A Facile Ionic Liquid Promoted Synthesis, Cholinesterase Inhibitory Activity and Molecular Modeling Study of Novel Highly Functionalized Spiropyrrolidines
Abstract
:1. Introduction
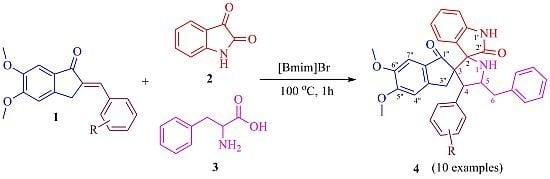
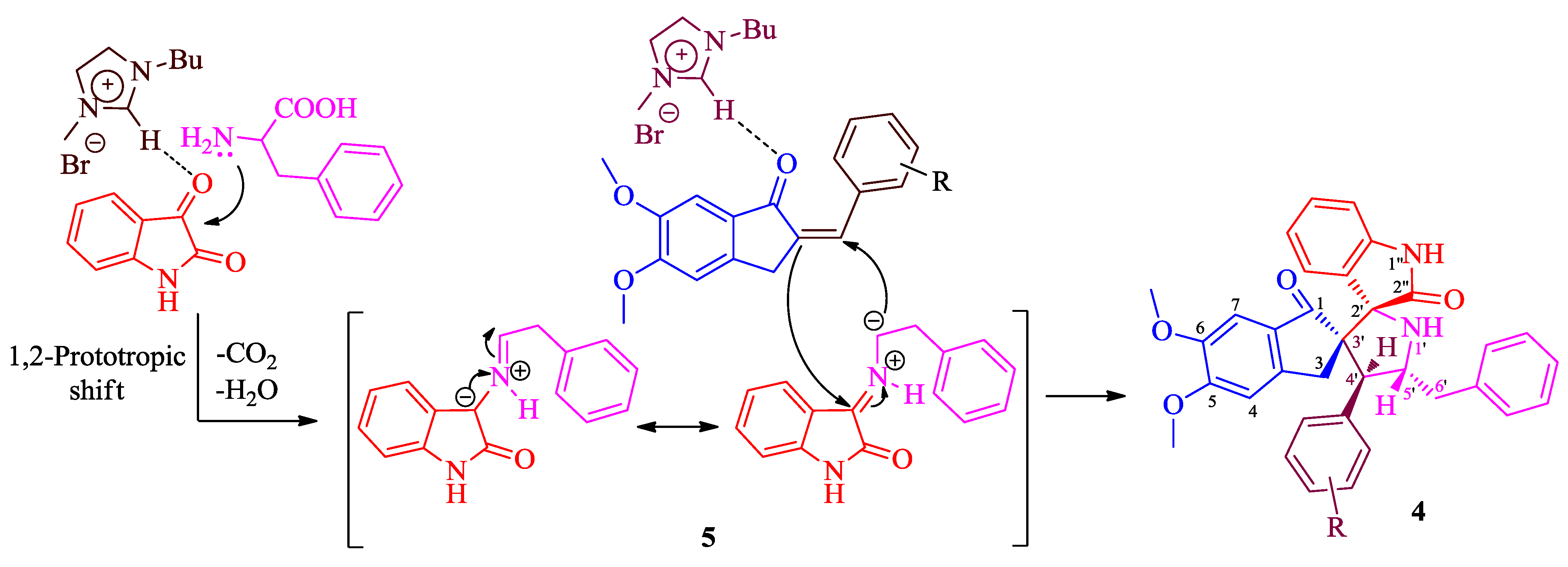
2. Results and Discussion
2.1. Chemistry

| Entry | Comp 4 | R | Yield (%) a | mp °C | AChE Inhibition IC50 µM (±SD) | BChE Inhibition IC50 µM (±SD) |
|---|---|---|---|---|---|---|
| 1 | a | H | 85 | 134–135 | 11.29 ± 0.21 | 15.73 ± 0.22 |
| 2 | b | 2-CH3 | 87 | 141–142 | 10.15 ± 0.17 | 21.32 ± 0.19 |
| 3 | c | 2-OCH3 | 82 | 126–127 | 12.23 ± 0.19 | 11.39 ± 0.12 |
| 4 | d | 2-Cl | 85 | 136–137 | 15.17 ± 0.22 | 9.63 ± 0.15 |
| 5 | e | 3-OCH3 | 84 | 127–128 | 2.13 ± 0.11 | 17.44 ± 0.23 |
| 6 | f | 3-O2N | 89 | 133–134 | 1.57 ± 0.09 | 15.32 ± 0.18 |
| 7 | g | 4-CH3 | 92 | 138–139 | 7.11 ± 0.13 | 10.46 ± 0.24 |
| 8 | h | 4-OCH3 | 80 | 129–130 | 3.67 ± 0.15 | 14.69 ± 0.17 |
| 9 | i | 4-Cl | 88 | 132–133 | 5.83 ± 0.20 | 16.11 ± 0.15 |
| 10 | j | 2,4-Cl2 | 90 | 135–136 | 6.72 ± 0.18 | 12.79 ± 0.25 |
| 11 | Galantamine | - | - | - | 2.09 ± 0.07 | 19.21 ± 0.12 |
| 12 | Donepezil | - | - | - | 0.21 ± 0.02 | 3.6 ± 0.11 |
2.2. Cholinesterase Inhibitory Activity Study
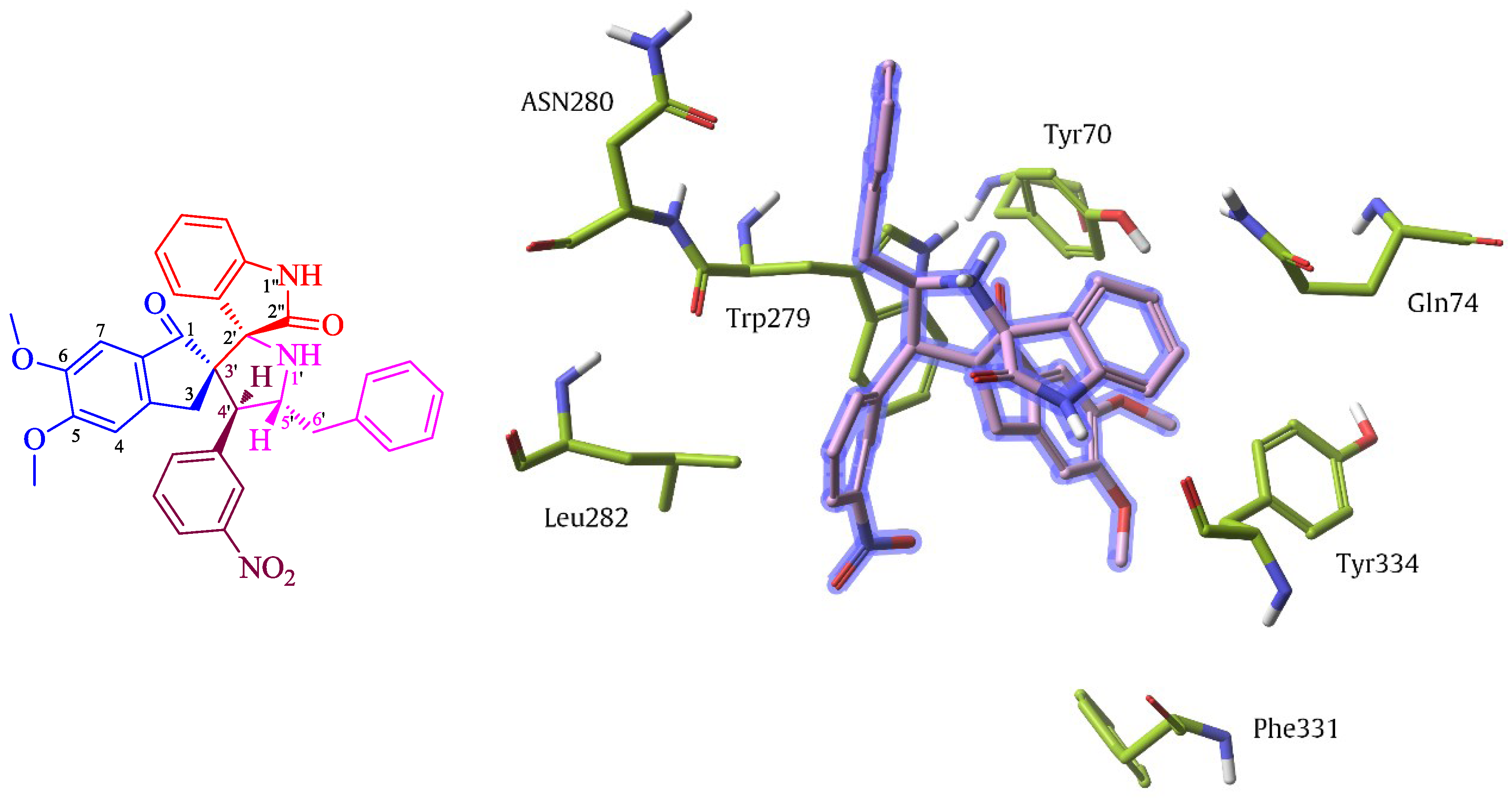
2.3. Molecular Docking Studies
3. Experimental Section
3.1. General Methods
3.2. General Procedure for the Synthesis of 4
3.3. Characterization Data for Compounds 4a–j
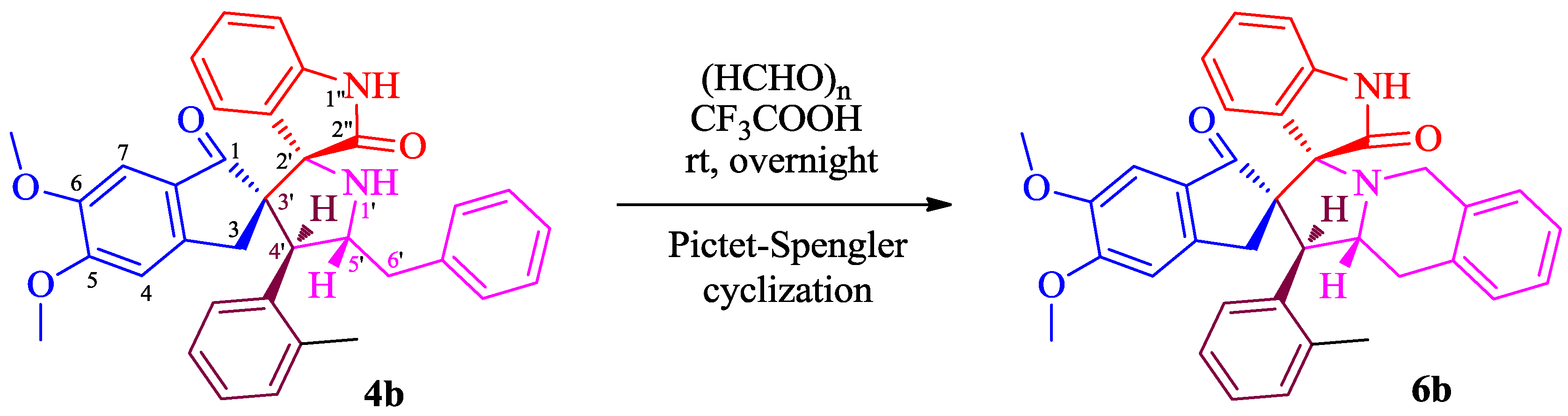
3.4. General Procedure for the Synthesis of 6
3.5. Cholinesterase Inhibition Assays
3.6. Molecular Modeling
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Terry, A.V.; Buccafusco, J.J. The cholinergic hypothesis of age and Alzheimer’s disease-related cognitive deficits: Recent challenges and their implications for novel drug development. J. Pharmacol. Exp. Ther. 2003, 306, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L. Alzheimer’s disease. N. Engl. J. Med. 2004, 351, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Mesulam, M.M.; Guillozet, A.; Shaw, P.; Levey, A.; Duysen, E.G.; Lockridge, O. Acetylcholinesterase knockouts establish central cholinergic pathways and can use butyrylcholinesterase to hydrolyze acetylcholine. Neuroscience 2002, 110, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhao, J.; Han, X.; Zhu, S. Stereoselective preparation of 1,2,4-oxadiazole derivatives substituted by pentafluorophenyl by 1,3-dipolar cycloaddition. Tetrahedron 2006, 62, 11008–11011. [Google Scholar] [CrossRef]
- Coutouli-Argyropoulou, E.; Lianis, P.; Mitakou, M.; Giannoulis, A.; Nowak, J. 1,3-Dipolar cycloaddition approach to isoxazole, isoxazoline and isoxazolidine analogues of C-nucleosides related to pseudouridine. Tetrahedron 2006, 62, 1494–1501; [Google Scholar] [CrossRef]
- Gomes, P.J. S.; Nunes, C.M.; Pais, A.A.C.C.; Pinho e Melo, T.M.V.D.; Arnaut, L.G. 1,3-Dipolar cycloaddition of azomethine ylides generated from aziridines in supercritical carbon dioxide. Tetrahedron Lett. 2006, 47, 5475–5479. [Google Scholar] [CrossRef]
- Abou-Gharbia, M.A.; Doukas, P.H. Synthesis of Tricyclic Arylspiro Compounds as Potential Antileukemic and Anticonvulsant Agents. Heterocycles 1979, 12, 637–640. [Google Scholar] [CrossRef]
- Kornett, M.J.; Thio, A.P. Oxindole-3-spiropyrrolidines and -piperidines. Synthesis and local anesthetic activity. J. Med. Chem. 1976, 19, 892–898. [Google Scholar]
- Lundahl, K.; Schut, J.; Schlatmann, J.L.M.A.; Paerels, G.B.; Peters, A. Synthesis and antiviral activities of adamantane spiro compounds. 1. Adamantane and analogous spiro-3'-pyrrolidines. J. Med. Chem. 1972, 15, 129–132. [Google Scholar]
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar]
