Study of the Biotransformation of Tongmai Formula by Human Intestinal Flora and Its Intestinal Permeability across the Caco-2 Cell Monolayer
Abstract
:1. Introduction
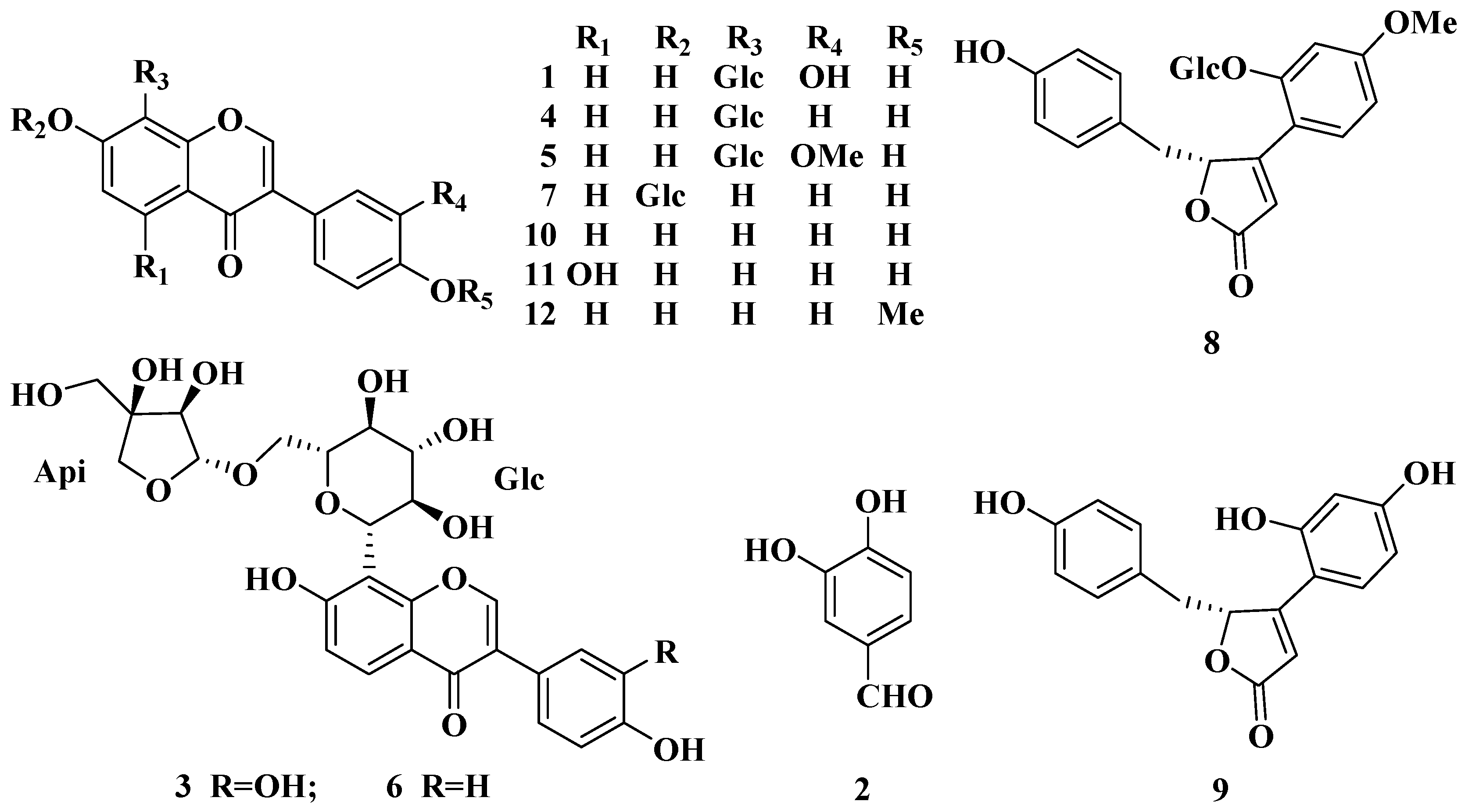
2. Results and Discussion
2.1. Methodological Validation of the Quantitative Analysis for the Twelve Constituents of TMF
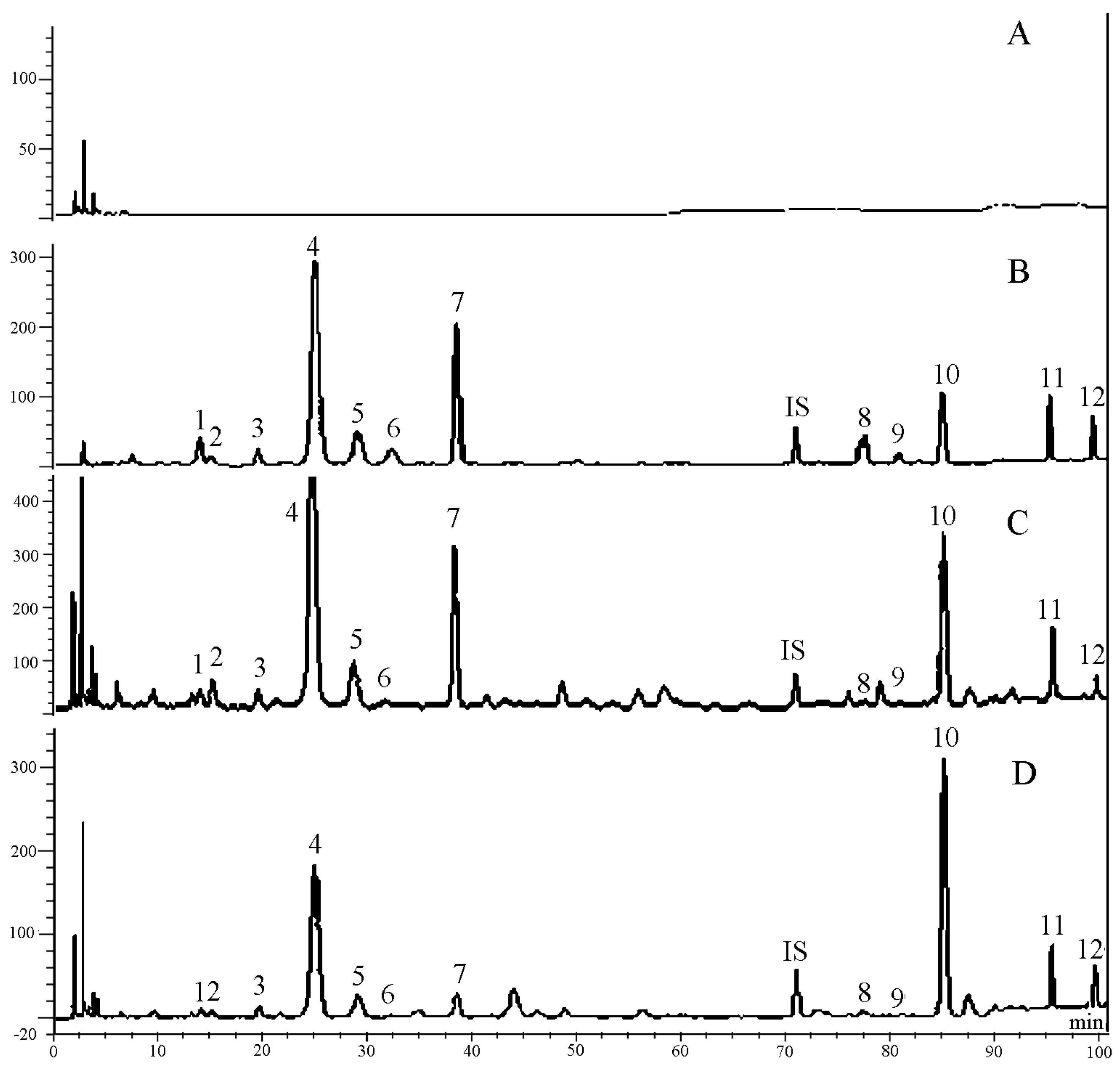
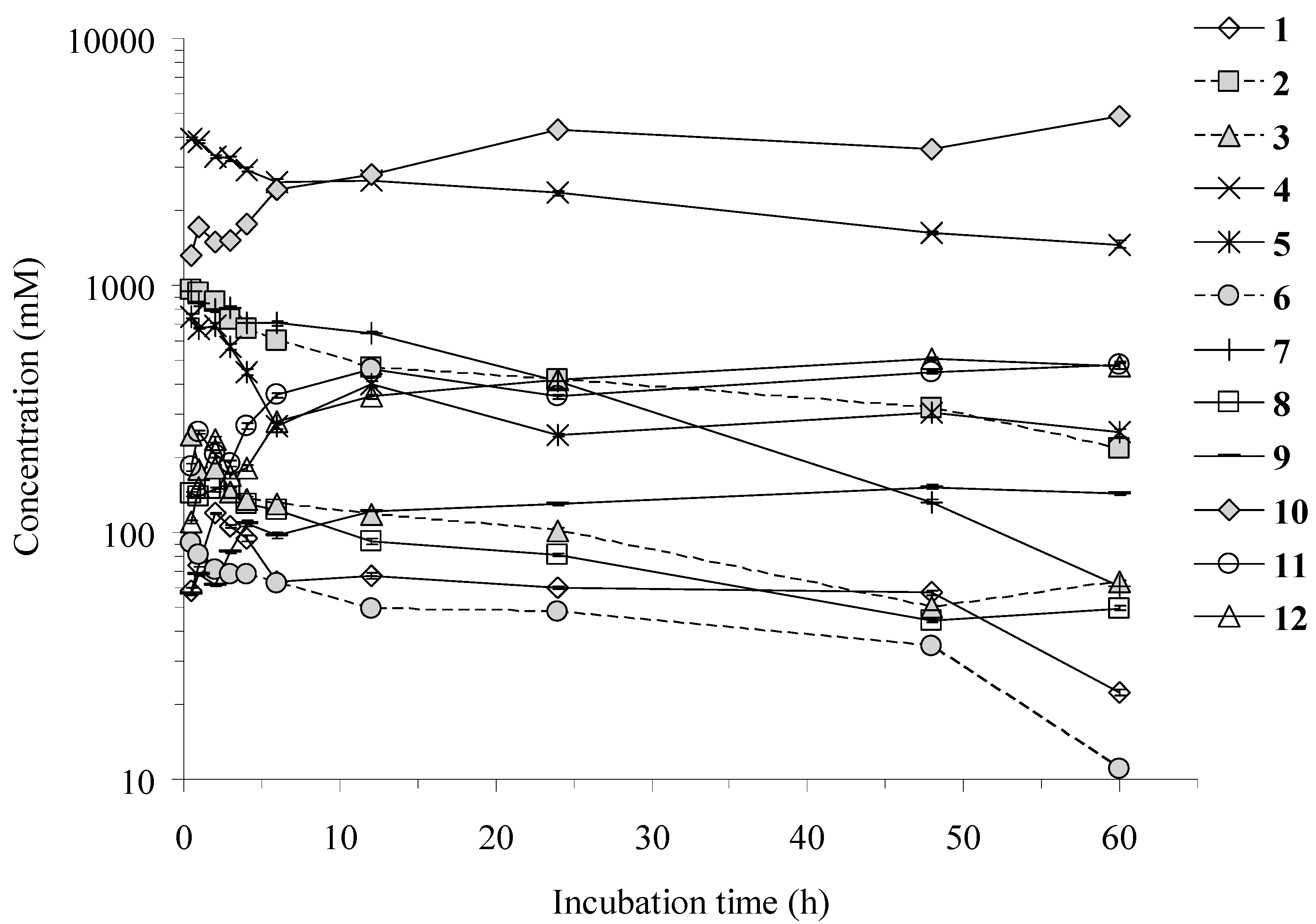
2.2. Time Course of the Twelve Constituents
2.3. Transport of the Eleven Compounds across the Caco-2 Cell Monolayer
| Compound | Papp AP→BL (×10−6, cm/s) | Papp BL→AP (×10−6, cm/s) | Papp BL→AP/Papp AP→BL | LogD (pH 7.4) |
|---|---|---|---|---|
| 1 | 0.45 ± 0.02 | 0.29 ± 0.01 | 0.64 | –1.02 |
| 3 | 0.97 ± 0.01 | 0.53 ± 0.01 | 0.55 | –1.61 |
| 4 | 0.68 ± 0.02 | 0.41 ± 0.01 | 0.60 | –0.81 |
| 5 | 0.75 ± 0.09 | 0.47 ± 0.03 | 0.63 | –0.69 |
| 6 | 0.29 ± 0.01 | 0.23 ± 0.01 | 0.79 | –0.94 |
| 7 | 0.40 ± 0.01 | 0.37 ± 0.01 | 0.92 | 0.23 |
