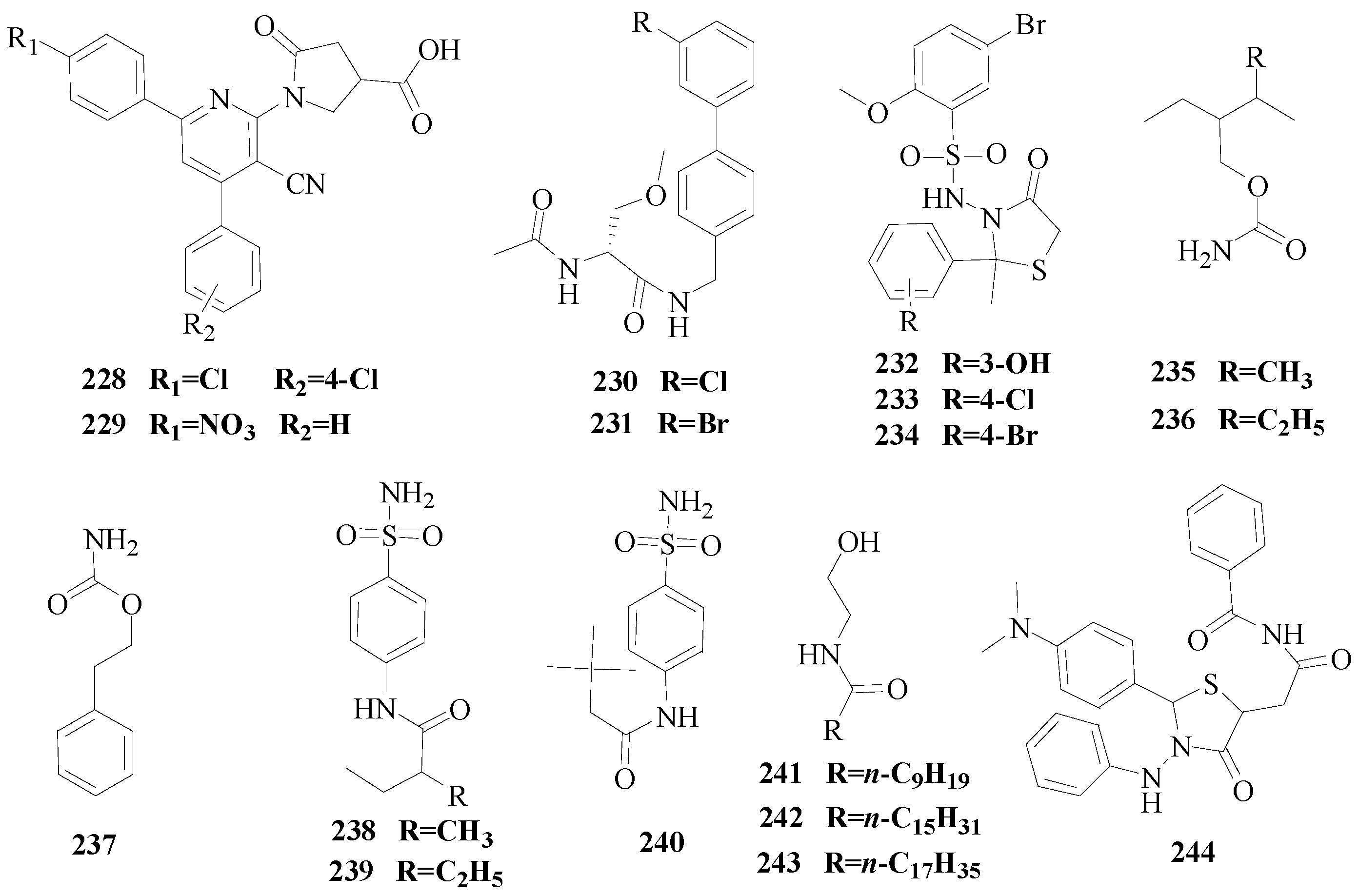2. The Quinoline Functional Group
Quinoline is nitrogen-containing heterocyclic aromatic compound. Pharmacologically active substances display a broad range of biological activity. Quinoline has been found to possess anti-malarial, antibacterial, antifungal, anthelmintic, cardiotonic, anticonvulsant, anti-inflammatory, and analgesic activity. Our laboratory has studied a lot of quinoline derivatives for antiepileptic activity [
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23].
Xie
et al., reported a new series of 7-alkoxyl-4,5-dihydro-[1,2,4]triazolo[4,3-
a]quinoline derivatives. Their anticonvulsant activities were evaluated by the MES test and the
sc-PTZ test, and their neurotoxicity was evaluated by the rotarod neurotoxicity test with a median toxic dose (TD
50) value of 54.5 mg/kg, MES and
sc-PTZ tests showed that compound
1 (
Table 1) was the most potent of this series with an effective dose (ED
50) value of 11.8 and 6.7 mg/kg and protective index (PI = TD
50/ED
50) value of 4.6 and 8.1, respectively [
12].
Table 1.
Quinoline anticonvulsant compounds.
![Molecules 20 19714 i001]()
Table 1.
Quinoline anticonvulsant compounds. ![Molecules 20 19714 i001]()
| Compound No. | R | R1 | Reference |
|---|
| 1 | -H | 7-OCH2Ph(4-F) | [12] |
| 2 | -Ph | 7-OCH2Ph | [13] |
| 3 | =O | 7-OCH2Ph | [14] |
| 4 | =O | 8-n-OC6H13 | [15] |
| 5 | =O | 8-n-OC7H15 |
| 6 | =O | 7-n-OC7H15, 2-COC2H5 | [16] |
| 7 | -H | 5-Ph(3-F) | [17] |
| 8 | -H | 7-n-C6H13, 5-Ph, 4=5 | [18] |
| 9 | -H | 5-n-OC6H13 | [19] |
| 10 | -CONH2 | 7-n-OC6H13 | [20] |
| 11 | 5-(1,3,4-triazole), 8-n-OC8H17, 1=2 and 3=4 | [21] |
| 12 | 6-(1,3,4-triazole), 1-n-OC6H13, 2=O | [22] |
| 13 | 1=2 and 3=4, 8-OCH2Ph, ![Molecules 20 19714 i002]() | [23] |
| 14 | 1=2 and 3=4, 8-OCH2Ph, ![Molecules 20 19714 i003]() | [24] |
| 15 | 1=2 and 3=4, 2-Cl, ![Molecules 20 19714 i004]() | [25] |
Cui
et al., reported a synthesis of 1-substituted-7-benzyloxy-4,5-dihydro-[1,2,4]triazolo[4,3-
a]-quinolines. Anticonvulsant activity was evaluated in the MES test,
sc-Met test, and rotarod neurotoxicity test. The safest compound was
2 (
Table 1), with TD
50 values of greater than 300 mg/kg which was better than most of the market drugs [
13].
Jin
et al., prepared a novel type of 7-hydroxyl-3,4-dihydro-2(1
H)-quinolines. In the anti-MES test, compound
3 showed ED
50 of 12.3 mg/kg (
Table 1), TD
50 of 547.5 mg/kg, and the PI of 44.5 which was much greater than the PI of the reference drugs phenytoin, phenobarbital, carbamazepin and valproate [
14]. Sun
et al., reported the synthesis of 8-alkoxy-4,5-dihydro-[1,2,4]triazole[4,3-
a]quinoline-l-one derivatives and evaluated their anticonvulsant activities by MES test,
sc-PTZ test, and rotarod test. The results demonstrated that compound
4 and compound
5 were the most potent anticonvulsants (
Table 1), with ED
50 values of 17.17 mg/kg and 24.55 mg/kg and PI values of 41.9 and 29.3 of compound
4 in the MES and
sc-PTZ tests, respectively, and compound
5 having ED
50 values of 19.7 mg/kg and 21.2 mg/kg and PI values of 36.5 and 33.9 in the MES and
sc-PTZ tests, respectively. The PI values of compounds
4 and
5 were many folds better than that of the reference drugs which mentioned above, which have PI values in the range of 1.6–8.1 in the MES test and <0.22–5.2 in the
sc-PTZ test [
15].
Wei
et al., synthesized a series of 2-substituted-7-heptyloxy-4,5-dihydro-[1,2,4]triazolo[4,3-
a]-quinolin-1(2
H)-ones and evaluated their anticonvulsant activities. Pharmacological tests showed that compound
6 was the most active and also had the lowest toxicity (
Table 1). In the anti-MES test, it showed ED
50 of 8.2 mg/kg, TD
50 of 318.3 mg/kg, and PI of 39.0 which was much greater than the PI of the reference drugs phenytoin and carbamazepine [
16].
Guan
et al., designed and synthesized a new series of substituted quinoline-2(1
H)-one and 1,2,4-triazolo[4,3-
a]quinoline derivatives. Their anticonvulsant activities were evaluated by MES test,
sc-PTZ test and rotarod test. Compound
7 showed the strongest anticonvulsant effect with ED
50 of 27.4 mg/kg and 22.0 mg/kg in the anti-MES and anti-PTZ test, respectively (
Table 1) [
17].
