Exploring the Effects of Pulsed Electric Field Processing Parameters on Polyacetylene Extraction from Carrot Slices
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of PEF Processing Parameters on Polyacetylenes
| Mild PEF Treatment Conditions * | Relative Content (%) **† | |||||||
|---|---|---|---|---|---|---|---|---|
| Electric Field (kV/cm) | Number of Pulses | Pulse Frequency (Hz) | Pulse Width (μs) | Specific Energy (KJ/kg) | Increase of Temperature | FaOH | FaDOH | FaDOAc |
| 1 | 100 | 10 | 10 | 0.1 | 0.05 ± 0.01 | 169.1 ± 8.0 | 130.4 ± 8.0 | 128.7 ± 8.2 |
| 4 | 100 | 10 | 10 | 2.4 | 0.90 ± 0.10 | 210.2 ± 11.0 | 179 ± 12.1 | 169.6 ± 10.4 |
| 1 | 1500 | 10 | 10 | 2.2 | 1.05 ± 0.20 | 190.8 ± 4.0 | 159.0 ± 3.8 | 115.4 ± 2.0 |
| 4 | 1500 | 10 | 10 | 35.6 | 6.00 ± 0.50 | 193.9 ± 2.0 | 88.6 ± 2.4 | 119.4 ± 3.0 |
| 1 | 100 | 200 | 10 | 0.1 | 0.06 ± 0.01 | 209.8 ± 1.0 | 135.7 ± 4.7 | 121.0 ± 1.3 |
| 4 | 100 | 200 | 10 | 2.4 | 1.00 ± 0.15 | 130.9 ± 2.0 | 166.3 ± 3.0 | 139.8 ± 2.5 |
| 1 | 1500 | 200 | 10 | 2.2 | 1.50 ± 0.22 | 140.0 ± 2.1 | 120.5 ± 7.0 | 70.5 ± 2.0 |
| 4 | 1500 | 200 | 10 | 35.6 | 6.40 ± 0.30 | 115.6 ± 8.0 | 97.4 ± 5.0 | 116.8 ± 4.3 |
| 1 | 100 | 10 | 30 | 0.4 | 0.31 ± 0.05 | 150.7 ± 2.0 | 142.7 ± 4.5 | 119.1 ± 3.2 |
| 4 | 100 | 10 | 30 | 7.1 | 1.30 ± 0.15 | 134.1 ± 6.0 | 126.0 ± 4.2 | 115.9 ± 4.3 |
| 1 | 1500 | 10 | 30 | 6.7 | 1.10 ± 0.14 | 127.8 ± 12.0 | 117.1 ± 6.5 | 99.3 ± 8.0 |
| 4 | 1500 | 10 | 30 | 106.7 | 10.0 ± 0.5 | 102.1 ± 7.2 | 73.6 ± 5.1 | 125.5 ± 4.0 |
| 1 | 100 | 200 | 30 | 0.4 | 0.05 ± 0.10 | 147.6 ± 3.2 | 150.2 ± 2.1 | 88.7 ± 1.3 |
| 4 | 100 | 200 | 30 | 7.1 | 1.00 ± 0.10 | 111.5 ± 5.4 | 125.5 ± 4.0 | 94.2 ± 2.0 |
| 1 | 1500 | 200 | 30 | 6.7 | 1.20 ± 0.10 | 122.8 ± 6.0 | 128.2 ± 9.1 | 115.6 ± 7.2 |
| 4 | 1500 | 200 | 30 | 106.7 | 10.0 ± 0.5 | 97.2 ± 5.0 | 72.8 ± 3.0 | 67.4 ± 2.5 |
| 1 | 800 | 105 | 20 | 2.4 | 2.20 ± 0.10 | 139.3 ± 1.4 | 139.9 ± 4.4 | 100.6 ± 4.2 |
| 4 | 800 | 105 | 20 | 37.9 | 6.20 ± 0.22 | 100.5 ± 3.0 | 119.3 ± 3.7 | 124.3 ± 2.0 |
| 2.5 | 100 | 105 | 20 | 1.9 | 0.65 ± 0.10 | 131.1 ± 4.2 | 130.7 ± 3.2 | 105.5 ± 3.5 |