| 8 | 1.29 ± 0.28 | 1.03 ± 0.09 | 0.80 | 0.28 |
| 9 | 6.27 ± 0.81 | 8.30 ± 1.60 | 1.32 | 2.09 |
| 10 | 25.51 ± 0.34 | 26.22 ± 0.01 | 1.03 | 2.51 |
| 11 | 32.41 ± 3.86 | 37.70 ± 1.93 | 1.16 | 1.65 |
| 12 | 30.64 ± 0.07 | 26.53 ± 1.10 | 0.87 | 2.92 |
2.4. Mass Balance of the Eleven Compounds in the Caco-2 Cell Model
| Compound | Total Recovery (%) | |
|---|---|---|
| AP→BL | BL→AP | |
| 1 | 98.08 | 94.43 |
| 3 | 100.61 | 98.28 |
| 4 | 96.62 | 95.70 |
| 5 | 94.62 | 86.15 |
| 6 | 99.42 | 98.51 |
| 7 | 99.75 | 98.55 |
| 8 | 77.01 | 91.42 |
| 9 | 86.05 | 90.87 |
| 10 | 93.44 | 100.35 |
| 11 | 86.72 | 75.68 |
| 12 | 101.82 | 97.99 |
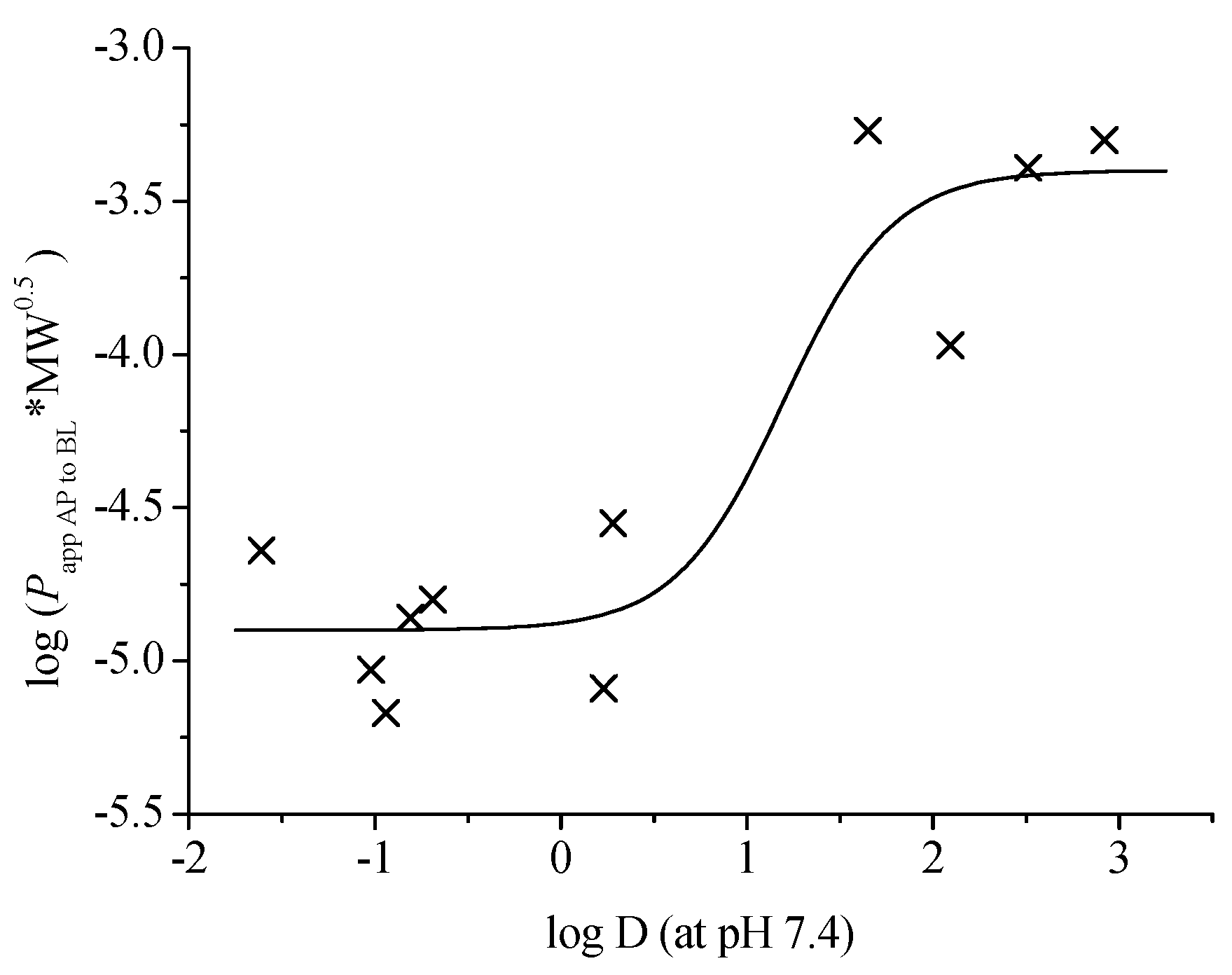
2.5. Structure-Intestinal Permeability Relationship
3. Experimental Section
3.1. Chemicals, Materials and Instruments
3.2. Preparation of GAM and HIF
3.3. Time Course the Twelve Constituents of TMF by HIF
3.4. Cell Culture
3.5. Caco-2 Permeation Experiment of the Eleven Compounds
