Inhibitors of the AAA+ Chaperone p97
Abstract
:1. Introduction
2. p97 Structure and Function

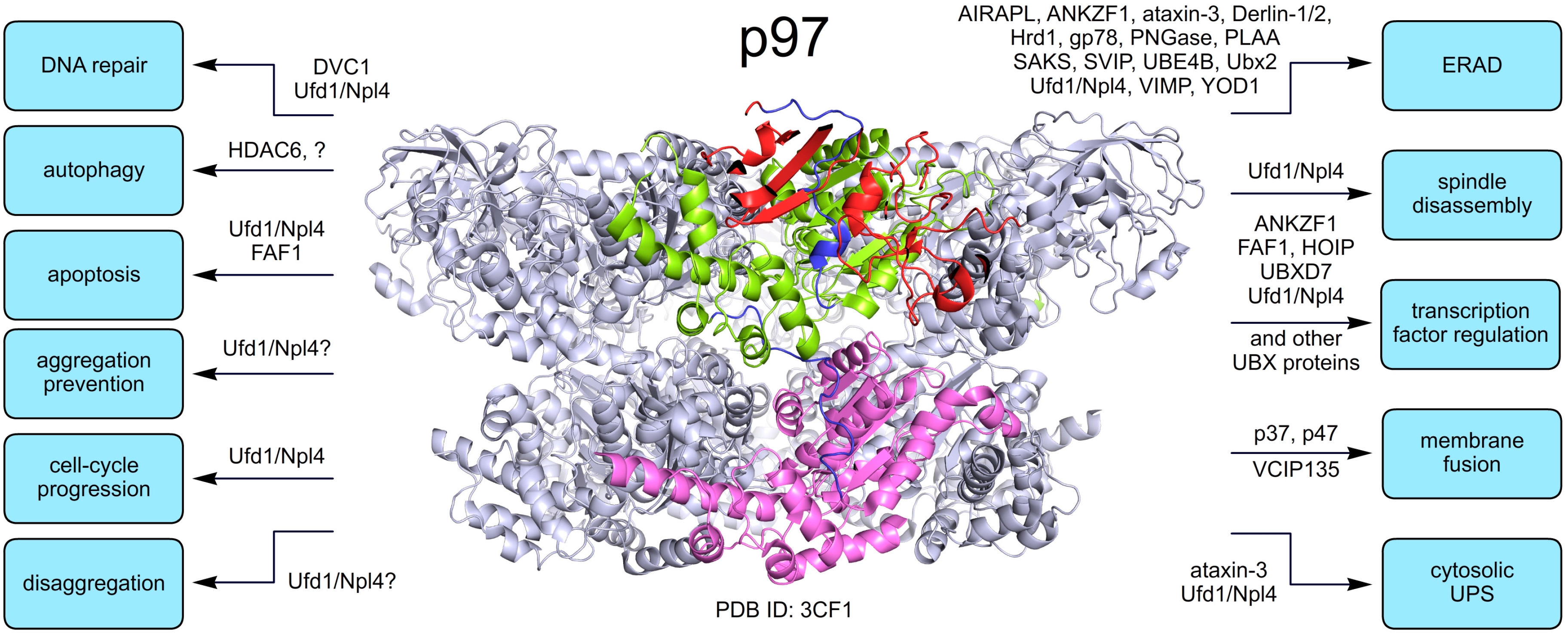
3. Pathologic Role of p97
4. Protein Quality Control as a Drug Target
5. Eeyarestatin I
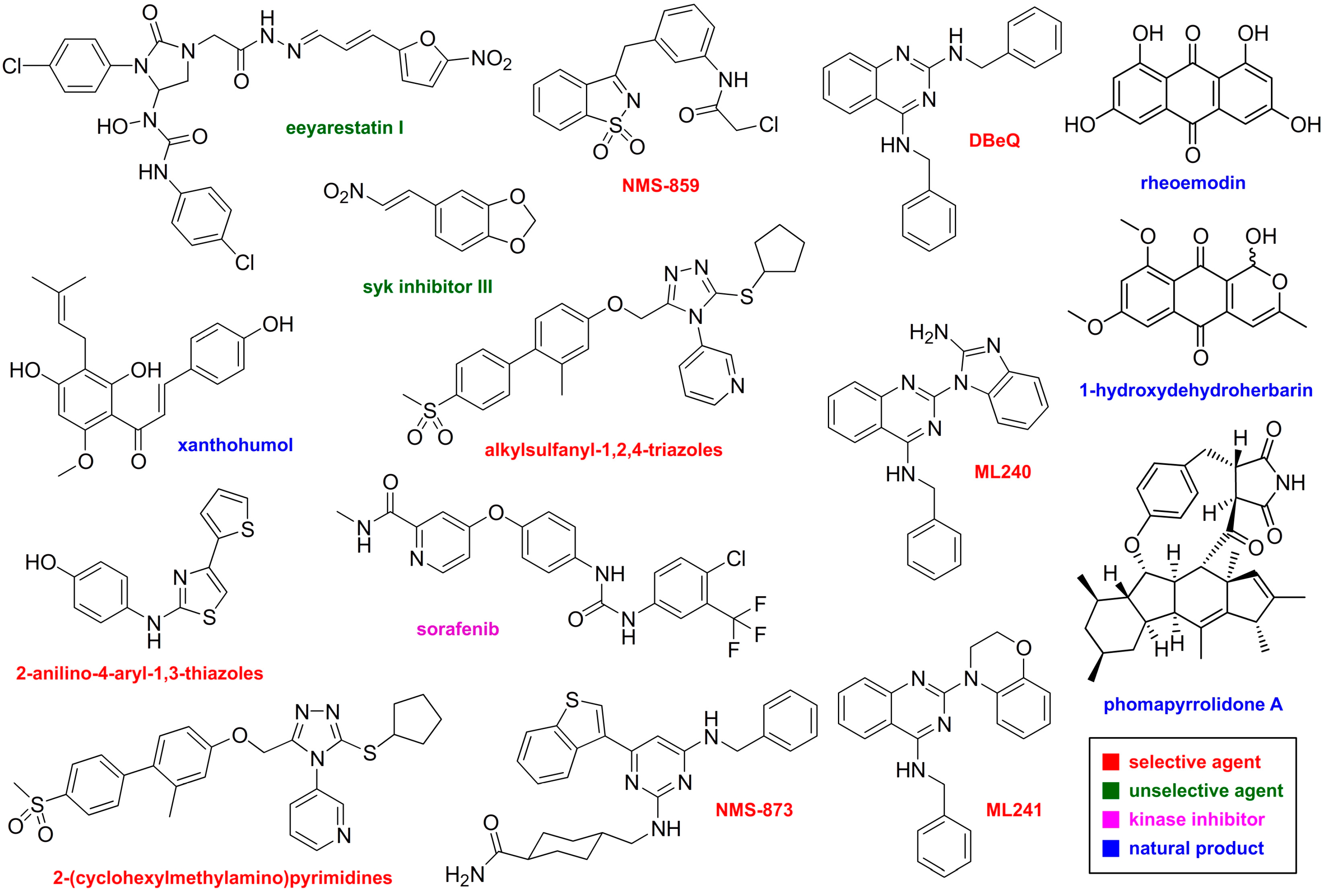
6. 2-Anilino-4-aryl-1,3-thiazoles
7. Syk Inhibitor III
8. Quinazolines
9. Alkylsulfanyl-1,2,4-triazoles
10. Xanthohumol
11. Natural Product p97 Inhibitors from Functional Chromatography
12. 2-(Cyclohexylmethylamino)pyrimidines
| Compound | Mechanism | p97 IC50 (µM) | Selectivity | Cellular |
|---|---|---|---|---|
| Eeyarestatin I | Allosteric | 5–10 (Kd) | Does not stabilize ODD-Luc or Luc-ODC | UbG76VGFP IC50 3.7 µM |
| 2-anilino-4-aryl-1,3-thiazole-2-thiophene | ATP-sensitive | 0.11 | Not reported | Ub-Luc stabilization EC50 0.09 µM |
| Syk inhibitor III | Covalent; modifies D2 ATPase pocket | 1.7 | ODD-Luc IC50 5.9 µM Luc-ODC IC50 > 30 µM | UbG76VGFP IC50 1.6 µM |
| Alkylsulfanyl-1,2-4-triazoles | Allosteric | 0.063 | IC50 0.38 (HCT116 cells) | |
| DBeQ | ATP-sensitive; binds D1 and D2 | 2.6 | ODD-Luc IC50 56 µM Luc-ODC IC50 45 µM | UbG76VGFP IC50 2.3 µM Extensive cellular studies |
| ML-240 | ATP-sensitive; D2 selective | 0.11 | ODD-Luc IC50 28 µM | UbG76VGFP IC50 0.9 µM Extensive cellular studies |
| ML-241 | ATP-sensitive; D2 selective | 0.11 | ODD-Luc IC50 46 µM | UbG76VGFP IC50 3.5 µM Extensive cellular studies |
| NMS-859 | Covalent; binds D2 ATP pocket | 0.37 | NSF, SPATA5, VPS4B, RuvBL1, HSP90, 50 kinases > 10 µM | Extensive cellular studies; toxicity and p97 specific pathways |
| NMS-873 | Allosteric; D1-D2 interface | 0.02 | NSF, SPATA5, VPS4B, RuvBL1, HSP90, 50 kinases > 10 µM | Extensive cellular studies; toxicity and p97 specific pathways |
| Xanthohumol | Binds to N-domains | Not reported | Not reported | Examined ERAD, UPR, and autophagy |
| Rheoemodin | ATP-sensitive; D1 selective | 39.8 | NSF, ClpX, GroEL > 200 µM; no protection of CD3δ | UbG76VGFP and TCRα stabilized, CD3δ not stabilized, poly-Ub increased, UPR activated, autophagy inhibited, apoptosis activated |
| 1–Hydroxydehydroherbarin | Allosteric; unknown binding site | 21.7 | NSF, GroEL > 200 µM; no protection of CD3δ | UbG76VGFP and TCRα stabilized, CD3δ not stabilized, poly-Ub increased, UPR activated, autophagy inhibited, apoptosis activated |
| Phomapyrrolidone A | Allosteric; unknown binding site | 6.6 | NSF, GroEL > 200 µM; no protection of CD3δ | UbG76VGFP and TCRα stabilized, CD3δ not stabilized, poly-Ub increased, UPR activated, autophagy inhibited, apoptosis activated |
| 2-(Cyclohexyl-methylamino)pyrimidine | ATP-sensitive; unknown binding site | 0.074 µM | NSF, SPATA5, VPS4B, RuvBL1, HSP90, 50 kinases > 10 µM | IC50 5.82 (HCT116); poly-Ub increased; UPR increased |
13. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Braun, R.J.; Zischka, H. Mechanisms of Cdc48/VCP-mediated cell death: From yeast apoptosis to human disease. Biochim. Biophys. Acta 2008, 1783, 1418–1435. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.S. p97-containing complexes in proliferation control and cancer: Emerging culprits or guilt by association? Genes Cancer 2010, 1, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Chapman, E.; Fry, A.N.; Kang, M. The complexities of p97 function in health and disease. Mol. Biosyst. 2011, 7, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Valle, C.W.; Min, T.; Bodas, M.; Mazur, S.; Begum, S.; Tang, D.; Vij, N. Critical role of VCP/p97 in the pathogenesis and progression of non-small cell lung carcinoma. PLoS One 2011, 6, e29073. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hei, Y.; Shu, Q.; Dong, J.; Gao, Y.; Fu, H.; Zheng, X.; Yang, G. VCP/p97, down-regulated by microRNA-129-5p, could regulate the progression of hepatocellular carcinoma. PLoS One 2012, 7, e35800. [Google Scholar] [CrossRef] [PubMed]
