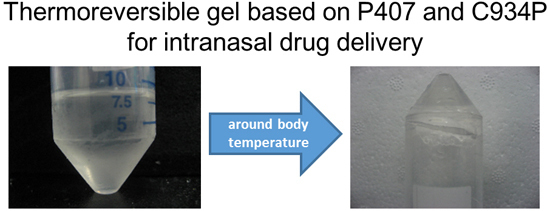
Carbopol-Incorporated Thermoreversible Gel for Intranasal Drug Delivery
Abstract
:1. Introduction
2. Results and Discussion
2.1. Gelation Temperature (Tsol-gel)
| Formulation | HP-ß-CD Concentration (%, w/v) | P407 Concentration (%, w/v) | Carbopol Type (0.1%, w/v) |
|---|---|---|---|
| P407 gel | 10 | 17 | - |
| P407/C934P gel | 10 | 17 | C934P |
| Formulation | Gelation Temperature ± S.D. (°C) |
|---|---|
| P407 gel | 29.9 ± 0.1 a |
| P407/C934P gel | 29.7 ± 0.1 |
2.2. Viscoelastic Properties of the Thermoreversible Gels
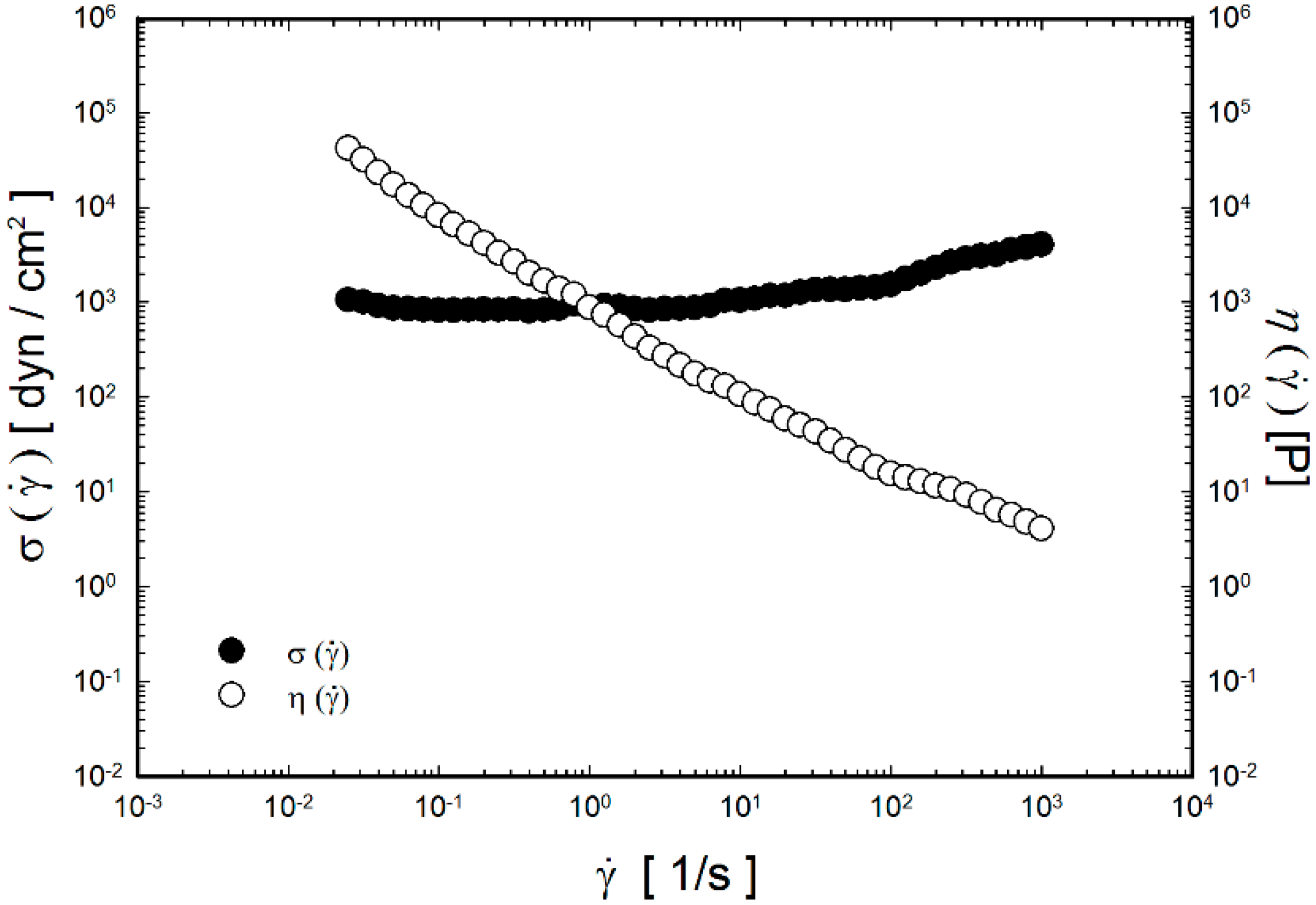
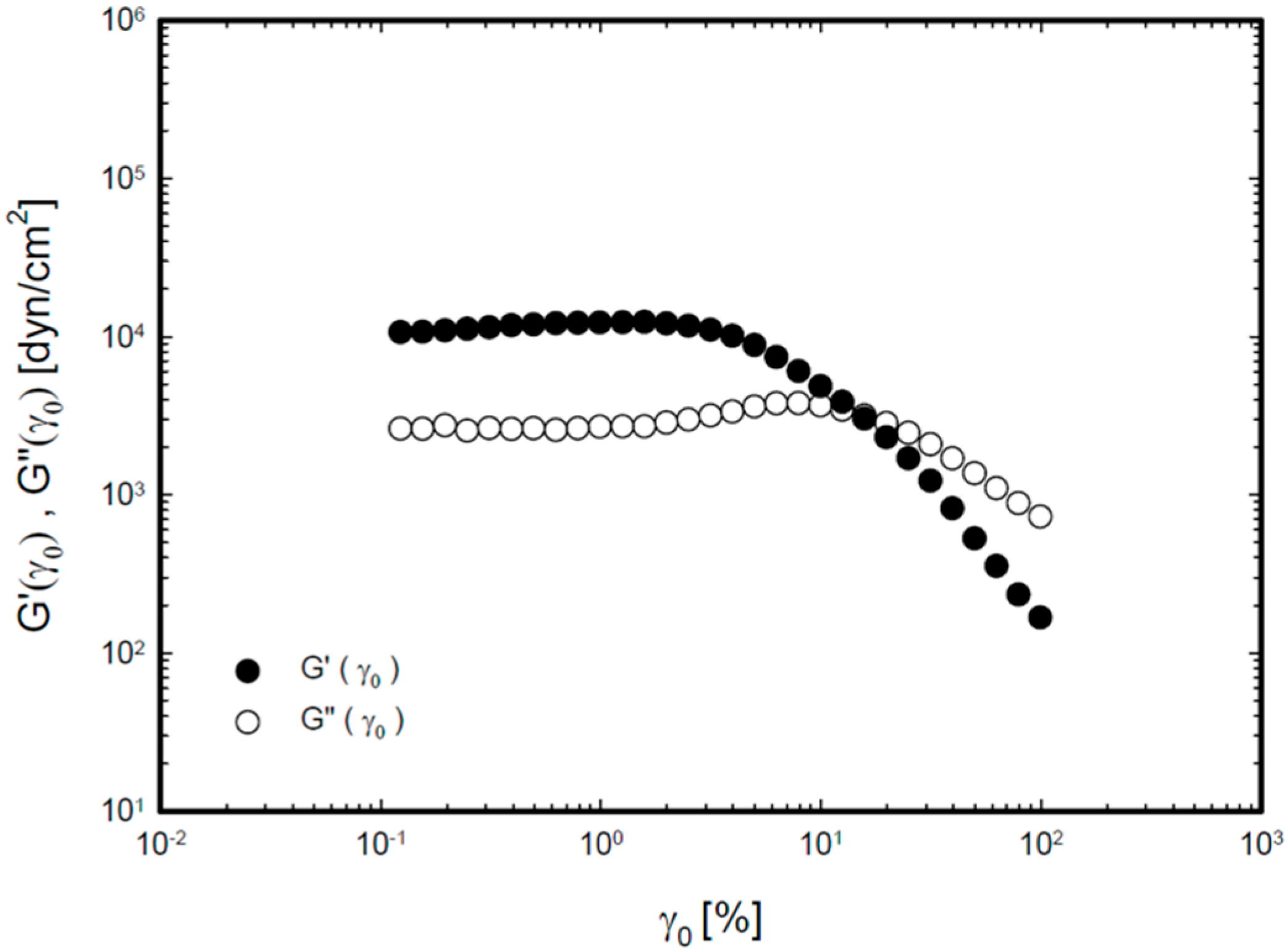
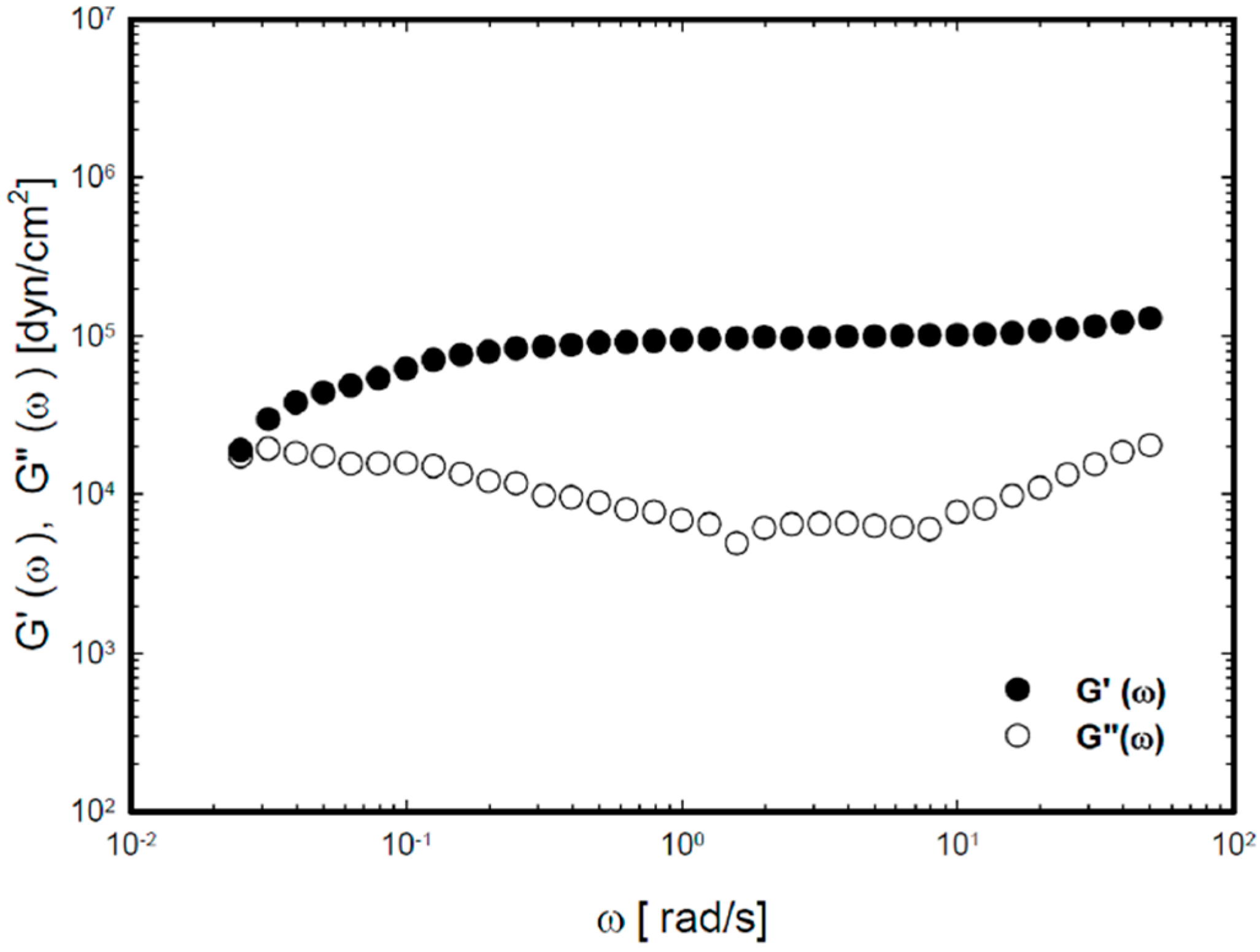
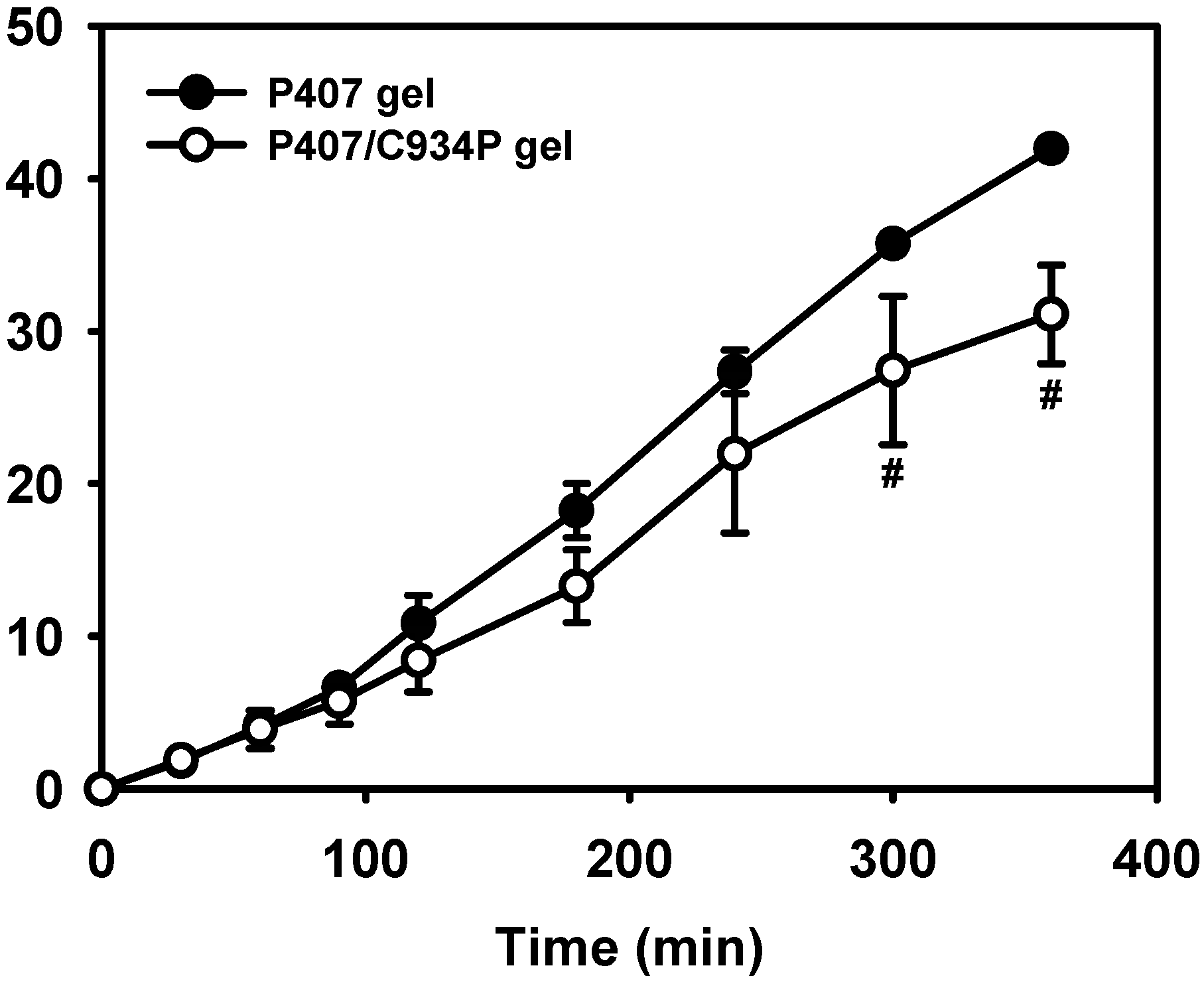
2.3. In Vitro Drug Release Study
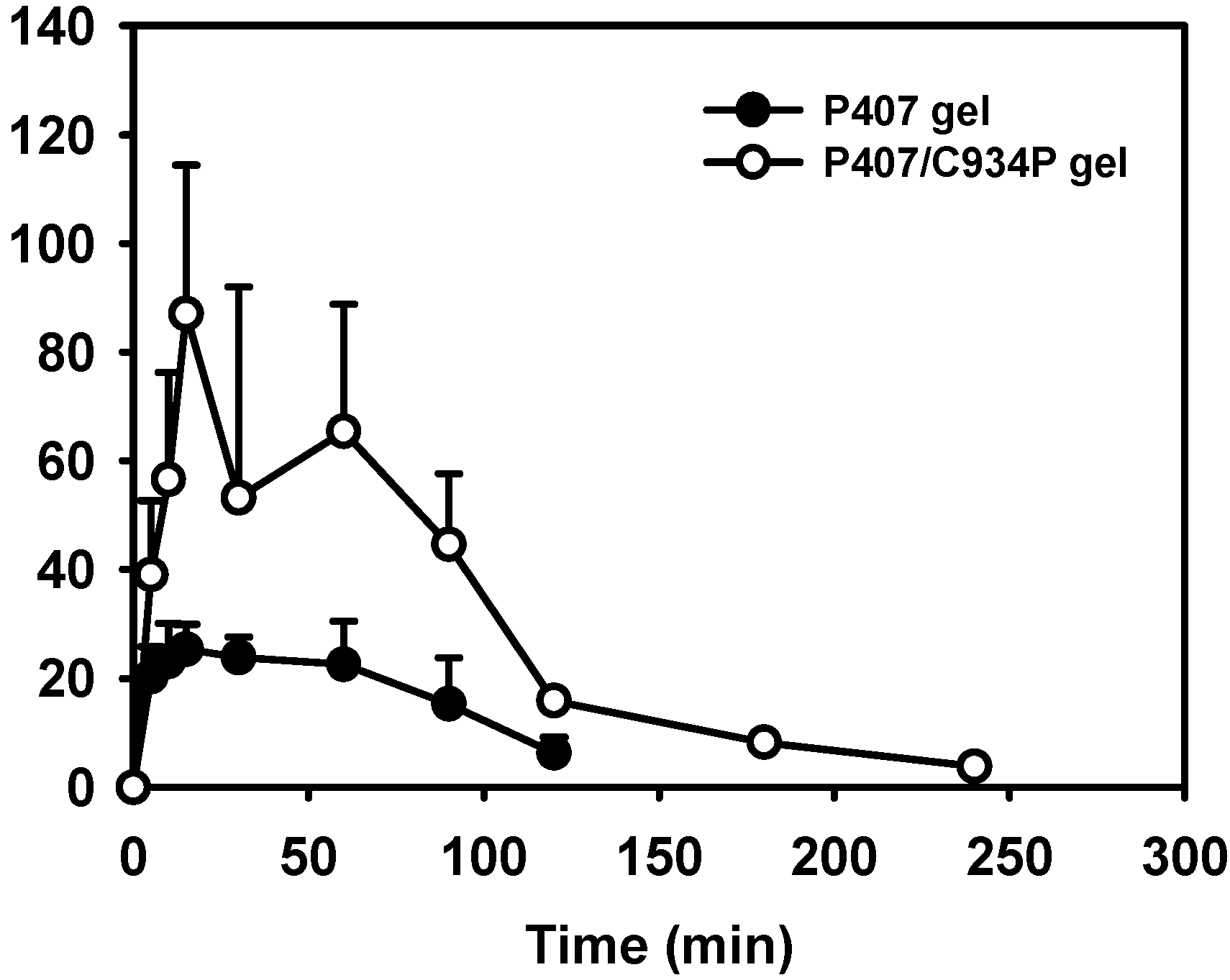
2.4. In Vivo Pharmacokinetic Study
| Administration Route (formulation) | Cmax (ng/mL) | AUC/D (kg·min/mL) × 10−6 | Relative Bioavailability (%) |
|---|---|---|---|
| IN (solution) a | 11.7 ± 1.3 * | 1264.4 ± 604.7 * | 100 |
| IN (P407 gel) | 29.5 ± 3.1 * | 5265.1 ± 1384.0 * | 416 |
| IN (P407/C934P gel) | 94.7 ± 14.3 * | 14323.6 ± 3773.6 * | 1133 |
3. Experimental Section
3.1. Materials
3.2. Preparation of P407-Based Thermoreversible Gel
3.3. Measurement of Gelation Temperature
3.4. Rheological Study
3.5. In Vitro Release Study
3.6. In Vivo Pharmacokinetic Study
