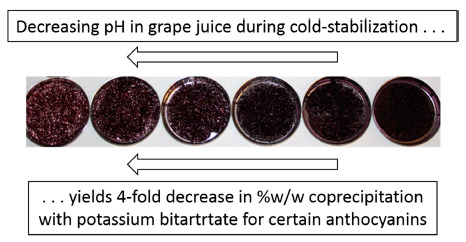
Decreasing pH Results in a Reduction of Anthocyanin Coprecipitation during Cold Stabilization of Purple Grape Juice
Abstract
:1. Introduction
2. Results and Discussion
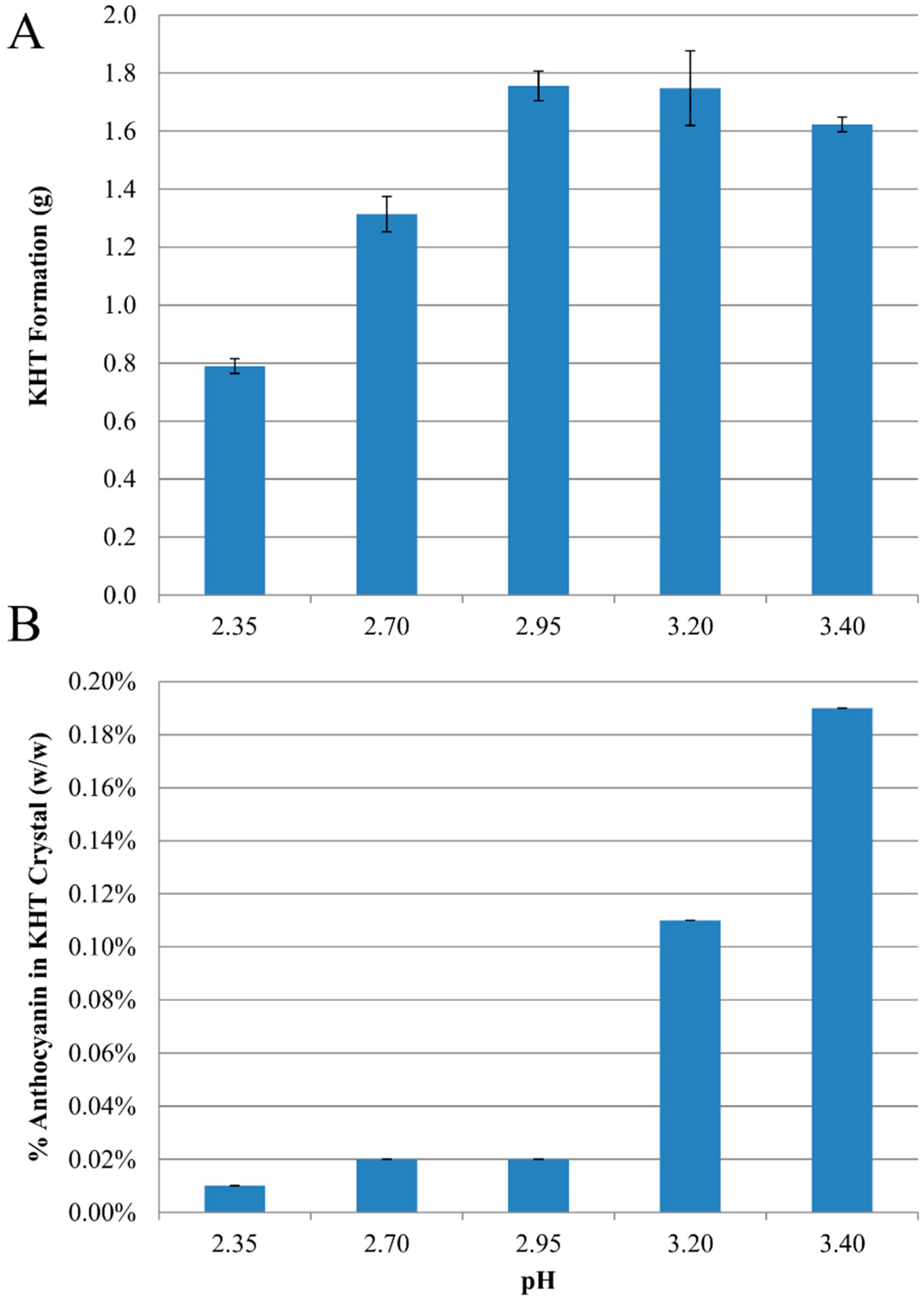
2.1. Anthocyanin-Bitartrate Coprecipitation in a Model Juice
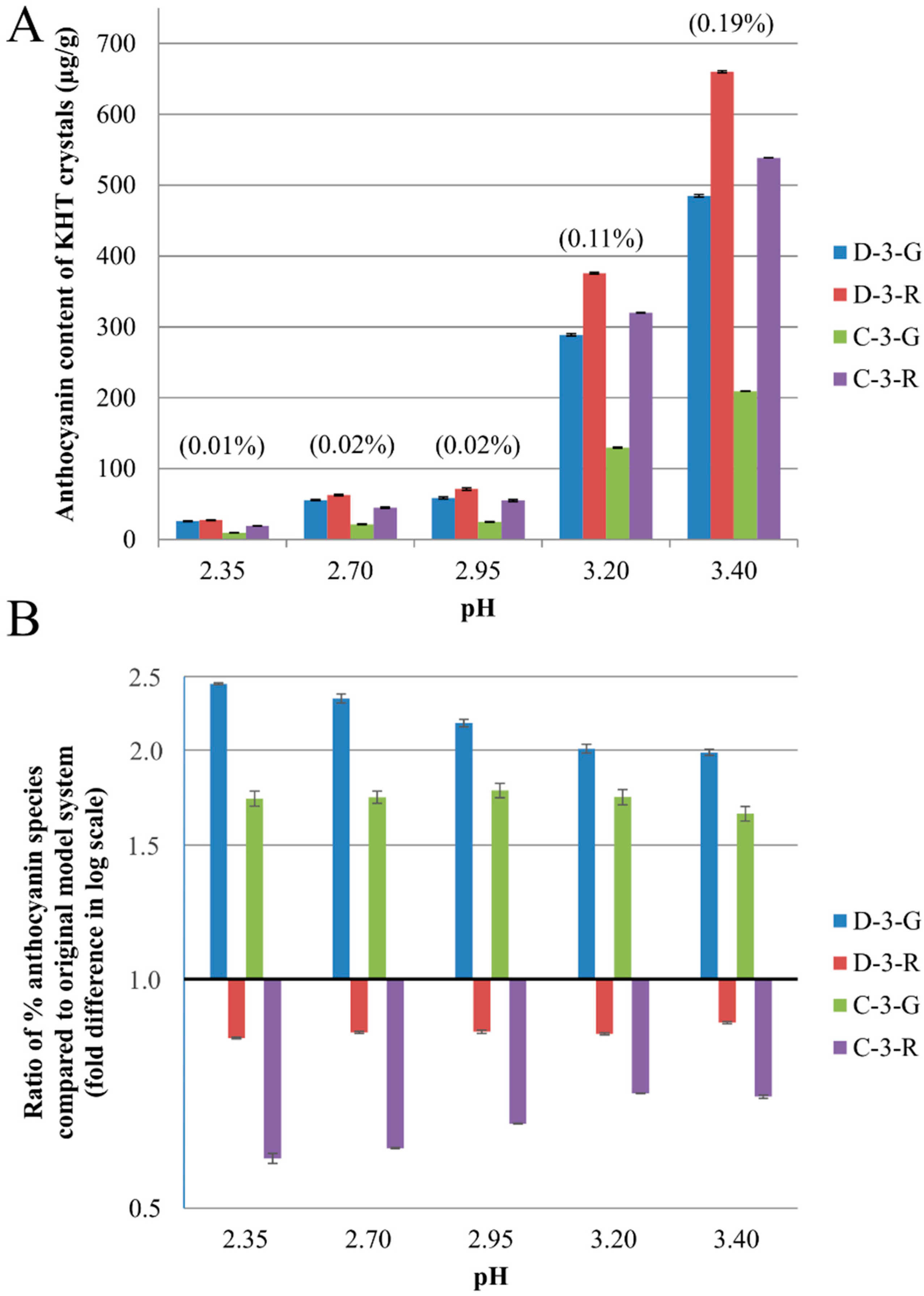
2.2. Anthocyanin-Bitartrate Coprecipitation in a Purple Concord Grape Juice
| Analyte | Concentration a |
|---|---|
| Anthocyanin Diglucosides | |
| Del-3,5-Di | 26.0 (0.38) |
| Cy-3,5-Di | 32.0 (0.63) |
| Pet-3,5-Di | 16.4 (0.65) |
| Peo-3,5-Di | 33.9 (0.68) |
| Mvn-3,5-Di | 30.2 (0.72) |
| Anthocyanin Monoglucosides | |
| Del-3-Glu | 94.8 (1.0) |
| Cy-3-Glu | 74.4 (0.82) |
| Pet-3-Glu | 28.4 (0.65) |
| Peo-3-Glu | 13.7 (0.37) |