- Laguë, M.N.; Romieu-Mourez, R.; Bonneil, É.; Boyer, A.; Pouletty, N.; Mes-Masson, A.M.; Thibault, P.; Nadeau, M.È.; Boerboom, D. Proteomic profiling of a mouse model for ovarian granulosa cell tumor identifies VCP as a highly sensitive serum tumor marker in several human cancers. PLoS One 2012, 7, e42470. [Google Scholar] [CrossRef] [PubMed]
- Nishikori, S.; Yamanaka, K.; Sakurai, T.; Esaki, M.; Ogura, T. p97 Homologs from Caenorhabditis elegans, CDC-48.1 and CDC-48.2, suppress the aggregate formation of huntingtin exon1 containing expanded polyQ repeat. Genes Cells 2008, 13, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Dolan, P.J.; Jin, Y.N.; Hwang, W.; Johnson, G.V. Decreases in valosin-containing protein result in increased levels of tau phosphorylated at Ser262/356. FEBS Lett. 2011, 585, 3424–3349. [Google Scholar] [CrossRef] [PubMed]
- Kuhlbrodt, K.; Janiesch, P.C.; Kevei, É.; Segref, A.; Barikbin, R.; Hoppe, T. The Machado-Joseph disease deubiquitylase ATX–3 couples longevity and proteostasis. Nat. Cell Biol. 2011, 13, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Laço, M.N.; Cortes, L.; Travis, S.M.; Paulson, H.L.; Rego, A.C. Valosin-containing protein (VCP/p97) is an activator of wild-type ataxin-3. PLoS One 2012, 7, e43563. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, Y.; Soetandyo, N.; Baek, K.; Hegde, R.; Ye, Y. A ubiquitin ligase-associated chaperone holdase maintains polypeptides in soluble states for proteasome degradation. Mol. Cell 2011, 42, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Donsante, A.; Kennerson, M.L.; Mercer, J.F.; Garbern, J.Y.; Kaler, S.G. Altered intracellular localization and valosin-containing protein (p97 VCP) interaction underlie ATP7A-related distal motor neuropathy. Hum. Mol. Genet. 2012, 21, 1794–1807. [Google Scholar] [CrossRef] [PubMed]
- Imamura, S.; Yabu, T.; Yamashita, M. Protective role of cell division cycle 48 (CDC48) protein against neurodegeneration via ubiquitin-proteasome system dysfunction during zebrafish development. J. Biol. Chem. 2012, 287, 23047–23056. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.Z.; Nalbandian, A.; Hsu, C.-I.; Li, S.; Llewellyn, K.J.; Mozaffar, T.; Kimonis, V.E.; Weiss, J.H. Slow development of ALS-like spinal cord pathology in mutant valosin-containing protein gene knock–in mice. Cell Death Dis. 2012, 3, e374. [Google Scholar] [CrossRef] [PubMed]
- Majouniea, E.; Traynora, B.J.; Chiòc, A.; Restagnod, G.; Mandriolie, J.; Benatarf, M.; Taylorg, J.P.; Singletona, A.B. Mutational analysis of the VCP gene in Parkinson’s disease. Neurobiol. Aging 2012, 33, 209.e1–209.e2. [Google Scholar] [PubMed]
- Meyer, H.; Weihl, C.C. The VCP/p97 system at a glance: Connecting cellular function to disease pathogenesis. J. Cell Sci. 2014, 127, 3877–3883. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.P. From neurodevelopment to neurodegeneration: The interaction of neurofibromin and valosin—containing protein/p97 in regulation of dendritic spine formation. J. Biomed. Sci. 2012, 19. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, L.; Ye, Y. Inhibition of p97-dependent protein degradation by Eeyarestatin I. J. Biol. Chem. 2008, 283, 7445–7454. [Google Scholar] [CrossRef] [PubMed]