- Petkovic, M.; Seddon, K.R.; Rebelo, L.P.N.; Pereira, C.S. Ionic liquids: a pathway to environmental acceptability. Chem. Soc. Rev. 2011, 40, 1383–1403. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q. OsO4 in ionic liquid [bmim]PF6: A recyclable and reusable catalyst system for olefin dihydroxylation. remarkable effect of DMAP. Org. Lett. 2002, 4, 2197–2199. [Google Scholar]
- Kumar, A.; Pawar, S.S. Converting exo-selective Diels-Alder reaction to endo-selective in chloroloaluminate ionic liquids. J. Org. Chem. 2004, 69, 1419–1420. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, K.; Yoshizawa, M.; Ohno, H. Room temperature ionic liquids from 20 natural amino acids. J. Am. Chem. Soc. 2005, 127, 2398–2399. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, S.A.; Frohlich, U.; Goodrich, P.; Gunaratne, H.Q. N.; Hardacre, C.; McKeown, A.; Seddon, K.R. Functionalised ionic liquids: Synthesis of ionic liquids with tethered basic groups and their use in Heck and Knoevenagel reactions. New J. Chem. 2010, 34, 723–731. [Google Scholar] [CrossRef]
- Gao, J.; Song, Q.-W.; He, L.-N.; Liu, C.; Yang, Z.-Z.; Han, X.; Li, X.-D.; Song, Q.-C. Preparation of polystyrene-supported Lewis acidic Fe(III) ionic liquid and its application in catalytic conversion of carbon. Tetrahedron 2012, 68, 3835–3842. [Google Scholar] [CrossRef]
- Narayana Kumar, G.G.K.S.; Aridoss, G.; Laali, K.K. Condensation of propargylic alcohols with indoles and carbazole in [bmim][PF6]/Bi(NO3)3·5H2O: A simple high yielding propargylation method with recycling and reuse of the ionic liquid. Tetrahedron Lett. 2012, 53, 3066–3069. [Google Scholar] [CrossRef]
- Ghahremanzadeh, R.; Ahadi, S.; Bazgir, A. A one-pot, four-component synthesis of α-carboline derivatives. Tetrahedron Lett. 2009, 50, 7379–7381. [Google Scholar] [CrossRef]
- Kabalka, G.W.; Venkataiah, B.; Das, B.C. Allylboration of carbonyl compounds in ionic liquids. Green Chem. 2002, 4, 472–473. [Google Scholar] [CrossRef]