Guan
et al., reported the synthesis of a series of novel 5-phenyl-[1,2,4]triazolo[4,3-
a]quinoline derivatives and evaluated their anticonvulsant activities. The MES test showed that compound
8 was found to be the most potent compound with an ED
50 value of 6.5 mg/kg and a PI value of 35.1 which was much higher than the PI of the reference drug phenytoin (
Table 1) [
18].
Guo
et al., synthesized a series of 5-alkoxy-[1,2,4]triazolo[4,3-
a]quinoline derivatives. Their anticonvulsant activities were evaluated by MES test and their neurotoxicity was measured by the rotarod test. The results demonstrated that compound
9 was the most potent anticonvulsant (
Table 1), with ED
50 of 19.0 mg/kg and PI value of 5.8 in the MES test [
19].
Sun
et al., synthesized a series of 8-alkoxy-5,6-dihydro-[1,2,4]triazino[4,3-
a]quinolin-1-one derivatives and evaluated their activities. The results showed that compound
10 was the most potent with an ED
50 value of 11.4 mg/kg (
Table 1), TD
50 of 114.1 mg/kg, PI value of 10.0 which is much greater than the PI of the reference drug carbamazepine [
20].
Wei
et al., established a series of 1-formamidotriazolo[4,3-
a]quinoline derivatives and evaluated their anticonvulsant activities. Compound
11 showed an ED
50 of 30.1 mg/kg (
Table 1), TD
50 of 286 mg/kg, and PI of 9.5 which is greater than the reference drug carbamazepine with the PI value of 6.0 [
21].
Wang
et al., synthesized two series of 8-alkoxy-5-(4
H-1,2,4-triazol-4-yl)quinolines and 8-alkoxy-5-(2
H-1,2,4-triazol-3-one-4-yl)quinolines. The anticonvulsant activities of these compounds were evaluated with MES test and rotarod test. Among the synthesized compounds, compound
12 was the most active, with and ED
50 of 8.80 mg/kg (
Table 1), TD
50 of 176.03 mg/kg and PI value of 20.0. Its neurotoxicity was the lowest among the synthesized compounds. Meanwhile, it was also significantly lower than carbamazepine that used as reference. Beyond that, the broad spectrum activity of compound
12 was inferred from the anti-seizure results of bicuculline-, PTZ- and 3-mercaptopropionic acid-induced seizure tests [
22].
Deng
et al., reported the synthesis of a series of 1-substituted-6-(4
H-1,2,4-triazol-4-yl)-3,4-dihydroquinolin-2(1
H)-ones and screened their anticonvulsant activities. In the MES screening, compound
13 showed anticonvulsant activity in moderation (
Table 1). At the dose of 100 mg/kg, all the animals were protected from seizure after treatment with compound
13, and all compounds synthesized exhibited no neurotoxicity [
23].
He
et al., synthesized 16 new 1-(2-(8-(benzyloxy)quinolin-2-yl)-1-butyrylcyclopropyl)-3-substituted urea derivatives and tested their anticonvulsant activity using the MES test and
sc-PTZ screening. The most active compound
14 showed anti-MES activity with an ED
50 value of 14.3 mg/kg and TD
50 value of 434 mg/kg after i.p. injection to mice (
Table 1), which showed compound
14 with a PI of 30.3 in the MES test [
24].
He
et al., prepared series of 16 new1-(8-(benzyloxy)quinolin-2-yl-6-substituted-4,6-dia-zaspiro [2,4]heptane-5,7-diones and evaluated their anticonvulsant activities using the MES and
sc-PTZ tests. The most active compound
15 showed the MES-induced seizures with ED
50 value of 8.6 mg/kg and TD
50 value of 365.3 mg/kg after i.p. to mice (
Table 1), compound
15 with a PI value of 26.8 in the MES test [
25].
Kumar
et al., demonstrated synthesis of a series of quinoline-incorporated substituted thiadiazole and evaluated their anticonvulsant activity. Compound
16 showed protection against the MES model at 30 mg/kg and showed activity at both 0.5 and 4 h period at dose level of 30 mg/kg indicating the compound to be highly potent and long acting (
Table 1) [
26].
3. The Quinazoline or Quinazolinone Functional Groups
As new horizons in anticonvulsant therapy, the quinazolines and quinazolinone structural class has been proved to be useful for the design and development of potent anticonvulsant agents [
27,
28].
Wang
et al., synthesized several series of novel 5-alkoxytetrazolo[1,5-
a]quinazoline derivatives. Anticonvulsant activities were evaluated using the MES test. Compound
17 protected completely against MES-induced seizure at a dose of 100 mg/kg (
Table 2), and was the best active compound in this series [
29].
Zheng
et al., prepared a series of novel 5-phenyl-[1,2,4]triazolo[4,3-
c]quinazolin-3-amine derivatives and screened their anticonvulsant activities by the MES test and their neurotoxicity was evaluated by the rotarod neurotoxicity test. The most promising compound was
18 (
Table 2), which showed an ED
50 value of 27.4 mg/kg and a PI value of 5.8. These values were superior to those provided by valproate (ED
50 and PI values of 272 and 1.6, respectively) in the MES test in mice [
30].
El-Azab
et al., established a new series of 2,3,8-trisubstituted-4 (3
H)-quinazoline derivatives. Compounds
19,
20 and
21 displayed median LD
50 values of 1000, 418 and 501 mg/kg with therapeutic index (LD
50/ED
50) values 10.2, 1.53 and 3.34 (
Table 2). Compounds
19,
20 and
21 showed better anticonvulsant activity and much lower toxicity comparable with the reference drugs valproate and methaqualone [
31].
El-Azab
et al., reported a novel series of 7-substituted-4(3
H)-quinazolinone and evaluated their antitumor and anticonvulsant activities. Compounds
22,
23,
24,
25,
26 and
27 showed advanced anticonvulsant activity as well as lower neurotoxicity than reference drugs valproate and methaqualone (
Table 2) [
32].
Abbas
et al., designed and synthesized a series of 2,3-disubstituted quinazolinone derivatives and a [1,2,4]triazino[2,3-
c]quinazolinone and screened their anticonvulsant activity using the
sc-PTZ and MES models. The study showed the most active compound
28 had a protective dose 50 (PD
50) of 200.53 μmol/kg (PD
50 of phenobarbitone = 62.18 μmol/kg) (
Table 2) [
33].
Rajasekaran
et al., synthesized a series of ten novel derivatives of 3-substituted-2-thioxoquinazolin-4(3
H)-ones. The titled compounds were evaluated for anticonvulsant activities by MES test. The compounds
29 and
30 showed potent anticonvulsant activity (
Table 2) [
34].
Prashanth
et al., reported a novel class of
N-substituted glycosmicine derivatives and evaluated their anticonvulsant activity by MES test and their neurotoxic effects were determined by rotorod test in mice. The most active compounds
31 and
32 exhibited anticonvulsant activity against MES-induced seizure at the dose of 100 mg/kg (
Table 2). Among all compouds
31 and
32 were recorded 70% of protection [
35].
Malik
et al., prepared various
N-(benzo[d]thiazol-2-ylcarbamoyl)-2-methyl-4-oxoquinazoline-3(4
H)-carbothioamide derivatives and evaluated their anticonvulsant activity with MES and
sc-PTZ models in mice. The most active one was compound
33 with ED
50 value of 82.5 mmol/kg (MES) and 510.5 mmol/kg (
sc-PTZ) (
Table 2). This molecule was more potent than phenytoin and ethosuximide which were used as reference antiepileptic drugs [
36].
Saravanan
et al., demonstrated some novel quinazolinone derivatives and screened their antiepileptic activity using MES and
sc-PTZ seizure tests. The most active one was compound
34 that revealed protection in MES at a dose of 30 mg/kg (ip) after 0.5 and 4 h (
Table 2). This molecule also provided protection in the
sc-PTZ at a dose of 100 mg/kg (0.5 h) and 300 mg/kg (4 h) [
37].
Table 2.
Anticonvulsant activity of quinazoline or quinazolinone compounds.
![Molecules 20 19714 i005]()
Table 2.
Anticonvulsant activity of quinazoline or quinazolinone compounds. ![Molecules 20 19714 i005]()
| Compound No. | Substituent Group | Reference No. |
|---|
| 19 | 2-CH3, 3-PH(2-CH3), 8-OCHCONHNH2 | [31] |
| 20 | 2-CH3, 3-PH(2-CH3), 8-OCHCONHNHCOOC2H5 |
| 21 | 2-CH3, 3-PH(2-CH3), 8-OCHCONHNHCSSCH3 |
| 22 | 2-CH3, 3-PH(2-CH3), 8-NHCOOC2H5 | [32] |
| 23 | 2-CH3, 3-PH(2-CH3), 8-NHCHPh(4-F) |
| 24 | 2-CH3, 3-PH(2-CH3), 8-NHCHPh(4-Cl) |
| 25 | 2-CH3, 3-PH(2-CH3), ![Molecules 20 19714 i006]() |
| 26 | 2-CH3, 3-PH(2-CH3), ![Molecules 20 19714 i007]() |
| 27 | 2-CH3, 3-PH(2-CH3), ![Molecules 20 19714 i008]() |
| 28 | 2-CH2OPh(2,4-Cl2), 3-NHCOCH2NHNHCOC5H4N | [33] |
| 29 | 3-Ph, 2-=S, ![Molecules 20 19714 i009]() (R=1,3-dichlorobenzene) (R=1,3-dichlorobenzene) | [34] |
| 30 | 3-naphthalene, 2-=S, ![Molecules 20 19714 i010]() |
| 31 | 1-CH3, 2-=O, 3-COC2H4NHPh(4-Cl) | [35] |
| 32 | 1-CH3, 2-=O, 3-COC2H4NHPh(4-F) |
| 33 | 2-CH3, ![Molecules 20 19714 i011]() | [36] |
| 34 | 2-CH3, ![Molecules 20 19714 i012]() | [37] |
4. The Thiazole or Benzothiazole Functional Groups
In the past few decades, the literature has been enriched with progressive findings about the anticonvulsant activities of various substituted thiazole derivatives [
38,
39].