- Bursavich, M.G.; Parker, D.P.; Willardsen, J.A.; Gao, Z.H.; Davis, T.; Ostanin, K.; Robinson, R.; Peterson, A.; Cimbora, D.M.; Zhu, J.F.; et al. 2-Anilino-4-aryl-1,3-thiazole inhibitors of valosin-containing protein (VCP or p97). Bioorg. Med. Chem. Lett. 2010, 20, 1677–1679. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.F.; Brown, S.J.; Minond, D.; Nordin, B.E.; Li, K.; Jones, A.C.; Chase, P.; Porubsky, P.R.; Stoltz, B.M.; Schoenen, F.J.; et al. Reversible inhibitor of p97, DBeQ, impairs both ubiquitin-dependent and autophagic protein clearance pathways. Proc. Natl. Acad. Sci. USA 2011, 108, 4834–4839. [Google Scholar] [CrossRef] [PubMed]
- Polucci, P.; Magnaghi, P.; Angiolini, M.; Asa, D.; Avanzi, N.; Badari, A.; Bertrand, J.; Casale, E.; Cauteruccio, S.; Cirla, A.; et al. Alkylsulfanyl-1,2,4-triazoles, a new class of allosteric valosine containing protein inhibitors. Synthesis and structure-activity relationships. J. Med. Chem. 2013, 56, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.F.; Kelin, L.; Porubsky, P.; Frankowski, K.; Schoenen, F.J.; Deshaies, R. Structure-activity relationship study reveals ML240 and ML241 as potent and selective inihibitors of p97 ATPase. Chem. Med. Chem. 2013, 8, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Magnaghi, P.; D’Alessio, R.; Valsasina, B.; Avanzi, N.; Rizzi, S.; Asa, D.; Gasparri, F.; Cozzi, L.; Cucchi, U.; Orrenius, C.; et al. Covalent and allosteric inhibitors of the ATPase VCP/p97 induce cancer cell death. Nat. Chem. Biol. 2013, 9, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.J.; Gui, L.; Zhang, X.; Moen, D.R.; Li, K.; Frankowski, K.J.; Lin, H.J.; Schoenen, F.J.; Chou, T.F. Evaluating p97 inhibitor analogues for their domain selectivity and potency against the p97–p47 complex. Chem. Med. Chem. 2014. [Google Scholar] [CrossRef]
- Kang, M.J.; Wu, T.; Wijeratne, E.M.; Lau, E.C.; Mason, D.J.; Mesa, C.; Tillotson, J.; Zhang, D.D.; Gunatilaka, A.A.; la Clair, J.J.; et al. Functional chromatography reveals three natural products that target the same protein with distinct mechanisms of action. Chem. Bio. Chem. 2014, 15, 2125–2131. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, S.; Rumpf, S. Cdc48 (p97): A “molecular gearbox” in the ubiquitin pathway? Trends Biochem. Sci. 2007, 32, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Halawani, D.; Latterich, M. The cell’s molecular purgatory? Mol. Cell 2006, 22, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y. Diverse functions with a common regulator: Ubiquitin takes command of an AAA ATPase. J. Struct. Biol. 2006, 156, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Neeraj, V. AAA ATPase p97/VCP: Cellular functions, disease and therapeutic potential. J. Cell Mol. Med. 2008, 12, 2511–2518. [Google Scholar] [CrossRef] [PubMed]
- Stolz, A.; Hilt, W.; Buchberger, A.; Wolf, D.H. Cdc48: A power machine in protein degradation. Trends Biochem. Sci. 2011, 36, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K.; Sasagawa, Y.; Ogura, T. Recent advances in p97/VCP/Cdc48 cellular functions. Biochim. Biophys. Acta 2012, 1823, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Fessart, D.; Marza, E.; Taouji, S.; Delom, F.; Chevet, E. P97/CDC-48: Proteostasis control in tumor cell biology. Cancer Lett. 2013, 337, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Pye, V.E.; Dreveny, I.; Briggs, L.C.; Sands, C.; Beuron, F.; Zhang, X.; Freemont, P.S. Going through the motions: The ATPase cycle of p97. J. Struct. Biol. 2006, 156, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.K.; Xia, D. Structural and functional deviations in disease-associated p97 mutants. J. Struct. Biol. 2012, 179, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Deshaies, R.J. Proteotoxic crisis, the ubiquitin-proteasome system, and cancer therapy. BMC Biol. 2014, 12. [Google Scholar] [CrossRef]