3.7. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Costantino, H.R.; Illum, L.; Brandt, G.; Johnson, P.H.; Quay, S.C. Intranasal delivery: Physicochemical and therapeutic aspects. Int. J. Pharm. 2007, 337, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Sharma, S.; Garg, S. Permeability issues in nasal drug delivery. Drug Discov. Today 2002, 7, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Ugwoke, M.I.; Verbeke, N.; Kinget, R. The biopharmaceutical aspects of nasal mucoadhesive drug delivery. J. Pharm. Pharmacol. 2001, 53, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.H.; Quay, S.C. Advances in nasal drug delivery through tight junction technology. Expert Opin. Drug Deliv. 2005, 2, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Balakrishnan, P.; Park, E.K.; Song, K.W.; Hong, S.S.; Jang, T.Y.; Kim, K.S.; Chung, S.J.; Shim, C.K.; Kim, D.D. Poloxamer/cyclodextrin/chitosan-based thermoreversible gel for intranasal delivery of fexofenadine hydrochloride. J. Pharm. Sci. 2011, 100, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Cornaz, A.L.; Buri, P. Nasal mucosa as an absorption barrier. Eur. J. Pharm. Biopharm. 1994, 40, 261–270. [Google Scholar]
- Taglialatela, M.; Timmerman, H.; Annunziato, L. Cardiotoxic potential and CNS effects of first-generation antihistamines. Trends Pharmacol. Sci. 2000, 21, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Petri, N.; Tannergren, C.; Rungstad, D.; Lennernás, H. Transport characteristics of fexofenadine in the Caco-2 cell model. Pharm. Res. 2004, 21, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Varma, M.V.; Panchagnula, R. Prediction of in vivo intestinal absorption enhancement on P-glycoprotein inhibition, from rat in situ permeability. J. Pharm. Sci. 2005, 94, 1694–1704. [Google Scholar] [CrossRef] [PubMed]
- Cvetkovic, M.; Leake, B.; Fromm, M.F.; Wilkinson, G.R.; Kim, R.B. OATP and P-glycoprotein transporters mediate the cellular uptake and excretion of fexofenadine. Drug Metab. Dispos. 1999, 27, 866–871. [Google Scholar] [PubMed]
- Shimizu, M.; Fuse, K.; Okudaira, K.; Nishigaki, R.; Maeda, K.; Kusuhara, H.; Sugiyama, Y. Contribution of OATP (organic anion-transporting polypeptide) family transporters to the hepatic uptake of fexofenadine in humans. Drug Metab. Dispos. 2005, 33, 1477–1481. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Gums, J.G. Fexofenadine: Biochemical, pharmacokinetic and pharmacodynamic properties and its unique role in allergic disorders. Expert Opin. Drug Metab. Toxicol. 2009, 5, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Lappin, G.; Shishikura, Y.; Jochemsen, R.; Weaver, R.J.; Gesson, C.; Houston, B.; Oosterhuis, B.; Bjerrum, O.J.; Rowland, M.; Garner, C. Pharmacokinetics of