Siddiqui
et al., prepared a series of 1,3-benzothiazol-2-yl-semicarbazones and evaluated their anticonvulsant activity. Compounds
35,
36 and
37 had shown 100% protection at both the time intervals, that is, 0.5 and 4 h (
Table 3). None of the compounds had shown the sign of neurotoxicity [
40].
Table 3.
Anticonvulsant thiazole or benzothiazole compounds.
![Molecules 20 19714 i013]()
Rana
et al., prepared a series of 1,3-benzothiazol-2-yl-benzamides and evaluated their anticonvulsant activity. Compounds
38,
39,
40 emerged as anticonvulsants with no neurotoxicity and can be claimed to detect compounds possessing effects against generalized toniceclonic (grand mal) and generalized absence (petit mal) seizures, respectively (
Table 3) [
41].
Hassan
et al., had reported synthesis of a series of
N-(substituted benzothiazol-2-yl)amide derivatives and evaluated their anticonvulsant effect. Compound
41 emerged as the most effective, with median doses of 40.96 mg/kg (MES ED
50), 85.16 mg/kg (
sc-PTZ ED
50) and 347.6 mg/kg (TD
50) (
Table 3) [
42].
Siddiqui
et al., demonstrated a synthesis of various
N-(5-chloro-6-substituted-benzothiazol-2-yl)-
N′-(substituted phenyl)-[1,3,4]thiadiazole-2,5-diamines. All the newly synthesized compounds were screened for their anticonvulsant activity and were compared with the standard drug phenytoin sodium. Compounds
42 and
43 showed complete protection against MES-induced seizures (
Table 3) [
43].
Siddiqui
et al., also synthesized a series of sulphonamide derivatives and evaluated their possible anticonvulsant activity and neurotoxicity. Compounds
44 and
45 were active at lower doses of 100 and 30 mg/kg, respectively, after 4.0 h (
Table 3). Compounds
44 and
45 showed activity at 300 mg/kg after 4 h in
sc-PTZ screening. Two compounds
44 and
45 showed delayed toxicity that was toxic only after 4.0 h, which were comparable with that of Carbamazepine (300 mg/kg) [
44].
Farag
et al., reported many derivatives of heterocyclic compounds containing a sulfonamide thiazole moiety and evaluated the anticonvulsant effect. Compound
46 obviously showed anticonvulsant activity with no tonic stretching stage and protected all the animals tested (
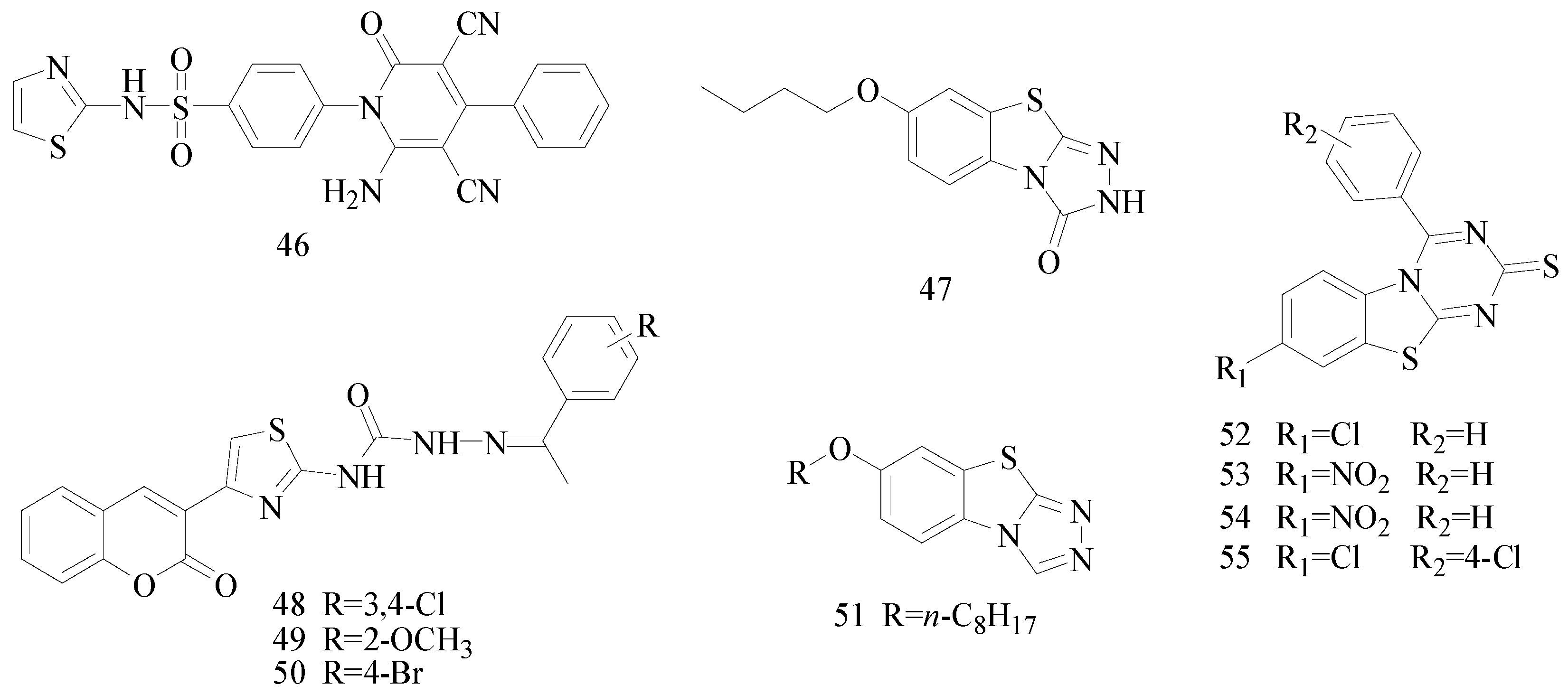
Figure 1) [
45].
Figure 1.
Structures of compounds 46–55.
Figure 1.
Structures of compounds 46–55.
Siddiqui
et al., designed and synthesized several heteroaryl semicarbazones. All synthesized compounds were tested for anticonvulsant activity utilizing pentylenetetrazole-induced seizure (PTZ) and MES tests. Three compounds of the series,
47,
48 and
49, exhibited significant anticonvulsant activity at dose of 30 mg/kg comparable to the standard drug phenytoin (
Figure 1) [
46].
Liu
et al., established a new series of 7-alkoxy[1,2,4]triazolo[3,4-
b]benzothiazol-3(2
H)-ones and evaluated their anticonvulsant activities. Compound
50 was the most active in MES-induced seizure test with ED
50 value of 13.6 mg/kg (
Figure 1). Meanwhile, its neurotoxicity was extremely low, with PI > 51 [
47].
Deng
et al., reported synthesis of 7-alkoxy-triazolo-[3,4-
b]benzo[
d]thiazoles. In the MES test, most of the compounds synthesized showed good effects on convulsion. Among the compounds studied, compound
51 was found to be the most potent compound with ED
50 value of 8.0 mg/kg and PI value of 15.0 (
Figure 1), possessing better anticonvulsant activity and higher safety than market drugs carbamazepine and phenytoin. Compound
51 exhibited activities of broad spectrum in several animal models [
48].
Siddiqui
et al., synthesized a number of new 8-substituted-4-(2/4-substituted phenyl)-2
H-[1,3,5]triazino[2,1-
b][1,3]benzothiazole-2-thiones and evaluated their anticonvulsant in a mouse seizure model and were comparable with the standard drug phenytoin. Compounds
52,
53,
54 and
55 showed complete protection after time periods of 0.5 h and 4 h (
Figure 1) [
49].
6. The Oxadiazole or Benzoxazinone Functional Groups
The oxadiazole scaffold is very versatile and has been subjected to extensive study in recent years. Compounds containing oxadiazole rings have been studied for many biological activities [
53].
Bhat
et al., prepared a series of 3-(4-acetyl-5
H/methyl-5-substituted phenyl-4,5-dihydro-1,3,4-oxadiazol-2-yl)-2
H-chromene-2-ones and evaluated their anticonvulsant activity and neurotoxicity. Compound
62 was found to be potent and had activity at a lower dose of 30 mg/kg in MES-test (
Table 4). All the compounds were less toxic as compared with the standard drug phenytoin [
54].