| 2.5 | 1500 | 105 | 20 | 27.8 | 5.20 ± 0.20 | 57.4 ± 10.0 | 109.0 ± 9.5 | 48.2 ± 9.4 |
| 2.5 | 800 | 10 | 20 | 14.8 | 3.00 ± 0.32 | 106.0 ± 9.3 | 102.0 ± 8.1 | 86.0 ± 6.1 |
| 2.5 | 800 | 200 | 20 | 14.8 | 3.20 ± 0.50 | 104.8 ± 5.5 | 65.5 ± 8.3 | 59.1 ± 3.0 |
| 2.5 | 800 | 105 | 10 | 7.4 | 1.60 ± 0.20 | 82.7 ± 6.2 | 91.8 ± 6.0 | 92.0 ± 3.0 |
| 2.5 | 800 | 105 | 30 | 22.2 | 3.30 ± 0.10 | 96.1 ± 5.2 | 66.4 ± 3.0 | 81.8 ± 4.0 |
| 2.5 | 800 | 105 | 20 | 14.8 | 2.60 ± 0.30 | 102.7 *** ± 12.0 | 110.2 *** ± 11.3 | 78.5 *** ± 11.0 |
| F-Value | |||
|---|---|---|---|
| Source 1 | FaOH | FaDOH | FaDOAc |
| Quadratic Model | 11.74 *** | 11.65 *** | 14.79 *** |
| E | 5.11 * | 8.33 * | 4.20 * |
| n | 7.68 ** | 27.85 *** | 13.81 * |
| f | 5.24 * | 1.25 | 13.97 * |
| τ | 15.64 ** | 7.48 ** | 9.06 ** |
| E2 | 32.25 *** | 10.58 ** | 50.16 *** |
| n2 | 0.11 | 3.86 | 0.04 |
| f2 | 1.28 | 1.83 | 0.43 |
| τ2 | 0.05 | 7.23 * | 0.82 |
| E · n | 3.50 | 16.14 ** | 0.32 |
| E · f | 4.00 * | 0.03 | 0.59 |
| E · τ | 0.25 | 4.83 * | 6.25 * |
| n · f | 0.70 | 1.01 | 0.05 |
| n · τ | 0.03 | 0.05 | 5.97 * |
| τ · f | 2.27 | 1.34 | 0.02 |
| R2 | 0.75 | 0.81 | 0.82 |
| Adjusted R2 | 0.69 | 0.75 | 0.77 |
2.1.1. Falcarinol (FaOH)
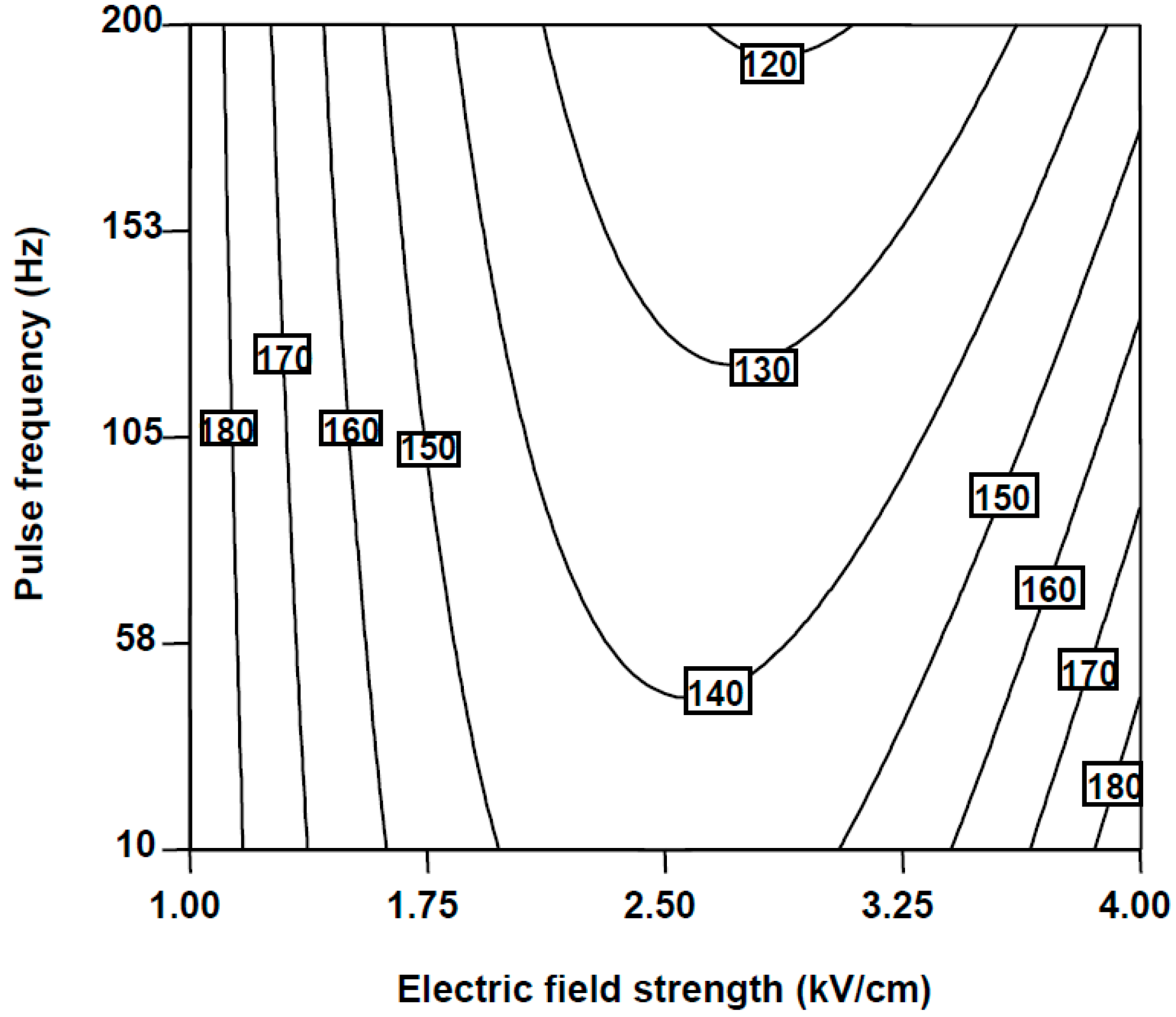
2.1.2. Falcarindiol (FaDOH)
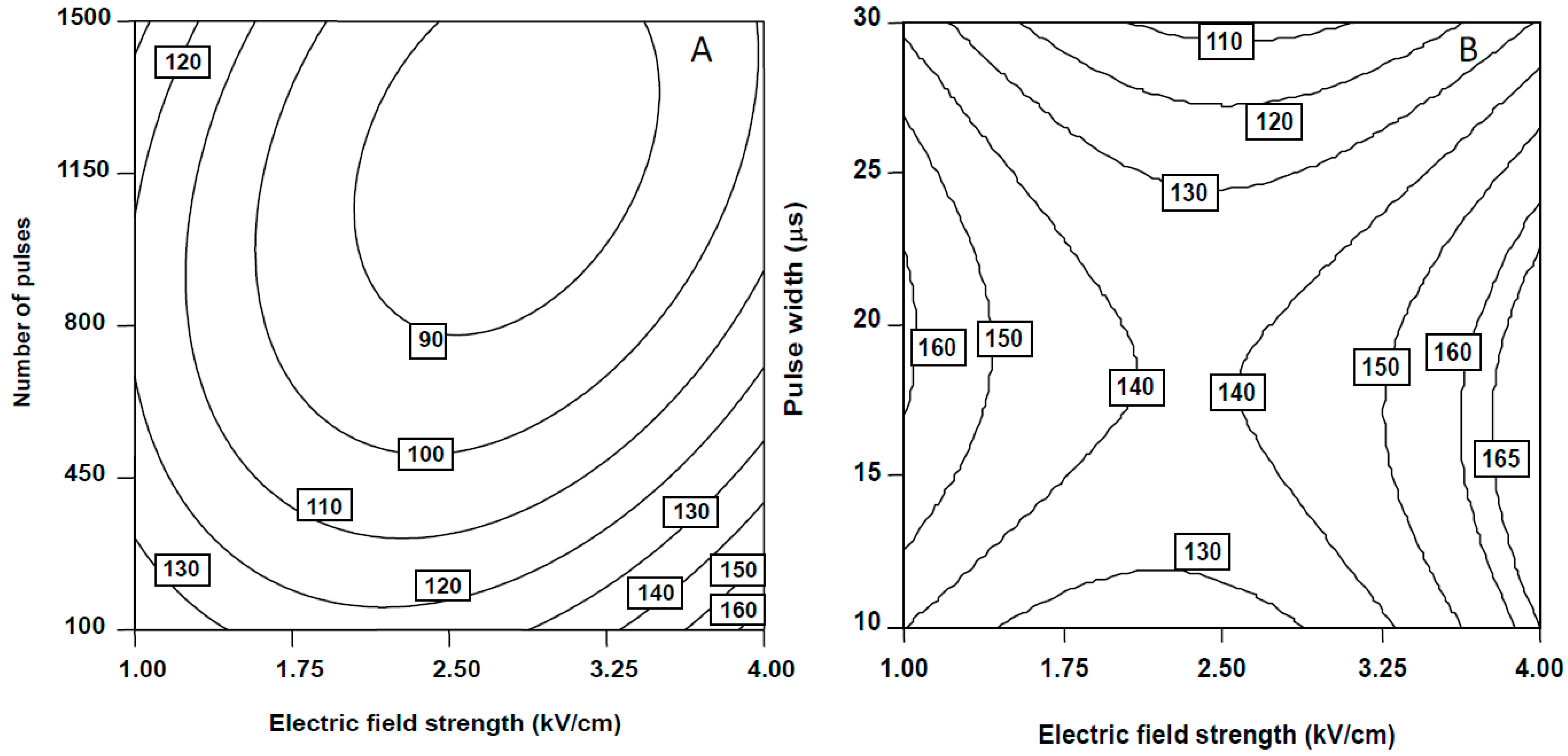
2.1.3. Falcarindiol-3-acetate (FaDOAc)

2.2. Optimization and Model Validation

| Actual Values at Optimal PEF | Predicted Values at Optimal PEF | E (%) | Desirability | |
|---|---|---|---|---|
| FaOH | 195 ± 6 | 188.0 | 3.58 | 0.910 |
| FaDOH | 170 ± 5 | 164.9 | 2.98 | 0.910 |
| FaDOAc | 168 ± 2 | 166.8 | 0.68 | 0.910 |
3. Experimental Section
3.1. Chemicals
3.2. Preparation of Carrot Slices
3.3. PEF Conditions
3.4. Extraction of Polyacetylenes
3.5. Separation and Quantification of Polyacetylenes
3.6. Experiment Design