| Mvn-3-Glu | 19.2 (0.59) |
| Acylated Anthocyanins | 122.6 (1.3) |
| Total Anthocyanins | 491.6 (0.84) |
| Organic Acids | |
| Citric Acid | 0.23 (0.01) |
| Tartaric Acid | 11.5 (0.07) |
| Malic Acid | 2.38 (0.03) |
| Total Soluble Solids | 20.0 (0.1) |
| Initial pH | 3.05 |
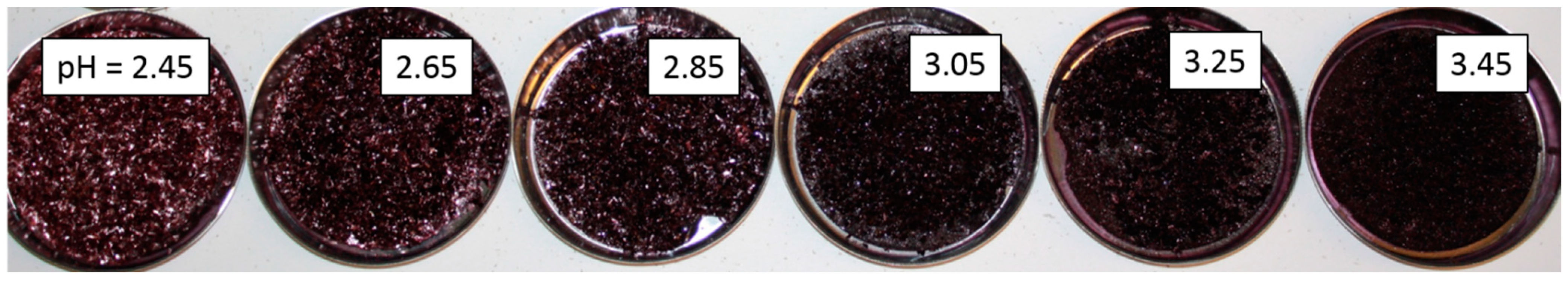

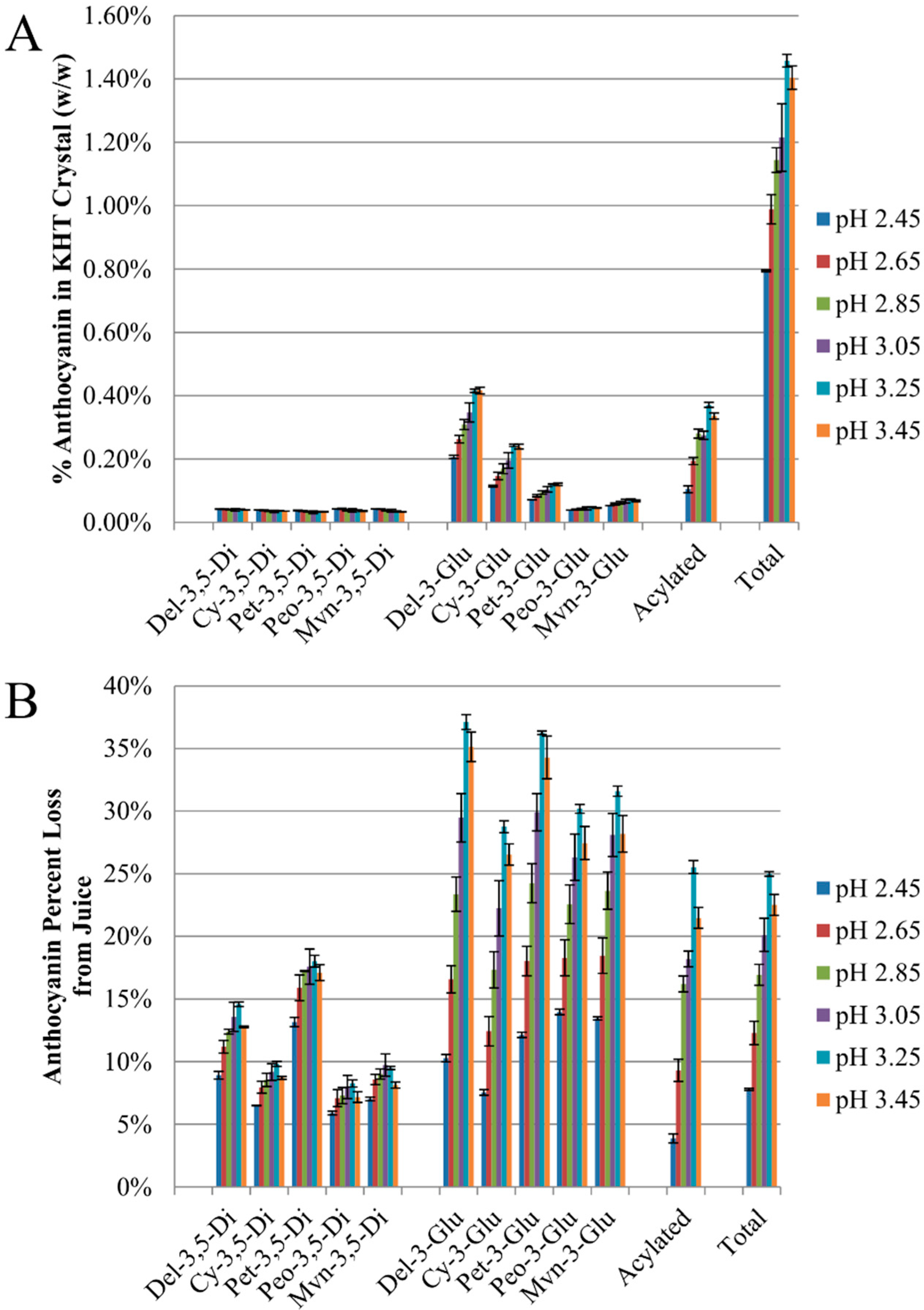
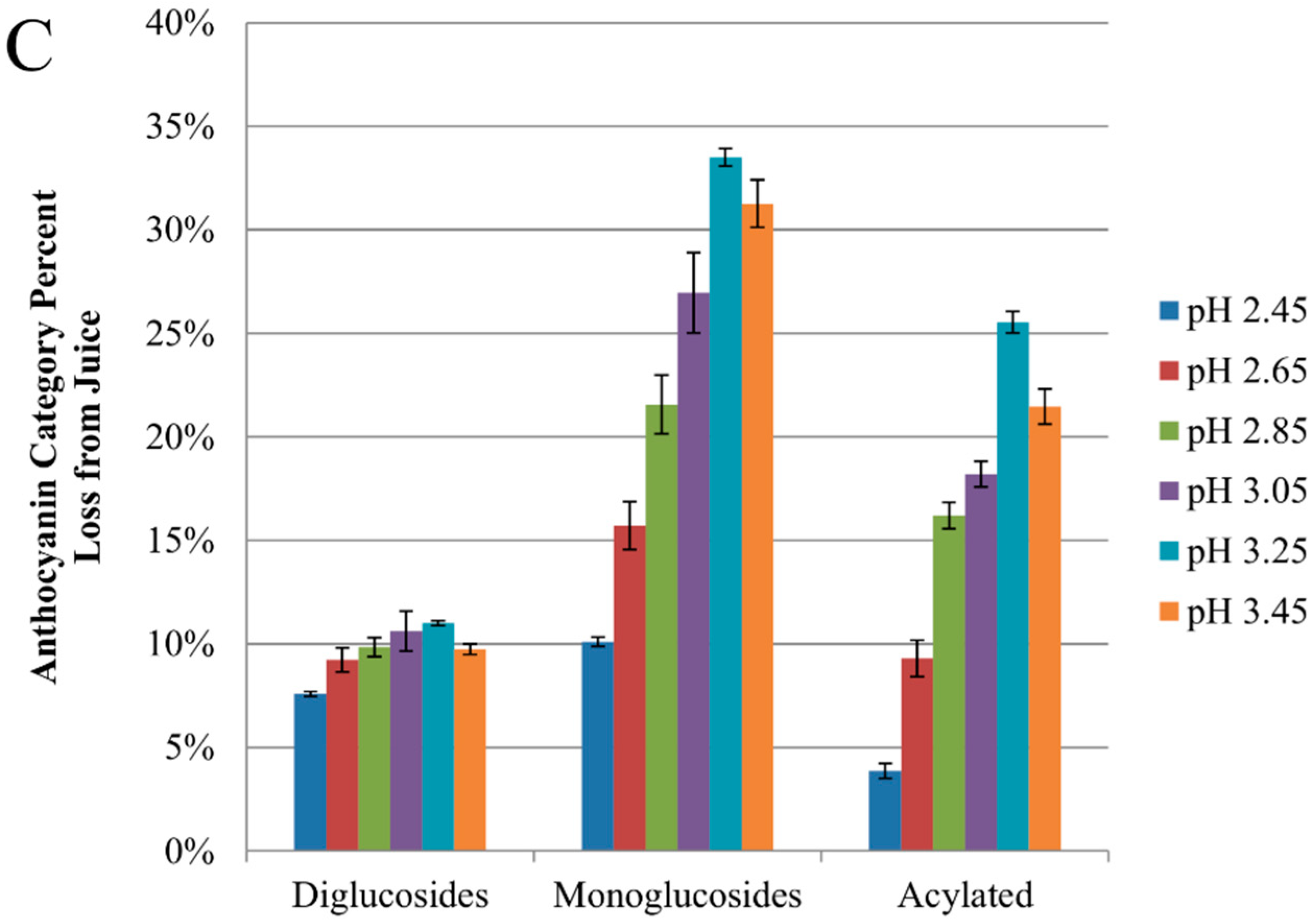
2.3. Discussion
2.3.1. Anthocyanin-Bitartrate Coprecipitation in a Purple Concord Juice
2.3.2. Potential Implications for Juice Processing and Retention or Isolation of Anthocyanins
3. Experimental Section
3.1. Chemicals
3.2. Preparation and Cold-Stabilization Treatments of Model Juices
3.3. Processing, Preparation, and Cold-Stabilization Treatments of Concord Juice