- Zhang, X.; Shaw, A.; Bates, P.A.; Newman, R.H.; Gowen, B.; Orlova, E.; Gorman, M.A.; Kondo, H.; Dokurno, P.; Lally, J.; et al. Structure of the AAA ATPase p97. Mol. Cell 2000, 6, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Rouiller, I.; Butel, V.M.; Latterich, M.; Milligan, R.A.; Wilson-Kubalek, E.M. A major conformational change in p97 AAA ATPase upon ATP binding. Mol. Cell 2000, 6, 1485–1490. [Google Scholar] [CrossRef]
- DeLaBarre, B.; Brunger, A.T. Complete structure of p97/valosin-containing protein reveals communication between nucleotide domains. Nat. Struct. Biol. 2003, 10, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Huyton, T.; Pye, V.E.; Briggs, L.C.; Flynn, T.C.; Beuron, F.; Kondo, H.; Ma, J.; Zhang, X.; Freemont, P.S. The crystal structure of murine p97/VCP at 3.6A. J. Struct. Biol. 2003, 144, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.M.; Tsuruta, H.; May, A.P.; Weis, W.I. Conformational changes of p97 during nucleotide hydrolysis determined by small-angle X-ray scattering. Structure 2005, 13, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.M.; Brunger, A.T.; Weis, W.I. Improved structures of full-length p97, an AAA ATPase: Implications for mechanisms of nucleotide-dependent conformational change. Structure 2008, 16, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Dreveny, I.; Pye, V.E.; Beuron, F.; Briggs, L.C.; Isaacson, R.L.; Matthews, S.J.; McKeown, C.; Yuan, X.; Zhang, X.; Freemont, P.S. p97 and close encounters of every kind: A brief review. Biochem. Soc. Trans. 2004, 32, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Dreveny, I.; Kondo, H.; Uchiyama, K.; Shaw, A.; Zhang, X.; Freemont, P.S. Structural basis of the interaction between the AAA ATPase p97/VCP and its adaptor protein p47. EMBO J. 2004, 23, 1030–1939. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Wang, Q.; Li, C.C. ATPase activity of p97-valosin-containing protein (VCP). D2 mediates the major enzyme activity, and D1 contributes to the heat-induced activity. J. Biol. Chem. 2003, 278, 3648–3655. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Song, C.; Yang, X.; Li, C.C. D1 ring is stable and nucleotide-independent, whereas D2 ring undergoes major conformational changes during the ATPase cycle of p97-VCP. J. Biol. Chem. 2003, 278, 32784–32793. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Meyer, H.H.; Rapoport, T.A. Function of the p97-Ufd1-Npl4 complex in retrotranslocation from the ER to the cytosol: Dual recognition of nonubiquitinated polypeptide segments and polyubiquitin chains. J. Cell Biol. 2003, 162, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Song, C.; Li, C.C. Hexamerization of p97-VCP is promoted by ATP binding to the D1 domain and required for ATPase and biological activities. Biochem. Biophys. Res. Commun. 2003, 300, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Madeo, F.; Schlauer, J.; Zischka, H.; Mecke, D.; Fröhlich, K.U. Tyrosine phosphorylation regulates cell cycle-dependent nuclear localization of Cdc48p. Mol. Biol. Cell 1998, 9, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Ewens, C.A.; Kloppsteck, P.; Förster, A.; Zhang, X.; Freemont, P.S. Structural and functional implications of phosphorylation and acetylation in the regulation of the AAA+ protein p97. Biochem. Cell Biol. 2010, 88, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Abaan, O.D.; Hendriks, W.; Uren, A.; Toretsky, J.A.; Erkizan, H.V. Valosin containing protein (VCP/p97) is a novel substrate for the protein tyrosine phosphatase PTPL1. Exp. Cell Res. 2013, 319, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Meyer, H.H.; Rapoport, T.A. The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature 2001, 414, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.M.; Livnat-Levanon, N.; Taylor, E.B.; Jones, K.T.; Dephoure, N.; Ring, J.; Xie, J.; Brodsky, J.L.; Madeo, F.; Gygi, S.P.; et al. A stress-responsive system for mitochondrial protein degradation. Mol. Cell 2010, 40, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Ghislain, M.; Dohmen, R.J.; Levy, F.; Varshavsky, A. Cdc48p interacts with Ufd3p, a WD repeat protein required for ubiquitin-mediated proteolysis in Saccharomyces cerevisiae. EMBO J. 1996, 15, 4884–4899. [Google Scholar] [PubMed]