3.7. Model Validation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zidorn, C.; Jöhrer, K.; Ganzera, M.; Schubert, B.; Sigmund, E.M.; Mader, J.; Greil, R.; Ellmerer, E.P.; Stuppner, H. Polyacetylenes from the apiaceae vegetables carrot, celery, fennel, parsley, and parsnip and their cytotoxic activities. J. Agric. Food Chem. 2005, 53, 2518–2523. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.P.; Brandt, K. Bioactive polyacetylenes in food plants of the Apiaceae family: Occurrence, bioactivity and analysis. J. Pharm. Biomed. Anal. 2006, 41, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, T.; Furumi, K.; Fujii, H.; Okabe, H.; Mihashi, K.; Nakano, Y.; Matsunaga, H.; Katano, M.; Mori, M. Antiproliferative constituents from Umbelliferae plants. V. A new furanocoumarin and falcarindiol furanocoumarin ethers from the root of Angelica japonica. Chem. Pharm. Bull. 1999, 47, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Purup, S.; Larsen, E.; Christensen, L.P. Differential effects of falcarinol and related aliphatic C17-polyacetylenes on intestinal cell proliferation. J. Agric. Food Chem. 1999, 57, 8290–8296. [Google Scholar] [CrossRef]
- Miyazawa, M.; Shimamura, H.; Bhuva, R.C.; Nakamura, S.; Kameoka, H. Antimutagenic activity of falcarindiol from Peucedanum praeruptorum. J. Agric. Food Chem. 1996, 44, 3444–3448. [Google Scholar] [CrossRef]
- Alanko, J.; Kurahashi, Y.; Yoshimoto, T.; Yamamoto, S.; Baba, K. Panaxynol, a polyacetylene compound isolated from oriental medicines; inhibits mammalian lipoxygenases. Biochem. Pharmacol. 1994, 48, 1979–1981. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.M.; Lundgaard, N.H.; Light, M.E.; Stafford, G.I.; van Staden, J.; Jäger, A.K. The polyacetylene falcarindiol with COX-1 activity isolated from Aegopodium podagraria L. J. Ethnopharmacol. 2007, 113, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Rawson, A.; Koidis, A.; Patras, A.; Tuohy, M.G.; Brunton, N.P. Modelling the effect of water immersion thermal processing on polyacetylene levels and instrumental colour of carrot disks. Food Chem. 2010, 121, 62–68. [Google Scholar] [CrossRef]
- Rawson, A.; Tiwari, B.K.; Tuohy, M.G.; O’Donnell, C.P.; Brunton, N. Effect of ultrasound and blanching pretreatments on polyacetylene and carotenoid content of hot air and freeze dried carrot discs. Ultrason. Sonochem. 2011, 18, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Bohlmann, F.; Burkhardt, F.; Zdero, C. Naturally Occurring Acetylenes; Academic Press Inc.: London, UK, 1973. [Google Scholar]