fexofenadine: Evaluation of a microdose and assessment of absolute oral bioavailability. Eur. J. Pharm. Sci. 2010, 40, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, A.; Kume, T.; Kazama, R. Effect of oral ketoconazole on intestinal first-pass effect of midazolam and fexofenadine in cynomolgus monkeys. Drug Metab. Dispos. 2007, 35, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.X.; Ritchie, D.M.; Cousineau, M.; Danser, E.; Dewire, R.; Floden, J. Altered oral bioavailability and pharmacokinetics of P-glycoprotein substrates by coadministration of biochanin A. J. Pharm. Sci. 2006, 95, 1984–1993. [Google Scholar] [CrossRef] [PubMed]
- Strelevitz, T.J.; Foti, R.S.; Fisher, M.B. In vivo use of the P450 inactivator 1-aminobenzotriazole in the rat: Varied dosing route to elucidate gut and liver contributions to first-pass and systemic clearance. J. Pharm. Sci. 2006, 95, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- Tas, C.; Ozkan, C.K.; Savaser, A.; Ozkan, Y.; Tasdemir, U.; Altunay, H. Nasal absorption of metoclopramide from different Carbopol 981 based formulations: In vitro, ex vivo and in vivo evaluation. Eur. J. Pharm. Biopharm. 2006, 64, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Casettari, L.; Illum, L. Chitosan in nasal delivery systems for therapeutic drugs. J. Control. Release 2014, 190, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, H.S.; Tyagi, V.K.; Patil, R.R.; Dusunge, S.B. Thiolated xyloglucan: Synthesis, characterization and evaluation as mucoadhesive in situ gelling agent. Carbohydr. Polym. 