- Latterich, M.; Fröhlich, K.U.; Schekman, R. Membrane fusion and the cell cycle: Cdc48p participates in the fusion of ER membranes. Cell 1995, 82, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Moir, D.; Botstein, D. Determination of the order of gene function in the yeast nuclear division pathway using cs and is mutants. Genetics 1982, 100, 565–577. [Google Scholar] [PubMed]
- Ju, J.S.; Fuentealba, R.A.; Miller, S.E.; Jackson, E.; Piwnica-Worms, D.; Baloh, R.H.; Weihl, C.C. Valosin-containing protein (VCP) is required for autophagy and is disrupted in VCP disease. J. Cell Biol. 2009, 187, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.M.; Chen, E.; Longo, D.L.; Gorbea, C.M.; Li, C.C. Involvement of valosin-containing protein, an ATPase Co-purified with IkappaBalpha and 26 S proteasome, in ubiquitin-proteasome-mediated degradation of IkappaBalpha. J. Biol. Chem. 1998, 273, 3562–3573. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, T.; Matuschewski, K.; Rape, M.; Schlenker, S.; Ulrich, H.D.; Jentsch, S. Activation of a membrane—bound transcription factor by regulated ubiquitin/proteasome-dependent processing. Cell 2000, 102, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Raasi, S.; Wolf, D.H. Ubiquitin receptors and ERAD: A network of pathways to the proteasome. Semin. Cell Dev. Biol. 2007, 18, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Franz, A.; Ackermann, L.; Hoppe, T. Create and preserve: Proteostasis in development and aging is governed by Cdc48/p97/VCP. Biochim. Biophys. Acta 2014, 1843, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Weissman, A.M. Ubiquitylation in ERAD: Reversing to go forward? PLoS Biol. 2011, 9, e1001038. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.H.; Stolz, A. The Cdc48 machine in endoplasmic reticulum associated protein degradation. Biochim. Biophys. Acta 2012, 1823, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Karbowski, M.; Youle, R.J. Regulating mitochondrial outer membrane proteins by ubiquitination and proteasomal degradation. Curr. Opin. Cell Biol. 2011, 23, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhou, X.; Zhao, G.; Schindelin, H.; Lennarz, W.J. Multiple modes of interaction of the deglycosylation enzyme, mouse peptide N-glycanase, with the proteasome. Proc. Natl. Acad. Sci. USA 2005, 102, 15809–15814. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Tomita, Y.; Hoshida, Y.; Sakon, M.; Kameyama, M.; Imaoka, S.; Sekimoto, M.; Nakamori, S.; Monden, M.; Aozasa, K. Expression of valosin-containing protein in colorectal carcinomas as a predictor for disease recurrence and prognosis. Clin. Cancer Res. 2004, 10, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Tomita, Y.; Hoshida, Y.; Nagano, H.; Dono, K.; Umeshita, K.; Sakon, M.; Ishikawa, O.; Ohigashi, H.; Nakamori, S.; et al. Increased expression of valosin-containing protein (p97) is associated with lymph node metastasis and prognosis of pancreatic ductal adenocarcinoma. Ann. Surg. Oncol. 