Tabatabai
et al., synthesized a series of some derivatives of 2-(2-phenoxy)phenyl-1,3,4-oxadiazole. Although the most effective compound
63 was a weaker anticonvulsant than diazepam (
Table 4), it should be mentioned that it had a good margin of safety and LD
50, which were 15-fold its ED
50 [
55].
Harish
et al., reported a series of novel 1-[5-(4-methoxyphenyl)-[1,3,4]oxadiazol-2-yl]-piperazine derivatives. The anticonvulsant effects of these derivatives on MES-induced seizures were experimented in male Wistar rats and phenytoin was used as reference drug. Compounds
64,
65,
66 and
67 showed excellent anticonvulsant activity in MES model (
Table 4) [
56].
Table 4.
Anticonvulsant oxadiazole or benzoxazinone compounds.
![Molecules 20 19714 i024]()
Harish
et al., investigated a series of new 2-methyl-2-[3-(5-piperazin-1-yl-[1,3,4]oxadiazol-2-yl)-phenyl]-propionitrile derivatives. All the compounds were screened for their anticonvulsant activity against MES seizure and their neurotoxic effects were determined by rotorod test. Compounds
68,
69 and
70 were found to be the most potent of this series (
Table 4). These compounds showed no neurotoxicity at the maximum dose administered (100 mg/kg) [
57].
Siddiqui
et al., reported a synthesis of new 5-(1
H-indol-3-yl)methyl-4-(substituted-aryl)-2,4-dihydro-3
H-1,2,4-triazole-3-thiones. All the newly synthesized compounds were screened for their anticonvulsant activity in the MES model and were compared with the standard drugs phenytoin sodium and carbamazepine. Among these compounds,
71 was found to be the most active in the series that showed protection against seizures both after 0.5 h and 4 h at 30 mg/kg body mass (
Table 4) [
58].
Siddiqui
et al., designed and synthesized a series of 5-carbomethoxybenzoxazole derivatives. Compounds
72,
73,
74 and
75 were found to be more lipophilic (
Figure 3), thus having potent anticonvulsant activity [
59].
Wei
et al., demonstrated a synthesis of novel 2-substituted-6-(4
H-1,2,4-triazol-4-yl)benzo[
d] oxazoles and evaluated the anticonvulsant activity with the MES test and
sc-PTZ test. Compound
76 was the most active and also had the lowest toxicity (
Figure 3). In the anti-MES potency test, it showed ED
50 of 29.5 mg/kg, a TD
50 of 285 mg/kg, and a PI of 9.7 which was greater than the reference drug, carbamazepine that has a PI of 6.4 [
60].
Figure 3.
Structures of compounds 71–79.
Figure 3.
Structures of compounds 71–79.
Malik
et al., prepared a series of 3-(benzo[
d]isoxazol-3-yl)-
N-substituted pyrrolidine-2,5-dione and evaluated their anticonvulsant activities. Preliminary anticonvulsant activity was performed using MES and
sc-PTZ tests after ip injection into mice. ED
50 value of compound
77 was 14.90 mg/kg (
Figure 3). Similarly the most potent one in
sc-PTZ was compound
78 with an ED
50 value of 42.30 mg/kg (
Figure 3). These compounds were more active and had lower neurotoxicity than the control drugs ethosuximide and phenytoin [
61].
Malik
et al., synthesized a novel series of (5-amino-3-substituted-1,2,4-triazin-6-yl)(2-(6-halo-substituted-benzo[d]isoxazol-3-yl) pyrrolidin-1-yl)methanone. The MES test showed that compound
79 was the most potent compound (
Figure 3), with an ED
50 value of 6.20 mg/kg (oral/rat) and a PI value of >48.38 which was much higher than the PI of the reference drug phenytoin [
62].
8. The Pyrazole Functional Group
Pyrazole and its derivatives consist of five-membered heterocycles with two
ortho-nitrogen-atoms. These compounds have received attention in the field of current medicinal and pharmacological research, and are reported to have a broad spectrum of biological activities, such as antitumor, antimicrobial, antioxidant and antimalarial activities [
67,
68,
69,
70].
Kaushik
et al., established synthesis of
N′-[(5-chloro-3-methyl-1-phenyl-1
H-pyrazol-4-yl)methylene]2/4-substituted hydrazides and evaluated their anticonvulsant activity against MES- and
sc-PTZ-induced seizure models in mice. All compounds showed protection in the MES model at 100 mg/kg, including compound
84 which showed activity at 0.5 h and 4.0 h periods indicating that
84 was potent having a rapid onset and long duration of action (
Figure 5). Compound
84 showed activity at a dose of 100 mg/kg comparable to sodium valproate in the
sc-PTZ test [
71].
Figure 5.
Structures of compounds 84–91.
Figure 5.
Structures of compounds 84–91.
Siddiqui
et al., had reported various 3,5-(substituted-diphenyl)-4,5-dihydro-pyrazole-1-acid phenylamides and evaluated their anticonvulsant activities. Compounds
85,
86,
87 and
88 were found to protect 100% of the animals in the MES screening at a dose of 25 mg/kg (
Figure 5). They were also found to have appreciable anticonvulsant activity in
sc-PTZ screening [
72].
Ahsan MJ
et al., designed and synthesized a series of fourteen 3a,4-dihydro-3
H-indeno[1,2-
c] pyrazole-2-carboxamide/carbothioamide analogues. Among the compounds synthesized, some exhibited marked effect on seizure model under minimal clonicity (6 Hz psychomotor seizure test). Compound
89 was found to be the most active compound of the series showing 75% (3/4, 0.25–2.0 h) and 50% (2/4, 4.0 h) protection against minimal clonic seizure at 100 mg/kg without any toxicity (
Figure 5). Compound
90 showed protection in MES seizure and subcutaneous metrazol (
sc-MET) seizure at 300 mg/kg (
Figure 5) [
73].
Farghaly A
et al., synthesized a series of new pyrazolo[3,4-
b]pyrazines containing, 1,2,4-oxadiazolyl, thiadiazolyl, imidazothiadiazolyl, thiazolidinonyl, substituents and other different substituents. Compound
91 showed best results at reducing PTZ-induced tonic convulsions and mortality (
Figure 5) [
74].
9. The Imidazole Functional Group
Imidazole and its derivatives are a class of 5-membered heterocyclic structures having two non-adjacent nitrogen atoms. Recent studies revealed that the substituted imidazole derivatives have attracted much attention due to their broad spectrum of pharmacological activities such as anti-inflammatory, analgesic [
75,
76]. Literature survey shows that imidazole-heterocyclic compounds could be new classes of anticonvulsant agents by the virtue of their potential anticonvulsant properties [
77].
Karakurt
et al., described a series of 2-acetylnaphthalene derivatives. Quantification of anti-convulsant protection was calculated via the i.p. route (ED
50 and TD
50) for the most active candidate (
92) (
Figure 6). Observed protection in the MES model was 38.46 mg/kg and 123.83 mg/kg in mice and 20.44 mg/kg, 56.36 mg/kg in rats, respectively [
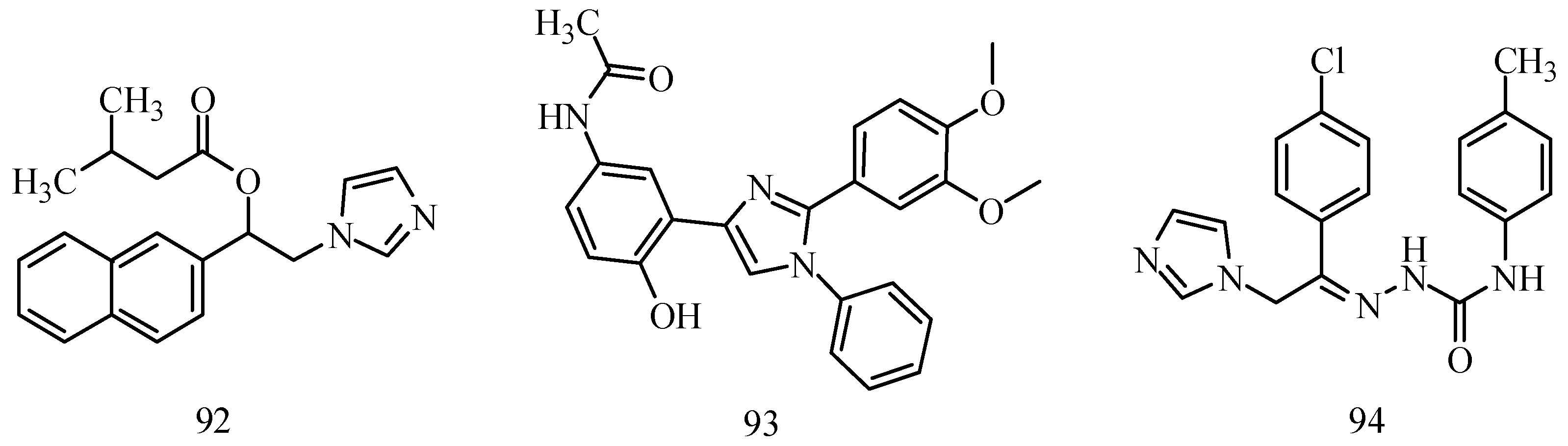
78].
