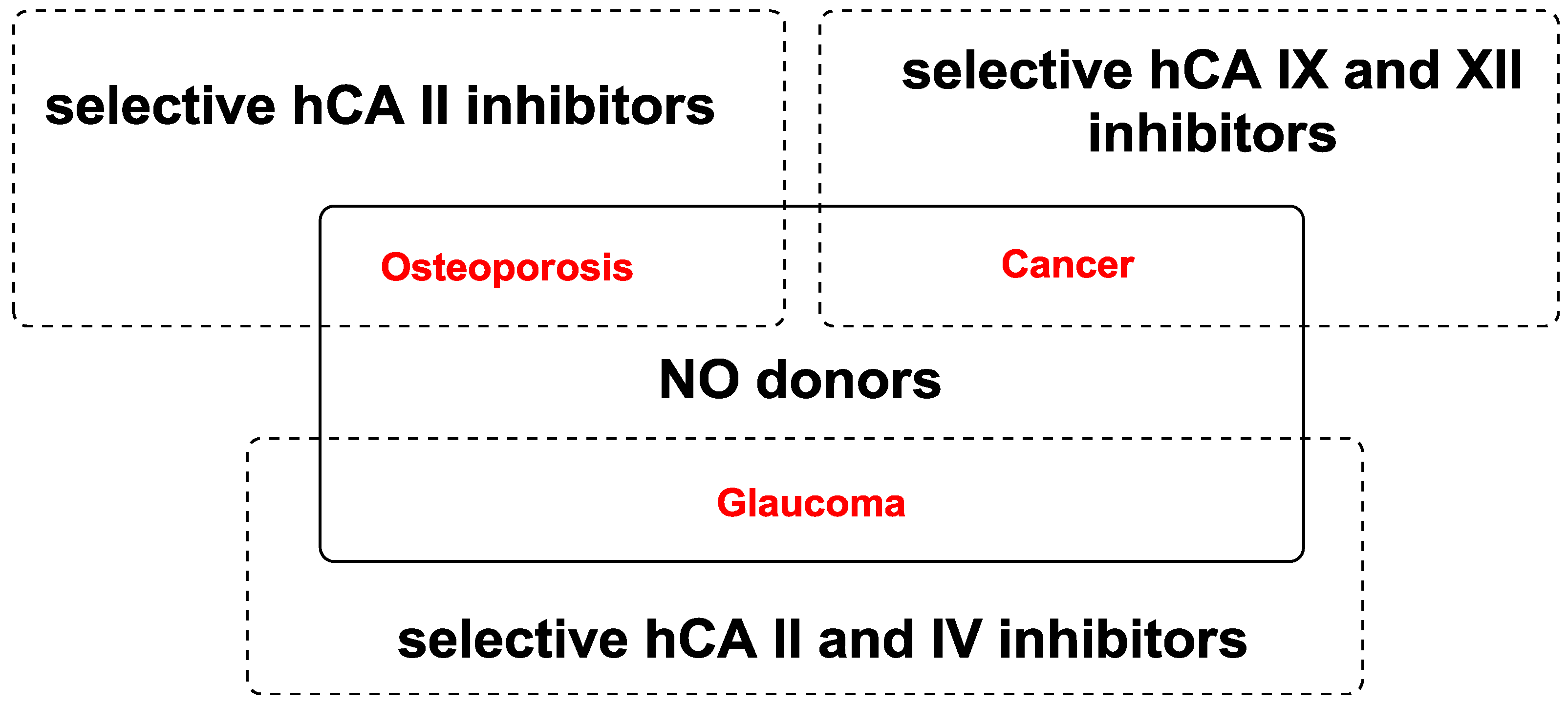
2. Discussion
2.1. Ocular Diseases
| Compound | Structure | Ki (nM) | ||
|---|---|---|---|---|
| hCA I | hCA II | hCA IV | ||
| Dorzolamide | 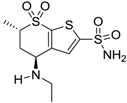 | 50,000 | 3.2 | 43 |
| Isosorbide mononitrate | 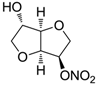 | >100,000 | >100,000 | >100,000 |
| 1 | 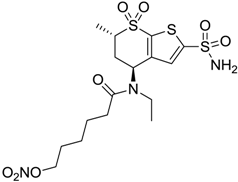 | 2950 | 14 | 4360 |
| 2 | 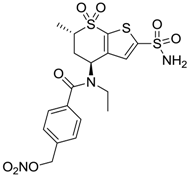 | 470 | 71 | 46 |
| 3 | 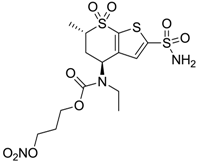 | 410 | 13 | 181 |
| 4 | 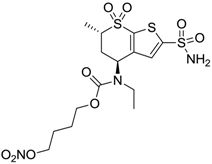 | 705 | 76 | 339 |
| 5 | 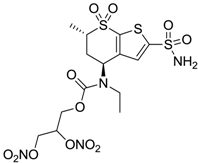 | 1520 | 63 | 3905 |
| 6 | 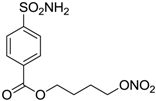 | 189 | 18 | 44 |
| 7 | 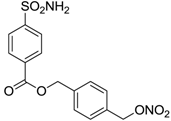 | 242 | 198 | 345 |
| 8 | 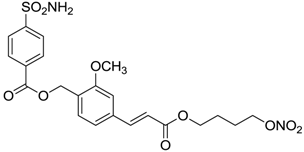 | 15 | 10 | 47 |
| 9 | 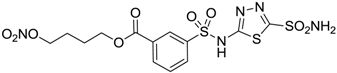 | 40 | 28 | 154 |
| 10 | 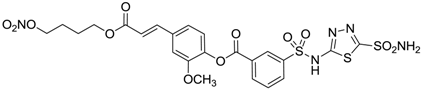 | 137 | 33 | 225 |
| 11 | 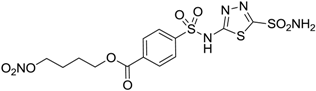 | 32 | 10 | 46 |
| 12 | 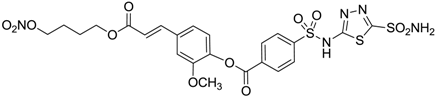 | 43 | 14 | 38 |
| 13 | 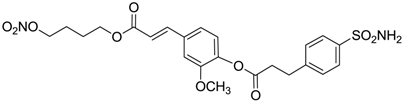 | 395 | 101 | 139 |
| 14 | 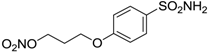 | 587 | 88 | 148 |
| 15 | 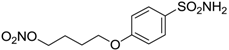 | 41 | 12 | 76 |
| 16 | 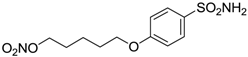 | 950 | 71 | 89 |
| 17 | 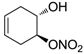 | >100,000 | >100,000 | nd |
| 18 | 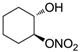 | >100,000 | >100,000 | nd |
| 19 | 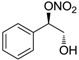 | >100,000 | >100,000 | nd |
| 20 | 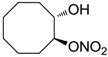 | >100,000 | >100,000 | nd |
| 21 | 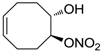 | >100,000 | >100,000 | nd |
| 22 | 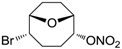 | >100,000 | >100,000 | nd |
| 23 | 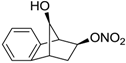 | >100,000 | >100,000 | nd |
| 24 | 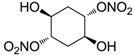 | >100,000 | >100,000 | nd |
| 25 | 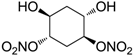 | >100,000 | >100,000 | nd |
| 26 | 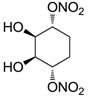 | >100,000 | >100,000 | nd |
| 27 | 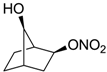 | >100,000 | >100,000 | nd |
| 28 | 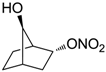 | >100,000 | >100,000 | nd |
| Dichlorophenamide | 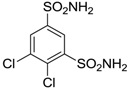 | 1200 | 38 | 15,000 |
| Acetazolamide | 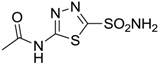 | 250 | 12 | 74 |
2.2. Cancer and Metastasis
| Compound | Structure | Ki (nM) | |
|---|---|---|---|
| hCA IX | hCA XII | ||
| Dorzolamide | 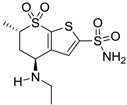 | 36 | 3.1 |