2004, 11, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Tomita, Y.; Uruno, T.; Hoshida, Y.; Qiu, Y.; Iizuka, N.; Nakamichi, I.; Miyauchi, A.; Aozasa, K. Increased expression of valosin-containing protein (p97) is correlated with disease recurrence in follicular thyroid cancer. Ann. Surg. Oncol. 2005, 12, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Tomita, Y.; Hoshida, Y.; Takiguchi, S.; Fujiwara, Y.; Yasuda, T.; Yano, M.; Nakamori, S.; Sakon, M.; Monden, M.; et al. Expression level of valosin-containing protein is strongly associated with progression and prognosis of gastric carcinoma. J. Clin. Oncol. 2003, 21, 2537–2544. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Tomita, Y.; Hoshida, Y.; Toyosawa, S.; Inohara, H.; Kishino, M.; Kogo, M.; Nakazawa, M.; Murakami, S.; Iizuka, N.; et al. Expression level of valosin-containing protein (VCP) as a prognostic marker for gingival squamous cell carcinoma. Ann. Oncol. 2004, 15, 1432–1438. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Yin, C.; Doong, H.; Fang, S.; Peterhoff, C.; Nixon, R.A.; Monteiro, M.J. Characterization of erasin (UBXD2): A new ER protein that promotes ER-associated protein degradation. J. Cell Sci. 2006, 119, 4011–4024. [Google Scholar] [CrossRef] [PubMed]
- Asaka, S.; Fujimoto, T.; Akaishi, J.; Ogawa, K.; Onda, M. Genetic prognostic index influences patient outcome for node-positive breast cancer. Surg. Today 2006, 36, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Cervi, G.; Magnaghi, P.; Asa, D.; Avanzi, N.; Badari, A.; Borghi, D.; Caruso, M.; Cirla, A.; Cozzi, L.; Felder, E.; et al. Discovery of 2-(cyclohexylmethylamino)pyrimidines as a new class of reversible valosine containing protein inhibitors. J. Med. Chem. 2014, 57, 10443–10454. [Google Scholar] [CrossRef] [PubMed]
- Watts, G.D.; Wymer, J.; Kovach, M.J.; Mehta, S.G.; Mumm, S.; Darvish, D.; Pestronk, A.; Whyte, M.P.; Kimonis, V.E. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat. Genet. 2004, 36, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Halawani, D.; LeBlanc, A.C.; Rouiller, I.; Michnick, S.W.; Servant, M.J.; Latterich, M. Hereditary inclusion body myopathy-linked p97/VCP mutations in the NH2 domain and the D1 ring modulate p97/VCP ATPase activity and D2 ring conformation. Mol. Cell Biol. 2009, 29, 4484–4494. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.K.; Xia, D. Altered intersubunit communication is the molecular basis for functional defects of pathogenic p97 mutants. J. Biol. Chem. 2013, 288, 36624–36635. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.K.; Li, D.; Li, C.C.; Esser, L.; Dai, R.; Guo, L.; Xia, D. A novel ATP-dependent conformation in p97 N-D1 fragment revealed by crystal structures of disease-related mutants. EMBO J. 2010, 29, 2217–2229. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sáiz, V.; Buchberger, A. Imbalances in p97 co-factor interactions in human proteinopathy. EMBO Rep. 2010, 11, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.O.; Mandrioli, J.; Benatar, M.; Abramzon, Y.; van Deerlin, V.M.; Trojanowski, J.Q.; Gibbs, J.R.; Brunetti, M.; Gronka, S.; Wuu, J.; et al. Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron 2010, 68, 857–964. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.L. Development of proteasome inhibitors as research tools and cancer drugs. J. Cell Biol. 2012, 199, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.M.; Ryter, S.W.; Levine, B. Autophagy in human health and disease. N. Engl. J. Med. 2013, 368, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Brenner, A.K.; Reikvam, H.; Lavecchia, A.; Bruserud, O. Therapeutic targeting the Cell Division Cycle 25 (CDC25) phosphatases in human acute myeloid leukemia-the possibility to target several linases through Inhibition of the various CDC25 isoforms. Molecules 2014, 19, 18414–18447. [Google Scholar] [CrossRef] [PubMed]