- Pferschy-Wenzig, E.M.; Getzinger, V.; Kunert, O.; Woelkart, K.; Zahrl, J.; Bauer, R. Determination of falcarinol in carrot (Daucus carota L.) genotypes using liquid chromatography/mass spectrometry. Food Chem. 2009, 114, 1083–1090. [Google Scholar] [CrossRef]
- Donsì, F.; Ferrari, G.; Pataro, G. Applications of pulsed electric field treatments for the enhancement of mass transfer from vegetable tissue. Food Eng. Rev. 2010, 2, 109–130. [Google Scholar] [CrossRef]
- Toepfl, S.; Mathys, A.; Heinz, V.; Knorr, D. Review: Potential of high hydrostatic pressure and pulsed electric fields for energy efficient and environmentally friendly food processing. Food Rev. Int. 2006, 22, 405–4203. [Google Scholar] [CrossRef]
- El-Belghiti, K.; Rabhi, Z.; Vorobiev, E. Effect of process parameters on solute centrifugal extraction from electropermeabilized carrot gratings. Food Bioprod. Process. 2007, 85, 24–28. [Google Scholar] [CrossRef]
- Jemai, A.B.; Vorobiev, E. Effect of moderate electric field pulses on the diffusion coefficient of soluble substances from apple slices. Int. J. Food Sci. Technol. 2002, 37, 73–86. [Google Scholar] [CrossRef]
- Loginova, K.V.; Shynkaryk, M.V.; Lebovka, N.I.; Vorobiev, E. Acceleration of soluble matter extraction from chicory with pulsed electric fields. J. Food Eng. 2010, 96, 374–379. [Google Scholar] [CrossRef]
- Aguiló-Aguayo, I.; Hossain, M.B.; Brunton, N.; Lyng, J.; Valverde, J.; Rai, D.K. Pulsed electric fields pre-treatment of carrot purees to enhance their polyacetylene and sugar contents. Innov. Food Sci. Emerg. 2014. [Google Scholar] [CrossRef]
- Czepa, A.; Hofmann, T. Quantitative studies and sensory analysis on the influence of cultivar; spatial tissue distribution and industrial processing on the bitter off-taste of carrots (Daucus carota L.) and carrot products. J. Agric. Food Chem. 2004, 52, 4508–4514. [Google Scholar] [CrossRef] [PubMed]