Figure 6.
Structures of compounds 92–94.
Figure 6.
Structures of compounds 92–94.
Husain
et al., established a synthesis of a series of 1,2,4-trisubstituted-1
H-imidazole derivatives. Anticonvulsant activity was shown by the majority of the synthesized compounds in the MES and
sc-PTZ screening when given i.p. to mice. In anticonvulsant screening, only one compound,
93, showed potent activity comparable to that of standard drugs phenytoin and carbamazepine (
Figure 6) [
79].
Amir
et al., demonstrated synthesis of a series of novel imidazole incorporated semicarbazones. Compound
94 showed the highest activity among the compounds synthesized with no neurotoxic and depressant effects on CNS (
Figure 6). Liver enzyme estimations (serum glutamate oxaloacetate transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT), alkaline phosphatase) of the compound also showed no significant change in the enzyme levels [
80].
Ulloora
et al., prepared a variety of five new series of imidazo[1,2-
a]pyridines carrying biologically active pyrazoline, cyanopyridone, cyanopyridine, 2-aminopyrimidine and pyrimidine-2-thione systems. The target compounds were screened for their
in vivo anticonvulsant activity following MES and
sc-PTZ methods at a small test dose of 10 mg/kg. Compounds
95,
96,
97,
98,
99 and
100 displayed potent anticonvulsant activity without displaying any toxicity (
Table 5) [
81].
Ulloora
et al., designed and synthesized new 2-arylimidazo[1,2-
a]pyridines carrying suitably substituted 1,2,3-triazoles. The anticonvulsant study was carried out by MES and
sc-PTZ screening methods, while their toxicity study was performed following rotarod method. The most active was compound
101 which displayed reasonably good activity in both the durations of 0.5 and 4 h indicating that they possess rapid onset and long duration of action (
Table 5). It exhibited complete protection against seizure and their activity at 20 mg/kg was comparable with that of standard drug diazepam [
82].
Table 5.
Anticonvulsant imidazole compounds.
![Molecules 20 19714 i030]()
10. The Pyrimidine Functional Group
Pyrimidine is an aromatic heterocyclic organic compound similar to pyridine. One of the three diazines, six-membered heterocyclics with two nitrogen atoms in the ring, has the nitrogens at positions 1 and 3 in the ring. Pyrimidines that have a broad spectrum of bioactivities (antibacteria, anticancer and anti-inflammation and so on) are an important one of the heterocyclic compounds [
83,
84,
85].
Alam
et al., synthesized a number of
N-(4,6-substituted diphenylpyrimidin-2-yl) semicarbazones and tested their anticonvulsant activity against the two seizure models, MES and
sc-PTZ. Three compounds (
102,
103 and
104) were found to be significantly active as they showed protection at the lowest dose of 30 mg/kg after 0.5 h and did not show any sign of neurotoxicity except in case of compound
102 which was found to be neurotoxic at 300 mg/kg after 4.0 h (
Figure 7) [
86].
Deng
et al., described the synthesis and anticonvulsant activities of 7-(substituted-phenyl)-6,7-dihydro-[1,2,4]triazolo[1,5-
a]pyrimidin-5(4
H)-ones and their derivatives. The majority of the compounds synthesized showed inhibition effects on MES-induced convulsion. The most promising compound
105 showed significant anticonvulsant activity in MES test with ED
50 value of 19.7 mg/kg (
Figure 7). It was safer than reference drugs with much higher PI value. In addition, the protective effect of compound
105 against seizures induced by PTZ, ISO, TSC, 3-MP, and bicuculline in the chemical-induced seizure tests suggested that compound
103 displayed broad spectrum activity in several models [
87].
Jiang
et al., reported a novel series of 7-substituted-5-phenyl-[1,2,4]triazolo[1,5-
a] pyrimidines. Their anticonvulsant activities were measured through the MES test, and carbamazepine (ED
50 = 11.8 mg/kg) and valproate (ED
50 = 272 mg/kg) were used as the reference drugs. Amongst the compounds tested, compound
106 was the most active in inhibiting convulsion with ED
50 value of 84.9 mg/kg that was higher than valproate but lower than carbamazepine (
Figure 7) [
88].
Shaquiquzzaman
et al., established syntheses of some new pyrimidine-5-carbonitrile derivatives. In the MES test, compounds
107,
108 and
109 were found to be highly active at a dose level of 30 mg/kg at 0.5 h time interval (
Figure 7), indicating their ability to prevent seizure spread at a relatively low dose [
89]. Shaquiquzzaman
et al., also reported a series of dihydropyrimidine-5-carbonitrile derivatives and evaluated their anticonvulsant activity against MES and
sc-PTZ models. Compounds
110 and
111 were found to be most active showing activity both in MES and
sc-PTZ screen at lower doses of 30 mg/kg at 0.5 h and 100 mg/kg at 4 h (
Figure 7). In the rotarod motor impairment screen, compound
110 did not show any motor impairment, even at the maximum dose of 300 mg/Kg. The pharmacophore hypothesis also fits best for compounds
110 and
111 [
90].
Figure 7.
Structures of compounds 102–111.
Figure 7.
Structures of compounds 102–111.
11. The Phthalazine Functional Group
As a heterocyclic compound, the molecular formula of phthalazine is C
8H
6N
2. Because of the broad spectrum of bioactivities such as anticonvulsion, vasorelaxation, anti-inflammation and cardiotonic effect, its derivatives are generally used for treating disease [
91,
92,
93].
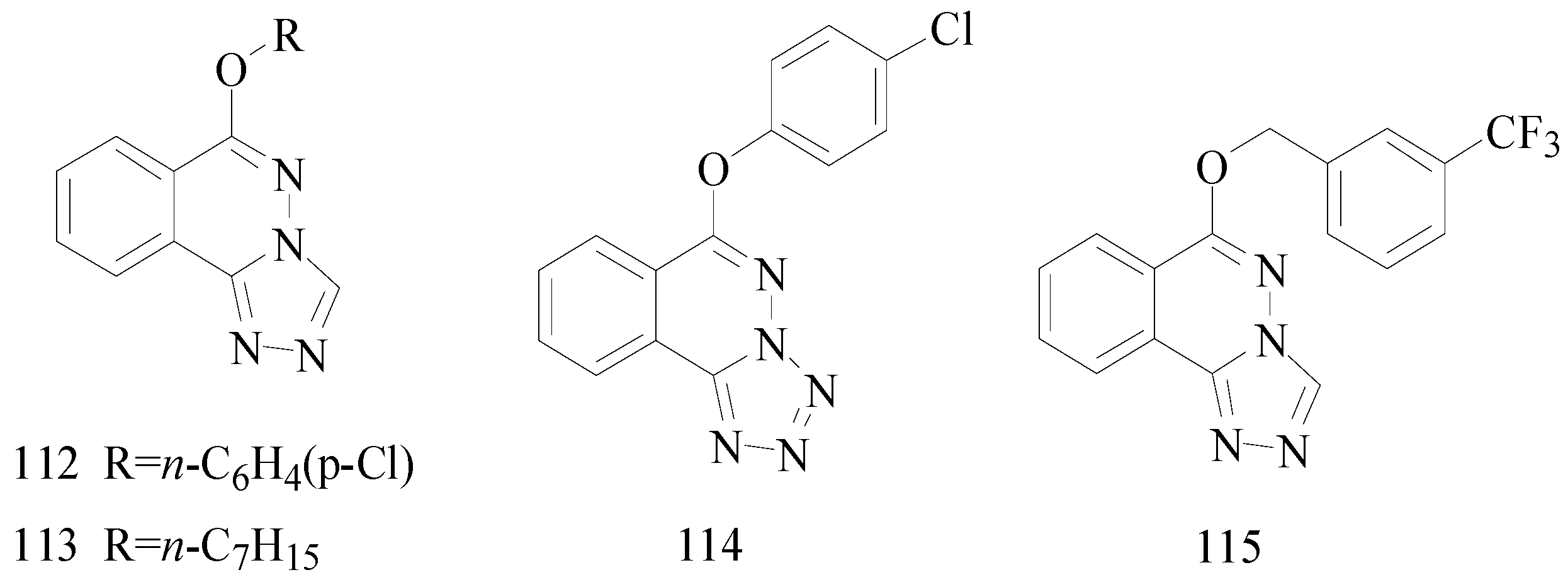
Zhang
et al., designed and synthesized a new series of 6-alkoxy-[1,2,4]triazolo[3,4-
a] phthalazines and evaluated their anticonvulsant activity and neurotoxicity by the MES test and the rotarod test respectively. The most promising compounds
112 and
113 showed a median effective dose of 7.1 and 11.0 mg/kg (
Figure 8), and had protective index values of 5.2 and 8.0, respectively. The two compounds were further found to have potent activity against seizures induced by PTZ, ISO, TSC, 3-MP but not seizures induced by strychnine [
94].