- Christianson, J.C.; Ye, Y. Cleaning up in the endoplasmic reticulum: Ubiquitin in charge. Nat. Struct. Mol. Biol. 2014, 21, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, D.; Meier, C.; Alla, V.; Pützer, B.M. A balancing act: Orchestrating amino-truncated and full-length p73 variants as decisive factors in cancer progression. Oncogene 2014. [Google Scholar] [CrossRef]
- Rayet, B.; Gélinas, C. Aberrant rel/nfkb genes and activity in human cancer. Oncogene 1999, 18, 6938–6947. [Google Scholar] [CrossRef] [PubMed]
- Pant, V.; Lozano, G. Limiting the power of p53 through the ubiquitin proteasome pathway. Genes Dev. 2014, 28, 1739–1751. [Google Scholar] [CrossRef] [PubMed]
- Fieger, E.; Hirsch, C.; Vyas, J.M.; Gordon, E.; Ploegh, H.L.; Tortorella, D. Dissection of dislocation pathway for type I membrane proteins with a new small molecule inhibitor, eeyarstatin. Mol. Biol. Cell 2004, 15, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shinkre, B.A.; Lee, J.; Weniger, M.A.; Liu, Y.; Chen, W.; Weistner, A.; Trenkle, W.C.; Ye, Y. The ERAD inhibitor eeyrestatin I is a bifunctional compound with a membrane-binding domain and a p97/VCP inhibitory group. PLoS One 2010, 5, e15479. [Google Scholar] [CrossRef] [PubMed]
- Cross, B.C.; McKibbin, C.; Callan, A.C.; Roboti, P.; Piacenti, M.; Rabu, C.; Wilson, C.M.; Whitehead, R.; Flitsch, S.L.; Pool, M.R.; et al. Eeyarestatin I inhibits Sec61-mediated protein translocation at the endoplasmic reticulum. J. Cell Sci. 2009, 122, 4393–4400. [Google Scholar] [CrossRef] [PubMed]
- Stack, J.H.; Whitney, M.; Rodems, S.M.; Pollok, B.A. A ubiquitin-based tagging system for controlled modulation of protein stability. Nat. Biotechnol. 2000, 18, 1298–1302. [Google Scholar] [CrossRef] [PubMed]
- Yi, P.; Higa, A.; Taouji, S.; Bexiga, M.G.; Marza, E.; Arma, D.; Castain, C.; le Bail, B.; Simpson, J.C.; Rosenbaum, J.; et al. Sorafenib-mediated targeting of the AAA+ ATPase p97/VCP leads to disruption of the secretory pathway, endoplasmic reticulum stress, and hepatocellular cancer cell death. Mol. Cancer. Ther. 2012, 11, 2610–2620. [Google Scholar] [CrossRef] [PubMed]
- Korennykh, A.; Walter, P. Structural basis of the unfolded protein response. Annu. Rev. Cell Dev. Biol. 2012, 28, 251–277. [Google Scholar] [CrossRef] [PubMed]
- McKibbin, C.; Mares, A.; Piacenti, M.; Williams, H.; Roboti, P.; Puumalainen, M.; Callan, A.C.; Lesiak-mieczkowska, K.; Linder, S.; Harant, H.; et al. Inhibition of protein translocation at the endoplasmic reticulum promotes activation of the unfolded protein response. Biochem. J. 2012, 442, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Mora-Jensen, H.; Weniger, M.A.; Perez-Galan, P.; Wolford, C.; Hai, T.; Ron, D.; Chen, W.; Trenkle, W.; Wiestner, A.; et al. ERAD inhibitors integrate ER stress with an epigenetic mechanism to activate BH3-only protein NOXA in cancer cells. Proc. Natl. Acad. Sci. USA 2009, 106, 2200–2225. [Google Scholar] [CrossRef] [PubMed]
- Auner, H.W.; Moody, A.M.; Ward, T.H.; Kraus, M.; Milan, E.; May, P.; Chaidos, A.; Driessen, C.; Cenci, S.; Dazzi, F.; et al. Combined inhibition of p97 and the proteasome causes lethal disruption of the secretory apparatus in multiple myeloma cells. PLoS One 2013, 8, e74415. [Google Scholar] [CrossRef] [PubMed]