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, Y.X. Tongmai Recipe in treating 40 cases of coronary heart disease in clinical observation. Chin. J. Geriatr. Care 2011, 9, 48–49. [Google Scholar]
- Ma, X.Y.; Lin, C.R.; Wang, M.; Liu, J.X. Effect of Rongban Tongmai granules on experimental hyperlipidemia and atherosclerosis in rabbits. Chin. Tradit. Pat. Med. 2006, 28, 1167–1170. [Google Scholar]
- Zhang, L.F.; Zhao, X.L.; Deng, R. Effect of Rongban Tongmai Granules on experimental myocardial infarction in dogs. Pharm. Clin. Res. 2009, 17, 447–449. [Google Scholar]
- Wang, F.R.; Ge, X.Z.; Yang, X.W. Chemical constituents of Tongmai formula. Chin. J. Exp. Tradit. Med. Form 2011, 17, 61–69. [Google Scholar]
- Jiang, W.L.; Yuan, X.H. Determination of salvianolic acid B in TongMai granules by HPLC. China Pharm. 2006, 15, 27. [Google Scholar]
- Lei, J.C.; Yu, J.Q.; Yao, Y. Determination of tanshinone IIA in TongMai granules by HPLC. Chin. J. Mod. Appl. Pharm. 2006, 23, 917–918. [Google Scholar]