Sun
et al., investigated a new phthalazine tetrazole derivative. Compound
114 exhibited higher activity (ED
50 = 6.8 mg/kg) and lower neurotoxicity (TD
50 = 456.4 mg/kg) (
Figure 8), resulting in a higher PI = 67.1 compared with carbamazepine (PI = 6.4). In addition, compound
114 exhibited significant oral anticonvulsant activity (ED
50 = 24 mg/kg) against MES-induced seizure with low neurotoxicity (TD
50 > 4500 mg/kg) in mice, resulting in a PI value of more than 187.5. Compound
114 was also tested in chemically induced animal models of seizure (PTZ, ISO, TSC and 3-MP) to further investigate the anticonvulsant activity. Compound
114 produced significant anticonvulsant activity against seizures induced by ISO, TSC and 3-MP [
95].
Bian
et al., reported a synthesis of new 6-substituted-[1,2,4]triazolo[3,4-
a](tetrazolo[5,1-
a]) phthalazine derivatives. All the compounds were evaluated for their anticonvulsant activities using the MES test. The most promising compound
115 showed significant anticonvulsant activity in MES test with ED
50 value of 9.3 mg/kg (
Figure 8). It displayed a wide margin of safety with protective index much higher than the standard drug carbamazepine [
96].
Figure 8.
Structures of compounds 112–115.
Figure 8.
Structures of compounds 112–115.
13. The Triazolethione Functional Group
Many compounds bearing a triazole moiety were found to possess anticonvulsant properties in various animal models of epilepsy. Therefore, some people want to loop through a combination of triazole-thione compounds to improve the antiepileptic activity.
Luszczki
et al., reported the effects of 4-(4-bromophenyl)-5-(3-chlorophenyl)-2,4-dihydro-3
H-1,2,4-triazole-3-thione (compound
121) and 5-(3-chlorophenyl)-4-(4-methylphenyl)-2,4-dihydro-3
H-1,2,4-triazole-3-thione (compound
122) on the protective action of four classical antiepileptic drugs—carbamazepine, phenobarbital, phenytoin and valproate—against MES test in mice (
Table 6). Results indicated that compound
121 administered intraperitoneally at doses of 75 and 100 mg/kg significantly elevated the threshold for electroconvulsions in mice. Compound
121 (50 mg/kg) significantly enhanced the anticonvulsant activity of carbamazepine, phenobarbital and valproate. Compound
122 administered intraperitoneally at 10 mg/kg significantly elevated the threshold for electroconvulsions in mice. Moreover, compound
122 (5 mg/kg) significantly enhanced the anticonvulsant activity of valproate, but not that of carbamazepine, phenobarbital or phenytoin in the MES test in mice. Pharmacokinetic experiments revealed that compound
122 significantly elevated total brain concentrations of valproate in mice [
103,
104].
Table 6.
Structures of compounds
121–
126.
![Molecules 20 19714 i038]()
Table 6.
Structures of compounds 121–126. ![Molecules 20 19714 i038]()
| Compound No. | R | R1 | Reference |
|---|
| 121 | -Ph(p-Br) | — | [103,104] |
| 122 | -Ph(p-CH3) | — |
| 123 | n-C6H13 | — | [105] |
| 124 | -Ph(p-F) | — |
| 125 | -Ph(o-CH3) | -Cl | [106] |
| 126 | -Ph(p-OCH3) | -Br | [107] |
Siddiqui
et al., prepared a various of 3-[4-(substituted phenyl)-1,3-thiazol-2-ylamino]-4-(substituted phenyl)-4,5-dihydro-1
H-1,2,4-triazole-5-thiones. Their
in vivo anticonvulsant screenings were performed using the two most adopted seizure models, MES and
sc-PTZ. Two compounds,
123 and
124 (
Table 6), showed significant anticonvulsant activity in both the screenings with ED
50 values of 23.9 mg/kg and 13.4 mg/kg, respectively, in the MES screen and 178.6 mg/kg and 81.6 mg/kg, respectively, in the
sc-PTZ test. They displayed a wide margin of safety with PI, median hypnotic dose (HD
50) and median lethal dose (LD
50) which were much higher than that of the standard drugs [
105].
Plech
et al., designed and synthesized 4-alkyl-1,2,4-triazole-3-thione derivatives. A group of derivatives showed strong anticonvulsant activity. The characteristic features of the most active compounds were rapid onset and long lasting effects. Among the tested compounds, compound
125 was assayed for the different PI values at different preprocessing times (
Table 6), and the results of that were ranging from 2.8 to 9.7 [
106].
Plech
et al., also reported a synthesis of 1,2,4-triazole-3-thione derivatives. Characteristic features of all active compounds were a rapid onset of action and long lasting effects. Compound
126 exhibited the most potent activity (ED
50 = 35.2 mg/kg) (
Table 6) [
107].
17. The Imidazoline-2,4-dione Functional Group
Imidazoline-2,4-diones, also called hydantoins, a class of cyclic imides, have been demonstrated to possess good anticonvulsant properties [
134]. Their substitution products have also been found a number of other pharmacological properties such as antitumor, anti-HIV and antibacterial activities [
135,
136,
137].
He
et al., synthesized new 6-methyl-1-substituted-4,6-diazaspiro[2.4]heptane-5,7-diones and tested the anticonvulsant activity using the MES and
sc-PTZ screens. Their neurotoxicity was determined by the rotarod test. The most active of the series was compound
166 (
Table 8), which showed a MES ED
50 value of 12.5 mg/kg in mice. The TD
50 was 310 mg/kg, providing compound
166 with a PI of 24.8 in the MES test which is better than that of Phenytoin [
138].
He
et al., investigated some new
N-3-arylamide substituted 5,5-cyclopropanespirohydantoin derivatives synthesized and tested for anticonvulsant activity using the maximal electroshock (MES), subcutaneous pentylenetetrazole (
sc-PTZ) screens, which are the most widely employed seizure models for early identification of candidate anticonvulsants. Their neurotoxicity was determined applying the rotorod test. The most active compound
167 showed the MES-induced seizures with ED
50 value of 9.2 mg/kg and TD
50 value of 421.6 mg/kg after i.p. to mice (
Table 8), which provided compound
167 with a protective index (TD
50/ED
50) of 45.8 in the MES test [
139].
Botros
et al., designed and synthesized new phenytoin derivatives and tested the anticonvulsant activity. Preliminary anticonvulsant screening was performed using standard MES and
sc-PTZ screens in mice. The neurotoxicity was determined by applying the rotarod test. Among these compounds,
168 and
169 showed the highest protection (80%) in the
sc-PTZ test at a dose of 100 mg/kg, whereas the compound
170 displayed promising anticonvulsant effect in the MES model (
Table 8) [
140].
Byrtus
et al., prepared a various of
N-Mannich bases derived from 5-cyclopropyl-5-phenyl- and 5-cyclopropyl-5-(4-chlorophenyl)-imidazolidine-2,4-diones. The quantitative evaluation after oral administration in rats showed that the most active was compound
171 with ED
50 values of 5.76 mg/kg (MES) and 57.31 mg/kg (
sc-PTZ) (
Table 8). Compared with the control drugs of ethosuximide and phenytoin, it was more active in the anti-convulsion assays. Additionally compound
171 with ED
50 of 26.06 mg/kg in a psychomotor seizure test (6-Hz) in mice showed comparable activity to a new generation anticonvulsant-levetiracetam [
141].
Dhanawat
et al., had reported a synthesis of
N-(3)-substituted-2,4-imidazolidine diones and oxazolidinediones derivatives and tested the anticonvulsant activity using the MES test. Compounds
172,
173,
174 and
175 exhibited significant anticonvulsant activity as compared to the standard drug phenytoin (
Table 8) [
142].
Botros
et al., described new bivalent ligands derived from phenytoin. Initial anticonvulsant screening was performed using MES and PTZ screens in mice. Most of the test compounds were found to be effective in at least one seizure model at a dose of 100 mg/kg. Compound
176 exhibited marked anticonvulsant activity in both MES and PTZ screens (
Table 8) [
143].
Byrtus
et al., established a synthesis of
N-Mannich from 5-cyclopropyl-5-phenyl- and 5-cyclopropyl-5-(4-chlorophenyl)-hydantoins and tested their anticonvulsant activity. The quantitative studies after oral administration to rats showed that several molecules were more potent than phenytoin and ethosuximide which were used as reference antiepileptic drugs. From the whole series, the most active was compound
177 with the ED
50 value of 5.29 mg/kg in the MES test (
Table 8) [
144].
Madaiah
et al., demonstrated a synthesis of new 3-[(2,4-dioxo-1,3,8-triazaspiro[4,6]undec-3-yl) methyl]benzonitrile derivatives and evaluated their possible anticonvulsant activity by MES and PTZ tests. Compounds
178,
179,
180 and
181 showed significant and protective effect on seizure when compared with the standard drug valproate (
Table 8). These compounds were found to exhibit advanced anticonvulsant activity as well as lower neurotoxicity than the reference drug [
145].