3.4. Characterization of Anthocyanins in KHT Crystals
3.4.1. KHT Solubilization
3.4.2. HPLC Analysis of Anthocyanins in Model Juice Samples
3.4.3. HPLC Analysis of Anthocyanins in Real Juice Samples
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Oszmianski, J.; Lee, C.Y. Isolation and HPLC determination of phenolic compounds in red grapes. Am. J. Enol. Vitic. 1990, 41, 204–206. [Google Scholar]
- Wang, Y.; Catana, F.; Yang, Y.; Roderick, R.; van Breemen, R.B. An LC-MS method for analyzing total resveratrol in grape juice, cranberry juice, and in wine. J. Agric. Food Chem. 2002, 50, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.R.; Striegler, R.K. Grape juice: Factors that influence quality, processing technology, and economics. In Processing Fruits: Science and Technology,, 2nd ed.; Barrett, D.M., Somogyi, L.P., Ramaswamy, H.S., Eds.; CRC: Boca Raton, FL, USA, 2005; pp. 585–616. [Google Scholar]
- Wallace, T.C. Anthocyanins in cardiovascular disease. Adv. Nutr. 2011, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Heiss, C.; Borges, G.; Crozier, A. Berry (poly)phenols and cardiovascular health. J. Agric. Food Chem. 2014, 62, 3842–3851. [Google Scholar] [CrossRef]
- Harbourne, N.; Marete, E.; Jacquier, J.C.; O’Riordan, D. Stability of phytochemicals as sources of anti-inflammatory nutraceuticals in beverages—A review. Food Res. Int. 2013, 50, 480–486. [Google Scholar] [CrossRef]
- Sacchi, K.L.; Bisson, L.F.; Adams, D.O. A review of the effect of winemaking techniques on phenolic extraction in red wines. Am. J. Enol. Vitic. 2005, 56, 197–206. [Google Scholar]