- Pingali, S.R.K.; Madhav, M.; Jursic, B.S. An efficient regioselective NBS aromatic bromination in the presence of an ionic liquid. Tetrahedron Lett. 2010, 51, 1383–1385. [Google Scholar] [CrossRef]
- Wang, X.-S.; Zhou, J.; Yang, K.; Zhang, M.-M. Divergent products obtained from the reactions of salicylaldehyde and 4-hydroxycoumarin in TEBAC-H2O, KF-Al2O3-EtOH, and ionic liquid. Synth. Commun. 2010, 40, 3332–3345. [Google Scholar] [CrossRef]
- Ramachary, D.B.; Narayana, V.V.; Ramakumar, K. Direct ionic liquid promoted organocatalyzed diazo-transfer reactions: diversity-oriented synthesis of diazo-compounds. Tetrahedron Lett. 2008, 49, 2704–2709. [Google Scholar] [CrossRef]
- Suresh Kumar, R.; Almansour, A.I.; Arumugam, N.; Basiri, A.; Kia, Y.; Kumar, R.R. Ionic Liquid-Promoted Synthesis and Cholinesterase Inhibitory Activity of Highly Functionalized Spiropyrrolidines. Aust. J. Chem. 2014. [Google Scholar] [CrossRef]
- Kumar, R.S.; Almansour, A.I.; Arumugam, N.; Osman, H.; Ali, M.A.; Basiri, A.; Kia, Y. An expedient synthesis and screening for antiacetylcholinesterase activity of piperidine embedded novel pentacyclic cage compounds. Med. Chem. 2014, 10, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Kia, Y.; Osman, H.; Suresh Kumar, R.; Murugaiyah, V.; Basiri, A.; Khaw, K.Y.; Rosli, M.M. An Efficient Ionic Liquid Mediated Synthesis, Cholinesterase Inhibitory Activity and Molecular Modeling Study of Novel Piperidone Embedded α,β-Unsaturated Ketones. Med Chem. 2014, 10, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Kia, Y.; Osman, H.; Suresh Kumar, R.; Basiri, A.; Murugaiyah, V. Synthesis and discovery of highly functionalized mono- and bis-spiro-pyrrolidines as potent cholinesterase enzyme inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 1815–1819. [Google Scholar] [CrossRef] [PubMed]