| 6 | 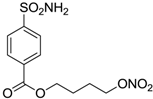 | 165 | 30 |
| 7 | 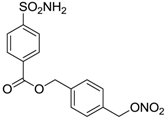 | 253 | 147 |
| 8 |  | 11 | 19 |
| 9 | 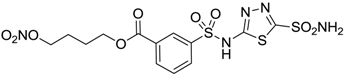 | 235 | 31 |
| 10 | 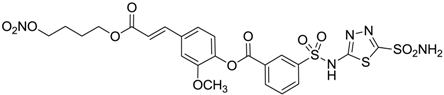 | 177 | 42 |
| 11 |  | 10 | 31 |
| 12 | 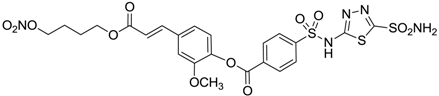 | 11 | 43 |
| 13 | 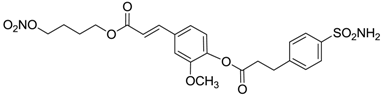 | 93 | 87 |
| 14 | 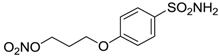 | 83 | 83 |
| 15 | 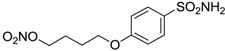 | 25 | 29 |
| 16 | 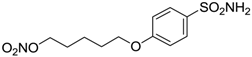 | 85 | 116 |
| Acetazolamide |  | 25 | 5.7 |
2.3. Osteoporosis
3. Biological Evaluation in vivo
| Compound * | IOP Lowering Effect | ||||
|---|---|---|---|---|---|
| Basal IOP (mmHg) | After Carbomer (mmHg) | Post-Treatment IOP (mmHg) # | ΔΔIOP (mmHg) ** | Tmax (min) *** | |
| 1 | 19.8 ± 0.7 | - | 15.9 ± 0.7 | −4.7 ± 0.7 | 60 |
| 3 | 19.2 ± 0.5 | - | 16.5 ± 0.4 | −3.7 ± 0.3 | 120 |
| 6 | 18.2 ± 0.7 | 32.4 ± 1.4 | 20.6 ± 1.8 | −15.7 ± 1.8 | 60 |
| Vehicle | 17.9 ± 0.3 | 32.4 ± 1.3 | 31.8 ± 1.9 | 0 ± 09 | - |
| Isosorbide mononitrate | 16.8 ± 0.3 | 35.3 ± 1.2 | 28.4 ± 1.6 | −6.8 ± 0.5 | 30 |
| Dorzolamide | 14.7 ± 0.9 | 35.4 ± 0.8 | 16.7 ± 0.8 | −7.4 ± 1.9 | 60 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Morphy, R.; Kay, C.; Rankovic, Z. From magic bullets to designed multiple ligands. Drug Discov. Today 2004, 9, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Megson, I.L. Recent developments in nitric oxide donor drugs. Br. J. Pharmacol. 2007, 151, 305–321. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.H.H.W.; Walter, U. NO at Work. Cell 1994, 78, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Gasco, A.; Fruttero, R.; Rolando, B. Focus on recent approaches for the development of new NO-donors. Mini-Rev. Med. Chem. 2005, 5, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Serafim, R.A.M.; Primi, M.C.; Trossini, G.H.G.; Ferreira, E.I. Nitric oxide: State of the art in drug design. Curr. Med. Chem. 2012, 19, 386–405. [Google Scholar] [CrossRef] [PubMed]
- Burgaud, J.; Ongini, E.; Soldato, P.D. Nitric oxide-releasing drugs: A novel class of effective and safe therapeutic agents. Ann. N. Y. Acad. Sci. 2002, 962, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.W.; Schoenfisch, M.H. Nitric oxide release: Part II. Therapeutic applications. Chem. Soc. Rev. 2012, 41, 3742–3752. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Structure-based drug discovery of carbonic anhydrase inhibitors. J. Enzym. Inhib. Med. Chem. 2012, 27, 759–772. [Google Scholar] [CrossRef]