2013, 91, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Dumortier, G.; Grossiord, J.L.; Zuber, M.; Couarraze, G.; Chaumeil, J.C. Rheological study of a thermoreversible morphine gel. Drug Dev. Ind. Pharm. 1991, 17, 1255–1265. [Google Scholar] [CrossRef]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Bandyopadhyay, A.K. Development and characterization of mucoadhesive in situ nasal gel of midazolam prepared with Ficus carica mucilage. AAPS PharmSciTech 2010, 11, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.M.; Kumar, A.; Pathak, K. Thermally triggered mucoadhesive in situ gel of loratadine: β-cyclodextrin complex for nasal delivery. AAPS PharmSciTech 2013, 14, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Gwak, H.; Chun, I. Formulation and evaluation of ondansetron nasal delivery systems. Int. J. Pharm. 2008, 349, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Turker, S.; Onur, E.; Ozer, Y. Nasal route and drug delivery systems. Pharm. World Sci. 2004, 26, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Zaki, N.M.; Awad, G.A.; Mortada, N.D.; Abd Elhady, S.S. Enhanced bioavailability of metoclopramide HCl by intranasal administration of a mucoadhesive in situ gel with modulated rheological and mucociliary transport properties. Eur. J. Pharm. Sci. 2007, 32, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Rathnam, G.; Narayanan, N.; Ilavarasan, R. Carbopol-based gels for nasal delivery of progesterone. AAPS PharmSciTech 2008, 9, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wiley, C.J.; Godwin, D.A.; Felton, L.A. Influence of hydroxypropyl-beta-cyclodextrin on transdermal penetration and photostability of avobenzone. Eur. J. Pharm. Biopharm. 2008, 69, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Meng, F.C.; Cui, Y.L.; Song, Y.F. Enhancing effect of hydroxypropyl-β-cyclodextrin on the intestinal absorption process of genipin. J. Agric. Food Chem. 2011, 59, 10919–10926. [Google Scholar] [CrossRef] [PubMed]
- Ameye, D.; Pringels, E.; Foreman, P.; Remon, J.P.; Adriaensens, P.; Storme, L.; Gelan, J. Correlation between the molecular morphology and the biocompatibility of bioadhesive carriers prepared from spray-dried starch/Carbopol® blends. Polymer 2005, 46, 2338–2345. [Google Scholar] [CrossRef]
- Kao, H.J.; Lin, H.R.; Lo, Y.L.; Yu, S.P. Characterization of pilocarpine-loaded chitosan/Carbopol nanoparticles. J. Pharm. Pharmacol. 