Madaiah
et al., synthesized a series of novel 1′-[2-(difluoromethoxy)benzyl]-2′
H,5′
H-spiro[8-azabicyclo[3,2,1]octane-3,4′-imidazolidine]-2′,5′-dione substituted hydantoins. The novel molecules were screened for anticonvulsant activity in mice by MES and
sc-PTZ-induced seizure tests. The neurotoxicity was assessed using the rotarod method. Compounds
182,
183,
184,
185 and
186 exhibited anticonvulsant potency against MES-induced seizure and in the
sc-PTZ model (
Table 8), with lesser neurotoxicity. Some title compounds showed lesser depression on central nervous system compared to phenytoin [
146].
Table 8.
Structures of compounds
166–
186.
![Molecules 20 19714 i048]()
Table 8.
Structures of compounds 166–186. ![Molecules 20 19714 i048]()
| Comp. No. | R | R1 | R2 | Reference |
|---|
| 166 | -CH3 | H | -Ph(p-SO2CH3) | [138] |
| 167 | -NHCOPh(p-CF3) | -CH3 | -CH3 | [139] |
| 168 | -CH2C(O)NNCSH | -Ph | -Ph | [140] |
| 169 | -CH2CONHNHCSNHPh(p-OCH3) | -Ph | -Ph |
| 170 | -CH2CONHNHCSNHPh | -Ph | -Ph |
| 171 | -H | -Ph | -C3H5 | [141] |
| 172 | -CH2N(CH2CH2)2Ph | -Ph | -C3H5 | [142] |
| 173 | -CH2CONHPh(p-Cl) | -Ph | -Ph |
| 174 | -CH2CONHPh(o-Cl) | -Ph | -Ph |
| 175 | -CH2CONHPh(p-OCH3) | -Ph | -Ph |
| 176 | -CH2CON(CH2CH2)2Ph(p-NO2) | -Ph | -Ph | [143] |
| 177 | -(CH2)2O(CH2)2 N(CH2CH2)2Ph(p-Cl) | -Ph | -Ph | [144] |
| 178 | -SO2Ph(o-F) | — | — | [145] |
| 179 | -SO2Ph(m-F) |
| 180 | -CO Ph(m-F) |
| 181 | -CO Ph(p-F) |
| 182 | -SO2Ph(o-F) | [146] |
| 183 | -SO2Ph(m-F) |
| 184 | -SO2Ph(o-F) |
| 185 | -CONHPh |
| 186 | -CONHPh(m-CH3) |
20. Miscellaneous Functional Groups
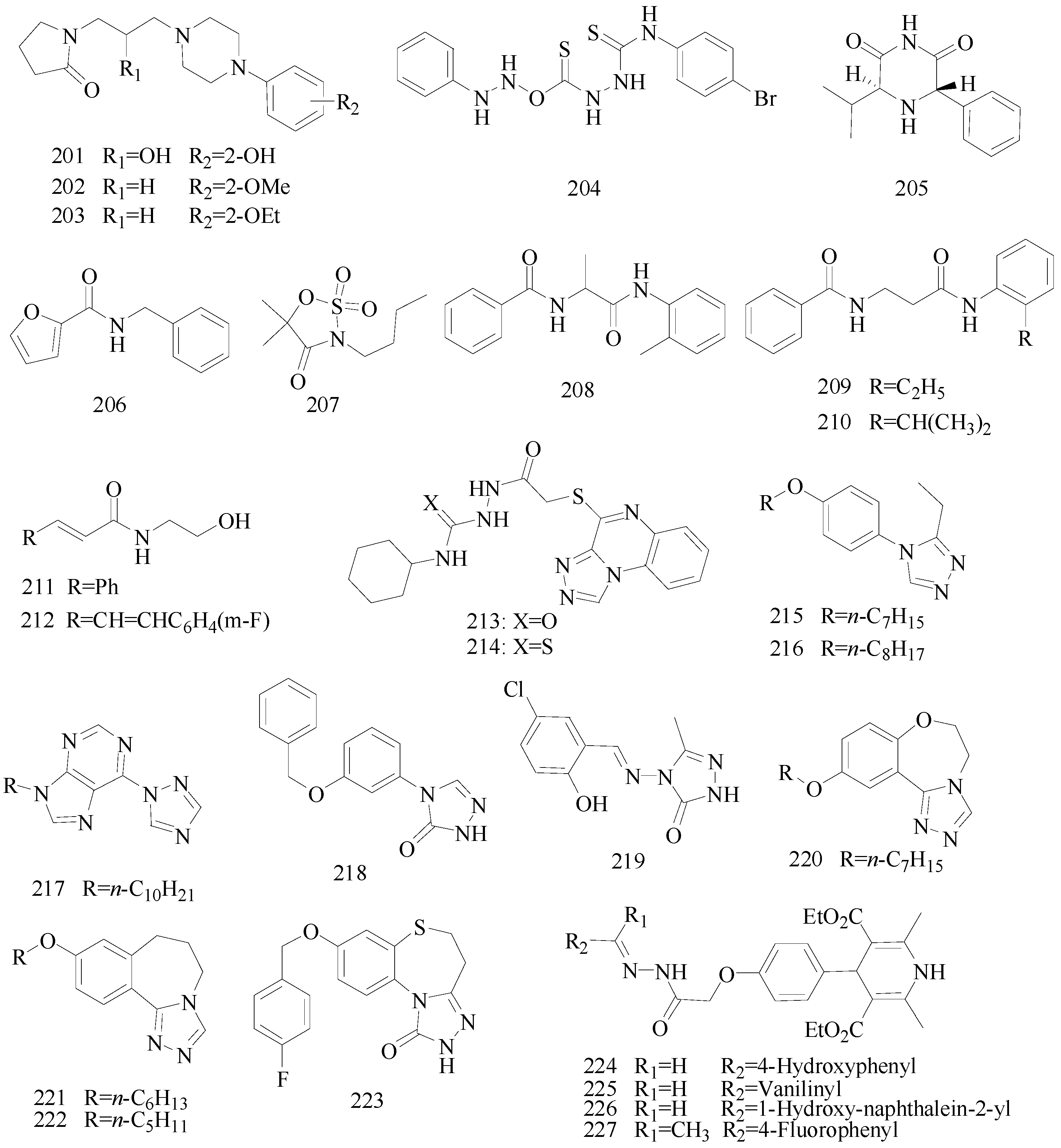
Sapa
et al., established a synthesis of some novel pyrrolidin-2-one derivatives and evaluated their possible anticonvulsant activity by MES and PTZ tests. Compound
201 significantly reduced the incidence of seizures in the MES test. The compounds
202 and
203 demonstrated activity in the PTZ-induced seizures test [
159,
160].
Nevagi
et al., demonstrated synthesis of novel thiosemicarbazide derivatives and evaluated their anticonvulsant activity and neurotoxicity. Amongst all the synthesized compounds, compound
204 is a broad-spectrum anticonvulsant agent since it was active in both MES- and PTZ-induced seizure models with no neurotoxicity (
Figure 12) [
161].
Dawidowski
et al., synthesized a series of novel diastereomerically pure, monocyclic 2,6-DKP derivatives using a diastereoselective method. In the MES test, some of them showed good or weak antiepileptic activities, however, there was no active compound in the
sc-Met screen. The most promising compound
205 exhibited notable action in the 6 Hz test (
Figure 12) [
162].
Strupińska
et al., synthesized a series of benzylamides of isocyclic and heterocyclic acids and tested their anticonvulsant activity. Nearly all synthesized heterocyclic acid derivatives showed anticonvulsant activity. Compound
206 appeared the most promising (
Figure 12). It showed in minimal clonic seizure (6 Hz) test (ASP) in rats after i.p. administration: MES ED
50 = 36.5 mg/kg, TOX TD
50 = 269.75 mg/kg, and PI = 7.39 [
163].
Pastore
et al., synthesized novel
N-derivative-1,2,3-oxathia-zolidine-4-one-2,2-dioxides heterocycles, bioisosteres of trimethadione (TMD, oxazolidine-2,4-dione) and phenytoin. Anticonvulsant screening was performed in mice after intraperitoneal administration in the MES test and
sc-PTZ test. Compound
207 (
Figure 12), the most active drug obtained, with an ED
50 of 60 μg/kg was 10,000 times more active than TMD, the reference compound in this work, and 90 times more active than valproic acid, an anticonvulsant drug presently in use in the clinic [
164].
Uysal
et al., designed and synthesized sixteen 2/3-benzoylaminopropionanilide derivatives. The anticonvulsant activity profile of the synthesized compounds was determined by MES and
sc-Met seizure tests. In the rotarod test, all of them exhibited no toxicity to the nervous system. Compounds
208,
209 and
210 were found to be more potent in the MES or
sc-Met tests (
Figure 12). Those compounds have emerged as lead compounds for future investigations [
165].
Guan
et al., prepared a variety of
N-(2-hydroxyethyl)cinnamamide derivatives and screened their anticonvulsant activities by the MES test and their neurotoxicity was evaluated by the rotarod test. In the anti-MES potency test, compounds
211 and
212 exhibited ED
50 dose of 17.7 and 17.0 mg/kg, respectively (
Figure 12), and TD
50 dose of 154.9 and 211.1 mg/kg, respectively, resulting in a PI of 8.8 and 12.4, respectively, which were much greater than the PI of the market antiepileptic drug carbamazepine [
166].