- Alp, C.; Maresca, A.; Alp, N.A.; Gültekin, M.S.; Ekinci, D.; Scozzafava, A.; Supuran, C.T. Secondary/tertiary benzenesulfonamides with inhibitory action against the cytosolic human carbonic anhydrase isoforms I and II. J. Enzym. Inhib. Med. Chem. 2013, 28, 294–298. [Google Scholar] [CrossRef]
- De Monte, C.; Carradori, S.; Secci, D.; D’Ascenzio, M.; Vullo, D.; Ceruso, M.; Supuran, C.T. Cyclic tertiary sulfamates: Selective inhibition of the tumor-associated carbonic anhydrases IX and XII by N- and O-substituted acesulfame derivatives. Eur. J. Med. Chem. 2014, 84, 240–246. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzio, M.; Carradori, S.; Secci, D.; Vullo, D.; Ceruso, M.; Akdemir, A.; Supuran, C.T. Selective inhibition of human carbonic anhydrases by novel amide derivatives of probenecid: Synthesis, biological evaluation and molecular modelling studies. Bioorg. Med. Chem. 2014, 22, 3982–3988. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzio, M.; Carradori, S.; de Monte, C.; Secci, D.; Ceruso, M.; Supuran, C.T. Design, synthesis and evaluation of N-substituted saccharin derivatives as selective inhibitors of tumor-associated carbonic anhydrase XII. Bioorg. Med. Chem. 2014, 22, 1821–1831. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, K.; Carradori, S.; Monti, S.M.; Secci, D.; Vullo, D.; Supuran, C.T.; de Simone, G. An out of the active site binding pocket for carbonic anhydrase inhibitors. Chem. Commun. 2015, 51, 302–305. [Google Scholar] [CrossRef]
- Gidaro, M.C.; Alcaro, F.; Carradori, S.; Costa, G.; Vullo, D.; Supuran, C.T.; Alcaro, S. Eriocitrin and apigenin as new carbonic anhydrase V A inhibitors, from a virtual screening of Calabrian natural products. Planta Med. 2015. [Google Scholar] [CrossRef]
- Carradori, S.; de Monte, C.; D’Ascenzio, M.; Secci, D.; Celik, G.; Ceruso, M.; Vullo, D.; Scozzafava, A.; Supuran, C.T. Salen and tetrahydrosalen derivatives act as effective inhibitors of the tumor-associated carbonic anhydrase XII—A new scaffold for designing isoform-selective inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 6759–6763. [Google Scholar] [CrossRef] [PubMed]
- Alterio, V.; di Fiore, A.; D’Ambrosio, K.; Supuran, C.T.; de Simone, G. Multiple binding modes of inhibitors to carbonic anhydrases: How to design specific drugs targeting 15 different isoforms? Chem. Rev. 2012, 112, 4421–4468. [Google Scholar] [CrossRef] [PubMed]
- Fabrizi, F.; Mincione, F.; Somma, T.; Scozzafava, A.; Galassi, F.; Masini, E.; Impagnatiello, F.; Supuran, C.T. A new approach to antiglaucoma drugs: Carbonic anhydrase inhibitors with or without NO donating moieties. Mechanism of action and preliminary pharmacology. J. Enzym. Inhib. Med. Chem. 2012, 27, 138–147. [Google Scholar] [CrossRef]