- Li, B.J.; Xiang, C.; Yang, X.W.; Wu, L.J.; Guo, D.A.; Ye, M. Fingerprints of Tongmai KeLi by HPLC-DAD-MS. Acta Pharm. Sin. 2010, 45, 1410–1414. [Google Scholar]
- Pan, Y. Simultaneous determination of danshensu, ferulic acid and puerarin in Tongmai granules by HPLC. China Pharm. 2011, 22, 376–378. [Google Scholar]
- Wang, R.F.; Yuan, M.; Yang, X.B.; Xu, W.; Yang, X.W. Intestinal bacterial transformation—A nonnegligible part of Chinese medicine research. J. Asian Nat. Prod. Res. 2013, 15, 532–549. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.W.; Xing, Z.T.; Cui, J.R.; Fan, Y.L.; Zhao, R.; Li, X.G. Studies on the metabolism of cinobufagin and cinobufotalin by human intestinal bacteria. J. Peking Univ. (Health Sci.) 2001, 33, 199–204. [Google Scholar]
- Yang, X.W.; Zhao, J.; Cui, J.R.; Guo, W. Studies on the biotransformation of escin Ia by human intestinal bacteria and the anti-tumor activities of desacylescin I. J. Peking Univ. (Health Sci.) 2004, 36, 31–35. [Google Scholar]
- Zhang, P.; Yang, X.W. Biotransformation of nodakenin and simultaneous quantification of nodakenin and its aglycone in incubated system of human intestinal bacteria by HPLC method. J. Asian Nat. Prod. Res. 2009, 11, 371–379. [Google Scholar] [CrossRef]
- Wu, S.; Xu, W.; Yang, X.W. Studies on biotransformation of chemical constituents of Tongmai Formula by human intestinal flora. China J. Chin. Mater. Med. 2013, 38, 3510–3519. [Google Scholar]
- Allen, R.H.; Robert, A.C.; Philip, S.B. Caco-2 cell monolayers as a model for drug transport across the intestinal mucosa. Pharm. Res. 1990, 7, 902–910. [Google Scholar]
- Guidance for Industry: Bioanalytical Method Validation (2001). Food and Drug Administration: Silver Spring, MD, USA; pp. 4–10. Available online: http://www.fda.gov/downloads/Drugs/.../Guidances/ucm070107.pdf (accessed on 10 October 2015).