- Kia, Y.; Osman, H.; Suresh Kumar, R.; Basiri, A.; Murugaiyah, V. Ionic liquid mediated synthesis of mono- and bis-spirooxindole-hexahydropyrrolidines as cholinesterase inhibitors and their molecular docking studies. Bioorg. Med. Chem. 2014, 22, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Basiri, A.; Murugaiyah, V.; Osman, H.; Suresh Kumar, R.; Kia, Y.; Hooda, A.; Parsons, R.B. Cholinesterase inhibitory activity versus aromatic core multiplicity: A facile green synthesis and molecular docking study of novel piperidone embedded thiazolopyrimidines. Bioorg. Med. Chem. 2014, 22, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Kia, Y.; Osman, H.; Kumar, R.S.; Murugaiyah, V.; Basiri, A.; Perumal, S.; Wahab, H.A.; Bing, C.S. Synthesis and discovery of novel piperidone-grafted mono- and bis-spirooxindole-hexahydropyrrolizines as potent cholinesterase inhibitors. Bioorg. Med. Chem. 2013, 21, 1696–1707. [Google Scholar] [CrossRef] [PubMed]
- Basiri, A.; Murugaiyah, V.; Osman, H.; Kumar, R.S.; Kia, Y.; Ali, M.A. Microwave assisted synthesis, cholinesterase enzymes inhibitory activities and molecular docking studies of new pyridopyrimidine derivatives. Bioorg. Med. Chem. 2013, 21, 3022–3031. [Google Scholar] [CrossRef] [PubMed]
- Kia, Y.; Osman, H.; Kumar, R.S.; Murugaiyah, V.; Basiri, A.; Perumal, S.; Razak, I.A. A facile chemo-, regio- and stereoselective synthesis and cholinesterase inhibitory activity of spirooxindole-pyrrolizine-piperidine hybrids. Bioorg. Med. Chem. Lett. 2013, 23, 2979–2983. [Google Scholar] [CrossRef] [PubMed]
- Basiri, A.; Murugaiyah, V.; Osman, H.; Kumar, R.S.; Kia, Y.; Awang, K.B.; Ali, M.A. An expedient, ionic liquid mediated multi-component synthesis of novel piperidone grafted cholinesterase enzymes inhibitors and their molecular modeling study. Eur. J. Med. Chem. 2013, 67, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Ismail, R.; Choon, T.S.; Kumar, R.S.; Asad, M.; Almansour, A.I.; Yoon, Y.K.; Wei, A.C.; Elumalai, K.; Pandian, S. Alzheimer Diseases: Substituted Spiro [2.3'] Oxindolespiro [3.2'']-5,6-Dimethoxy-Indane-1''-One-Indolizine Analogue as Inhibitors of Acetylcholinesterase. Med. Chem. 2012, 2, 7–10. [Google Scholar]
- Ali, M.A.; Yar, M.S.; Hasan, M.Z.; Ahsan, M.J.; Pandian, S. Design, synthesis and evaluation of novel 5,6-dimethoxy-1-oxo-2,3-dihydro-1H-2-indenyl-3,4-substituted phenyl methanone analogues. Bioorg. Med. Chem. Lett. 2009, 19, 5075–5077. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.; Martinez, A. Targeting beta-amyloid pathogenesis through acetylcholinesterase inhibitors. Curr. Pharm. Des. 2006, 12, 4377–4387. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almansour, A.I.; Kumar, R.S.; Arumugam, N.; Basiri, A.; Kia, Y.; Ali, M.A.; Farooq, M.; Murugaiyah, V. A Facile Ionic Liquid Promoted Synthesis, Cholinesterase Inhibitory Activity and Molecular Modeling Study of Novel Highly Functionalized Spiropyrrolidines. Molecules 2015, 20, 2296-2309. https://doi.org/10.3390/molecules20022296
Almansour AI, Kumar RS, Arumugam N, Basiri A, Kia Y, Ali MA, Farooq M, Murugaiyah V. A Facile Ionic Liquid Promoted Synthesis, Cholinesterase Inhibitory Activity and Molecular Modeling Study of Novel Highly Functionalized Spiropyrrolidines. Molecules. 2015; 20(2):2296-2309. https://doi.org/10.3390/molecules20022296
Chicago/Turabian StyleAlmansour, Abdulrahman I., Raju Suresh Kumar, Natarajan Arumugam, Alireza Basiri, Yalda Kia, Mohamed Ashraf Ali, Mehvish Farooq, and Vikneswaran Murugaiyah. 2015. "A Facile Ionic Liquid Promoted Synthesis, Cholinesterase Inhibitory Activity and Molecular Modeling Study of Novel Highly Functionalized Spiropyrrolidines" Molecules 20, no. 2: 2296-2309. https://doi.org/10.3390/molecules20022296