- Goldstein, I.M.; Otswaldp, P.; Roth, S. Nitric oxide: A review of its role in retinal function and disease. Vis. Res. 1996, 36, 2979–2994. [Google Scholar] [CrossRef] [PubMed]
- Cavet, M.E.; Vittitow, J.L.; Impagnatiello, F.; Ongini, E.; Bastia, E. Nitric oxide (NO): An emerging target for the treatment of glaucoma. Investig. Ophtalmol. Vis. Sci. 2014, 55, 5005–5015. [Google Scholar] [CrossRef]
- Scozzafava, A.; Supuran, C.T. Glaucoma and the applications of carbonic anhydrase inhibitors. Subcell. Biochem. 2014, 75, 349–359. [Google Scholar] [PubMed]
- Carta, F.; Supuran, C.T.; Scozzafava, A. Novel therapies for glaucoma: A patent review 2007–2011. Expert Opin. Ther. Pat. 2012, 22, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Mincione, F.; Benedini, F.; Biondi, S.; Cecchi, A.; Temperini, C.; Formicola, G.; Pacileo, I.; Scozzafava, A.; Masini, E.; Supuran, C.T. Synthesis and crystallographic analysis of new sulfonamides incorporating NO-donating moieties with potent antiglaucoma action. Bioorg. Med. Chem. Lett. 2011, 21, 3216–3221. [Google Scholar] [CrossRef] [PubMed]
- Steele, R.M.; Benedini, F.; Biondi, S.; Borghi, V.; Carzaniga, L.; Impagnatiello, F.; Miglietta, D.; Chong, W.K.M.; Rajapakse, R.; Cecchi, A.; et al. Nitric oxide-donating carbonic anhydrase inhibitors for the treatment of open-angle glaucoma. Bioorg. Med. Chem. Lett. 2009, 19, 6565–6570. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, D.; Çavdar, H.; Talaz, O.; Şentürk, M.; Supuran, C.T. NO-releasing esters show carbonic anhydrase inhibitory action against human isoforms I and II. Bioorg. Med. Chem. 2010, 18, 3559–3563. [Google Scholar] [CrossRef] [PubMed]
- Wistrand, P.J.; Lindqvist, A. Carbonic Anhydrase-From Biochemistry and Genetics to Physiology and Clinical Medicine; Botrè, F., Gros, G., Storey, B.T., Eds.; VCH: Weinhein, Germany, 1991; pp. 352–378. [Google Scholar]
- Mincione, F.; Scozzafava, A.; Supuran, C.T. Antiglaucoma carbonic anhydrase inhibitors as ophthalomologic drugs. In Drug Design of Zinc-Enzyme Inhibitors: Functional, Structural, and Disease Applications; Supuran, C.T., Winum, J.Y., Eds.; Wiley: Hoboken, NJ, USA, 2009; pp. 139–154. [Google Scholar]
- Pinard, M.A.; Boone, C.D.; Rife, B.D.; Supuran, C.T.; McKenna, R. Structural study of interaction between brinzolamide and dorzolamide inhibition of human carbonic anhydrases. Bioorg. Med. Chem. 2013, 21, 7210–7215. [Google Scholar] [CrossRef] [PubMed]