- Kidmose, U.; Hansen, S.L.; Christensen, L.P.; Edelenbos, M.; Larsen, E.; Nørbæk, R. Effects of genotype; root size, storage, and processing on bioactive compounds in organically grown carrots (Daucus carota L.). J. Food Sci. 2004, 69, S388–S394. [Google Scholar] [CrossRef]
- Mercier, J.; Ponnampalam, R.; Bérard, L.S.; Arul, J. Polyacetylene content and UV-induced 6-methoxymellein accumulation in carrot cultivars. J. Sci. Food Agric. 2006, 63, 313–317. [Google Scholar] [CrossRef]
- López, N.; Puértolas, E.; Condón, S.; Raso, J.; Alvarez, I. Enhancement of the extraction of betanine from red beetroot by pulsed electric fields. J. Food Eng. 2009, 90, 60–66. [Google Scholar] [CrossRef]
- Puértolas, E.; Cregenzán, O.; Luengo, E.; Alvarez, I.; Raso, J. Pulsed-electric-field-assisted extraction of anthocyanins from purple-fleshed potato. Food Chem. 2013, 136, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Søltoft, M.; Eriksen, M.R.; Träger, A.W.B.; Nielsen, J.; Laursen, K.H.; Husted, S.; Halekoh, U.; Knuthsen, P. Comparison of polyacetylene content in organically and conventionally grown carrots using a fast ultrasonic liquid extraction method. J. Agric. Food Chem. 2010, 58, 7673–7679. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguiló-Aguayo, I.; Abreu, C.; Hossain, M.B.; Altisent, R.; Brunton, N.; Viñas, I.; Rai, D.K. Exploring the Effects of Pulsed Electric Field Processing Parameters on Polyacetylene Extraction from Carrot Slices. Molecules 2015, 20, 3942-3954. https://doi.org/10.3390/molecules20033942
Aguiló-Aguayo I, Abreu C, Hossain MB, Altisent R, Brunton N, Viñas I, Rai DK. Exploring the Effects of Pulsed Electric Field Processing Parameters on Polyacetylene Extraction from Carrot Slices. Molecules. 2015; 20(3):3942-3954. https://doi.org/10.3390/molecules20033942
Chicago/Turabian StyleAguiló-Aguayo, Ingrid, Corina Abreu, Mohammad B. Hossain, Rosa Altisent, Nigel Brunton, Inmaculada Viñas, and Dilip K. Rai. 2015. "Exploring the Effects of Pulsed Electric Field Processing Parameters on Polyacetylene Extraction from Carrot Slices" Molecules 20, no. 3: 3942-3954. https://doi.org/10.3390/molecules20033942