2006, 58, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Schmolka, I.S. Artificial skin I. Preparation and properties of Pluronic F-127 gels for treatment of bums. J. Biomed. Mater. Res. 1972, 6, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Tirnaksiz, F.; Robinson, J.R. Rheological, mucoadhesive and release properties of pluronic F-127 gel and pluronic F-127/polycarbophil mixed gel systems. Pharmazie 2005, 60, 518–523. [Google Scholar] [PubMed]
- Huh, Y.; Cho, H.J.; Yoon, I.S.; Choi, M.K.; Kim, J.S.; Oh, E.; Chung, S.J.; Shim, C.K.; Kim, D.D. Preparation and evaluation of spray-dried hyaluronic acid microspheres for intranasal delivery of fexofenadine hydrochloride. Eur. J. Pharm. Sci. 2010, 40, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balakrishnan, P.; Park, E.-K.; Song, C.-K.; Ko, H.-J.; Hahn, T.-W.; Song, K.-W.; Cho, H.-J. Carbopol-Incorporated Thermoreversible Gel for Intranasal Drug Delivery. Molecules 2015, 20, 4124-4135. https://doi.org/10.3390/molecules20034124
Balakrishnan P, Park E-K, Song C-K, Ko H-J, Hahn T-W, Song K-W, Cho H-J. Carbopol-Incorporated Thermoreversible Gel for Intranasal Drug Delivery. Molecules. 2015; 20(3):4124-4135. https://doi.org/10.3390/molecules20034124
Chicago/Turabian StyleBalakrishnan, Prabagar, Eun-Kyoung Park, Chung-Kil Song, Hyun-Jeong Ko, Tae-Wook Hahn, Ki-Won Song, and Hyun-Jong Cho. 2015. "Carbopol-Incorporated Thermoreversible Gel for Intranasal Drug Delivery" Molecules 20, no. 3: 4124-4135. https://doi.org/10.3390/molecules20034124