- Masini, E.; Carta, F.; Scozzafava, A.; Supuran, C.T. Antiglaucoma carbonic anhydrase inhibitors: A patent review. Expert Opin. Ther. Pat. 2013, 23, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Coulter, J.A.; Mccarthy, H.O.; Xiang, J.; Roedl, W.; Wagner, E.; Robson, T.; Hirst, D.G. Nitric oxide—A novel therapeutic for cancer. Nitric Oxide 2008, 19, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.M.; Witzeling, S.D.; Damodaran, V.B.; Medeiros, T.N.; Knodle, R.D.; Edwards, M.A.; Lookian, P.P.; Brown, M.A. Applications for nitric oxide in halting proliferation of tumor cells. Biochem. Biophys. Res. Commun. 2013, 431, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Wang, L.; Mollica, M.; Re, A.T.; Wu, S.; Zuo, L. Nitric oxide in cancer metastasis. Cancer Lett. 2014, 353, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.K.V.; Azad, N.; Wangb, L.; Rojanasakul, Y. Role of S-nitrosylation in apoptosis resistance and carcinogenesis. Nitric Oxide 2008, 19, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Jamal, S.A.; Reid, L.S.; Hamilton, C.J. The effects of organic nitrates on osteoporosis: A systematic review. Osteoporos. Int. 2013, 24, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Ohta, K.; Kubozono, K.; Ishida, Y.; Naruse, T.; Takechi, M.; Kamata, N. Zolendronate inhibits receptor activator of nuclear factor kappa-B ligand induced osteoclast differentiation via suppression of expression of nuclear factor of activated T-cell c1 and carbonic anhydrase 2. Arch. Oral. Biol. 2014, 60, 557–565. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carradori, S.; Mollica, A.; De Monte, C.; Granese, A.; Supuran, C.T. Nitric Oxide Donors and Selective Carbonic Anhydrase Inhibitors: A Dual Pharmacological Approach for the Treatment of Glaucoma, Cancer and Osteoporosis. Molecules 2015, 20, 5667-5679. https://doi.org/10.3390/molecules20045667
Carradori S, Mollica A, De Monte C, Granese A, Supuran CT. Nitric Oxide Donors and Selective Carbonic Anhydrase Inhibitors: A Dual Pharmacological Approach for the Treatment of Glaucoma, Cancer and Osteoporosis. Molecules. 2015; 20(4):5667-5679. https://doi.org/10.3390/molecules20045667
Chicago/Turabian StyleCarradori, Simone, Adriano Mollica, Celeste De Monte, Arianna Granese, and Claudiu T. Supuran. 2015. "Nitric Oxide Donors and Selective Carbonic Anhydrase Inhibitors: A Dual Pharmacological Approach for the Treatment of Glaucoma, Cancer and Osteoporosis" Molecules 20, no. 4: 5667-5679. https://doi.org/10.3390/molecules20045667
APA StyleCarradori, S., Mollica, A., De Monte, C., Granese, A., & Supuran, C. T. (2015). Nitric Oxide Donors and Selective Carbonic Anhydrase Inhibitors: A Dual Pharmacological Approach for the Treatment of Glaucoma, Cancer and Osteoporosis. Molecules, 20(4), 5667-5679. https://doi.org/10.3390/molecules20045667