- Brem, G.J.; Myolans, I.; Bruning, A. Eeyarestatin causes cervical cancer cell sensitization to bortezomib treatment by augmenting ER stress and CHOP expression. Gynecol. Oncol. 2013, 128, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Cubedo, E.; Cordeu, L.; Bandres, E.; Rebollo, A.; Malumbres, R.; Sanmartin, C.; Font, M.; Palop, J.A.; Gacía-Foncillas, J. New symmetrical quinazoline derivatives selectively induce apoptosis in human cancer cells. Cancer Biol. Ther. 2006, 5, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Arrojo, E.; Drigo, R.; Egri, P.; Jo, S.; Gereben, B.; Bianco, A.C. The type II deiodinase is retrotranslocated to the cytoplasm and proteasomes via p97/Atx3 complex. Mol. Endocrinol. 2013, 27, 2105–2115. [Google Scholar] [CrossRef] [PubMed]
- Minogue, P.J.; Beyer, E.C.; Berthoud, V.M. A connexin50 mutant, CX50fs, that causes cataracts is unstable, but is rescued by a proteasomal inhibitor. J. Biol. Chem. 2013, 288, 20427–20434. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Song, W.; Brancati, G.; Segatori, L. Inhibition of endoplasmic reticulum-associated degradation rescues native folding in loss of function protein misfolding diseases. J. Biol. Chem. 2011, 286, 43454–43464. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.F.; Bufler, S.L.; Weihl, C.C.; Li, K.; Lis, L.G.; Walters, M.A.; Schoene, F.J.; Lin, H.J.; Deshaies, R.J.; Arkin, M.R. Specific inhibition of p97/VCP ATPase and kinetic analysis demonstrated interaction between D1 and D2 ATPase domains. J. Mol. Biol. 2014, 426, 2886–2899. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-F.; Raymond, J. Deshaies quantitative cell-based protein degradation assays to identify and classify drugs that target the ubiquitin-proteasome system. J. Biol. Chem. 2011, 286, 16546–16554. [Google Scholar] [CrossRef] [PubMed]
- Briggs, L.C.; Baldwin, G.S.; Miyata, N.; Kondo, H.; Zhang, X.; Freemont, P.S. Analysis of nucleotide binding to P97 reveals the properties of a tandem AAA hexameric ATPase. J. Biol. Chem. 2008, 283, 13745–13752. [Google Scholar] [CrossRef] [PubMed]
- Segel, I.H. Multisite and allosteric enzymes. In Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems; Wiley: Hoboken, NJ, USA, 1993; pp. 346–462. [Google Scholar]
- Reinhart, G.D. Quantitative analysis and interpretation of allosteric behavior. Methods Enzymol. 2004, 380, 187–203. [Google Scholar] [PubMed]
- Cortés, A.; Cascante, M.; Cárdenas, M.L.; Cornish-Bowden, A. Relationships between inhibition constants, inhibitor concentrations for 50% inhibition and types of inhibition: New ways of analysing data. Biochem. J. 2001, 357, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Fenton, A.W. Allostery: An illustrated definition for the “second secret of life”. Trends Biochem. Sci. 2008, 33, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Sasazawa, Y.; Kanagaki, S.; Tashiro, E.; Nogawa, T.; Muroi, M.; Kondoh, Y.; Osada, H.; Imoto, M. Xanthohumol impairs autophagosome maturation through direct inhibition of valosin-containing protein. ACS Chem. Biol. 2012, 7, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.C.; Mason, D.J.; Eichhorst, N.; Engelder, P.; Mesa, C.; Wijeratne, E.M.K.; Gunaherath, G.M.; Gunatilaka, A.A.L.; la Clair, J.J.; Chapman, E. Functional chromatographic technique for natural product isolation. Org. Biomol. Chem. 2015. [Google Scholar] [CrossRef]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chapman, E.; Maksim, N.; De la Cruz, F.; La Clair, J.J. Inhibitors of the AAA+ Chaperone p97. Molecules 2015, 20, 3027-3049. https://doi.org/10.3390/molecules20023027
Chapman E, Maksim N, De la Cruz F, La Clair JJ. Inhibitors of the AAA+ Chaperone p97. Molecules. 2015; 20(2):3027-3049. https://doi.org/10.3390/molecules20023027
Chicago/Turabian StyleChapman, Eli, Nick Maksim, Fabian De la Cruz, and James J. La Clair. 2015. "Inhibitors of the AAA+ Chaperone p97" Molecules 20, no. 2: 3027-3049. https://doi.org/10.3390/molecules20023027