Alswah
et al., reported synthesis of some [1,2,4]triazolo[4,3-
a]quinoxaline derivatives as novel anticonvulsant agents. The anticonvulsant evaluation was used metrazol-induced convulsion model and phenobarbitone sodium was as a standard. Among this set of tested compounds, two of them (
213 and
214) showed the best anticonvulsant activities (
Figure 12) [
167].
Chen
et al., reported synthesis of 4-(4-alkoxylphenyl)-3-ethyl-4
H-1,2,4-triazole derivatives. Their anticonvulsant activities were evaluated by the MES test and their neurotoxicity was evaluated by the rotarod test. MES test showed that compound
215 was found to be the most potent with ED
50 value of 8.3 mg/kg and PI value of 5.5, but compound
216 exhibited better PI value of 9.3 (
Figure 12), which was much greater than PI value of the prototype drug phenytoin [
168].
Figure 12.
Structures of compounds 201–227.
Figure 12.
Structures of compounds 201–227.
Wang
et al., synthesized a series of new purines containing triazole and other heterocycle substituents and evaluated their preliminary anticonvulsant activity and neurotoxicity by using the MES,
sc-PTZ and rotarod tests. Among the compounds studied, compound
217 was the most potent compound, with a ED
50 of 23.4 mg/kg and a high protective index of more than 25.6 after intraperitoneal administration in mice (
Figure 12). Compound
217 showed significant oral activity against MES-induced seizures in mice, with an ED
50 of 39.4 mg/kg and a PI above 31.6 [
169].
Shu
et al., reported synthesis of 4-(3-alkoxy-phenyl)-2,4-dihydro-[1,2,4]triazol-3-ones. All target compounds exhibited anticonvulsant activity of varying degrees in the maximal electroshock test. Compound
218 was the most promising compound with an ED
50 value of 30.5 mg/kg and a PI of 18.63 (
Figure 12), showing a higher safety than the standard drug carbamazepine (PI = 6.45). In addition, the potency of compound
218 against seizures induced by pentylenetetrazole and 3-mercaptopropionic acid suggested its broad-spectrum activity [
170].
Kahveci
et al., designed and synthesized a series of new 1,2,4-triazole-3-one derivatives bearing the salicyl moiety. The anticonvulsant activities of all compounds were evaluated by the Anticonvulsant Screening Program of the U.S. National Institutes of Health. The most active compound
219 showed significant anticonvulsant activity with an ED
50 of 81.1 mg/kg at an approximate TPE (time of peak effect) of 1 h (
Figure 12) [
171].
Deng
et al., reported a synthesis of 10-alkoxy-5,6-dihydrotriazolo[4,3-
d]benzo[
f][1,4] oxazepine derivatives and screened their anticonvulsant activities by the MES test and their neurotoxicity was evaluated by the rotarod test. In the MES test, compound
220 was found to possess better anticonvulsant activity and higher safety than market drugs carbamazepine and phenytoin with an ED
50 value of 6.9 mg/kg a PI value of 9.5 (
Figure 12) [
172].
Piao
et al., reported a novel series of 9-alkoxy-6,7-dihydro-5
H-benzo[
c][1,2,4]triazolo[4,3-
a] azepine derivatives and screened their anticonvulsant activity by the MES test and the
sc-PTZ test. The results revealed that all of the compounds exhibited anticonvulsant activity, compound
221 was found to possess the most potent anticonvulsant activity in the anti-MES potency test (
Figure 12), it had a ED
50 value of 12.3 mg/kg, a TD
50 value of 73.5 mg/kg, and a PI of 6.0, which was slightly lower than the PI of the prototype drug carbamazepine (ED
50 = 8.8, PI = 8.1). In the
sc-PTZ test, compound
222 was the most active, with an ED
50 value of 19.8 mg/kg, a TD
50 value of 80.8 mg/kg and a PI value of 4.1, which are greatly higher than that of carbamazepine (ED
50 > 100, PI < 0.72) [
173].
Deng
et al., synthesized two series of 8-alkoxy-4,5-dihydrobenzo[
b][1,2,4]triazolo[4,3-
d] [1,4]thiazepine derivatives. All of the prepared compounds were effective in the MES screens, among which, compound
223 was considered as the most promising one with an ED
50 value of 26.3 mg/kg and a superior PI value of 12.6 (
Figure 12). The potency of compound
223 against seizures induced by pentylenetetrazole, 3-mercaptopropionic acid and bicuculline was great too [
174].
Ulloora
et al., synthesized new substituted 1,4-dihydropyridin-4-yl-phenoxyacetohydrazones. The final compounds were screened for their
in vivo anticonvulsant activity by MES,
sc-PTZ and 6 Hz methods. The active compounds,
224,
225,
226 and
227 exhibited their activities at 4 h after i.p. injection with 100 mg/kg (
Figure 12). All these tested compounds exhibited activity in 6 Hz method within 1 h [
175].
Siddiqui
et al., reported synthesis of various 1-[6-(4-substituted phenyl)-3-cyano-4-(substituted phenyl)-pyridin-2-yl]-5-oxopyrrolidine-3-carboxylic acids. Their
in vivo anticonvulsant evaluation was performed by MES and
sc-PTZ tests. Compounds
228 and
229 displayed comparable anticonvulsant activity to the standard drugs with ED
50 values of 13.4 and 18.6 mg/kg in electroshock screen, respectively (
Figure 13). The compounds
228 and
229 were also found to have encouraging anticonvulsant activity (ED
50 = 86.1 and 271.6 mg/kg, respectively) in
sc-PTZ screen. Interestingly, they did not show any sign of motor impairment at the maximum dose administered and were not toxic to the liver [
176].
Lee
et al., prepared 13 derivatives of
N-(biphenyl-4′-yl)methyl-(
R)-2-acetamido-3-methoxy-propionamide that were tested for anticonvulsant activity at the Anticonvulsant Screening Program (ASP) of the National Institute of Neurological Disorders and Stroke (NINDS) of the U.S. National Institutes of Health. The excellent activities in the MES test (mice, i.p.) of the compound
230 and
231 (ED
50 = 9.8 and 12 mg/kg, respectively) coupled with their low neurotoxicities (TD
50 = 74 and 86 mg/kg, respectively) provided compounds with notably higher PI (7.6 and 7.2, respectively) (
Figure 13) [
177].
Siddiqui
et al., prepared a series of 4-thiazolidinones bearing a sulfonamide group and tested their anticonvulsant activity utilizing MES and
sc-PTZ animal models. Compounds
232,
233 and
234 displayed promising activity and could be considered as leads for further investigations (
Figure 13) [
178].
Hen
et al., synthesized a novel class of 19 carbamates and evaluated their anticonvulsant activity in the rat MES and
sc-Met seizure tests and pilocarpine-induced status epilepticus (SE) model. The carbamates
235 (MES ED
50 = 64 mg/kg),
236 (MES ED
50 = 52 mg/kg) and
237 (MES ED
50 = 16 mg/kg) offered an optimal anticonvulsant efficacy and safety profile and consequently are potential candidates for further development as new AEDs (
Figure 13) [
179].
Hen
et al., synthesized a novel class of aromatic amides composed of phenylacetic acid or branched aliphatic carboxylic acids, with five to nine carbons in their carboxylic moiety, and aminobenzenesulfonamide. The final compounds were screened for their anticonvulsant activity by MES and
sc-Met tests. The amides
238,
239 and
240 were the most potent compounds possessing MES-ED
50 values of 7.6, 9.9, and 9.4 mg/kg and remarkable PI values of 65.7, 50.5, and 53.2, respectively (
Figure 13) [
180].
Figure 13.
Structures of compounds 228–244.
Figure 13.
Structures of compounds 228–244.
Guan
et al., demonstrated a synthesis of novel series of
N-(2-hydroxyethyl)amide derivatives and screened their anticonvulsant activities by the MES test, and their neurotoxicity was evaluated by the rotarod test. The MES test showed that compounds
241,
242 and
243 were found to show a better anticonvulsant activity and also had lower toxicity than the market anti-epileptic drug valproate (
Figure 13).
In the anti-MES potency test, these compounds exhibited ED
50 doses of 22.0, 23.3, 20.5 mg/kg, respectively, and TD
50 doses of 599.8, >1000, >1000 mg/kg, respectively, resulting in a PI of 27.5, >42.9, >48.8, respectively, which are much higher than valproate (PI = 1.6) [
181].
Senthilraja
et al., synthesized a new series of 2-(4-dimethylaminophenyl)-3-substituted thiazolidin-4-one-5-yl-acetyl acetamides/benzamides. The title compounds were investigated for their anticonvulsant activities, among the test compounds, compound
244 emerged as the most active compound of the series and as moderately more potent than the reference standard diazepam (
Figure 13) [
182].






 (R=1,3-dichlorobenzene)
(R=1,3-dichlorobenzene)