- Zhao, B.; Yang, X.B.; Yang, X.W.; Wu, Q.; Wang, Y.; Zhang, L.X.; Xu, W. Intestinal permeability of the constituents from the roots of Saposhnikovia divaricatain the human Caco-2 cell monolayer model. Planta Med. 2011, 77, 1531–1535. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.W.; Yang, X.D.; Wang, Y.; Ma, L.; Zhang, Y.; Yang, X.G.; Wang, K. Establishment of Caco-2 cell monolayer model and standard operation procedure for assessing intestinal absorption of chemical components of traditional Chinese medicine. J. Chin. Integr. Med. 2007, 5, 634–641. [Google Scholar] [CrossRef]
- Yee, S.Y. In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man-fact or myth. Pharm. Res. 1997, 14, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.E.; Son, Y.K.; Min, B.S.; Jung, H.A.; Choi, J.S. Anti-inflammatory and antioxidant activities of constituents isolated from Pueraria lobata roots. Arch. Pharm. Res. 2012, 35, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.W.; Huang, X.; Ma, L.; Wu, Q.; Xu, W. The intestinal permeability of neolignans from the seeds of Myristica fragrans in the Caco-2 cell monolayer model. Planta Med. 2010, 76, 1587–1591. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.F.; Xu, W.; Song, W.; Ye, M.; Yang, X.W. Transport of twelve coumarins from Angelicae Pubescentis Radix across a MDCK-pHaMDR cell monolayer—An in vitro model for blood-brain barrier permeability. Molecules 2015, 20, 11719–11732. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.W.; Xu, W. Establishment of model and standard operation procedure for biotransformation of chemical constituents of traditional Chinese medicine by human intestinal bacteria. China J. Chin. Mater. Med. 2011, 36, 19–26. [Google Scholar]
- Yang, X.W.; Wang, N.; Li, W.; Xu, W.; Wu, S. Biotransformation of 4,5-O-dicaffeoylquinic acid methyl ester by human intestinal flora and evaluation on their inhibition of NO production and antioxidant activity of the products. Food Chem. Toxicol. 2013, 55, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Rashid, Z.; Basson, M.D. Topoisomerase II inhibition differentially modulates Caco-2 intestinal epithelial cell phenotype. Biochem. Biophys. Res. Commun. 1996, 219, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Artursson, P.; Karlsson, J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem. Biophys. Res. Commun. 1991, 175, 880–885. [Google Scholar] [CrossRef]
- Lennernäs, H.; Palm, K.; Fagerholm, U.; Artursson, P. Comparison between active and passive drug transport in human intestinal epithelial (Caco-2) cells in vitro and human jejunum in vivo. Int. J. Pharm. 1996, 127, 103–107. [Google Scholar] [CrossRef]
- Tian, X.J.; Yang, X.W.; Yang, X.D.; Wang, K. Studies of intestinal permeability of 36 flavonoids using Caco-2 cell monolayer model. Int. J. Pharm. 2009, 367, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Xu, W.; Wang, F.-R.; Yang, X.-W. Study of the Biotransformation of Tongmai Formula by Human Intestinal Flora and Its Intestinal Permeability across the Caco-2 Cell Monolayer. Molecules 2015, 20, 18704-18716. https://doi.org/10.3390/molecules201018704
Wu S, Xu W, Wang F-R, Yang X-W. Study of the Biotransformation of Tongmai Formula by Human Intestinal Flora and Its Intestinal Permeability across the Caco-2 Cell Monolayer. Molecules. 2015; 20(10):18704-18716. https://doi.org/10.3390/molecules201018704
Chicago/Turabian StyleWu, Shuai, Wei Xu, Fu-Rong Wang, and Xiu-Wei Yang. 2015. "Study of the Biotransformation of Tongmai Formula by Human Intestinal Flora and Its Intestinal Permeability across the Caco-2 Cell Monolayer" Molecules 20, no. 10: 18704-18716. https://doi.org/10.3390/molecules201018704