- Delgado-Vargas, F.; Jiménez, A.R.; Paredes-López, O. Natural pigments: Carotenoids, anthocyanins, and betalains—Characteristics, biosynthesis, processing, and stability. Crit. Rev. Food Sci. Nutr. 2000, 40, 173–289. [Google Scholar] [CrossRef] [PubMed]
- Ingalsbe, D.; Neubert, A.; Carter, G. Concord grape pigments. J. Agric. Food Chem. 1963, 11, 263–268. [Google Scholar] [CrossRef]
- Alongi, K.S.; Padilla-Zakour, O.I.; Sacks, G.L. Effects of concentration prior to cold-stabilization on anthocyanin stability in Concord grape juice. J. Agric. Food Chem. 2010, 58, 11325–11332. [Google Scholar] [CrossRef] [PubMed]
- Correa-Gorospe, I.; Polo, M.; Hernandez, T. Characterization of the proteic and the phenolic fraction in tartaric sediments from wines. Food Chem. 1991, 41, 135–146. [Google Scholar] [CrossRef]
- Vernhet, A.; Dupre, K.; Boulange-Petermann, L.; Cheynier, V.; Pellerin, P.; Moutounet, M. Composition of tartrate precipitates deposited on stainless steel tanks during the cold stabilization of wines. Part II. Red wines. Am. J. Enol. Vitic. 1999, 50, 398–403. [Google Scholar]
- Amerine, M.A.; Ough, C.S. Methods for Analysis of Musts and Wines; Wiley: New York, NY, USA, 1980. [Google Scholar]
- Mattick, L.; Shaulis, N.; Moyer, J. The effect of potassium fertilization on the acid content of Concord grape juice. Am. J. Enol. Vitic. 1972, 23, 26–30. [Google Scholar]
- Zoecklein, B.W.; Fugelsang, K.C.; Gump, B.H.; Nury, F.S. Wine Analysis and Production; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1999. [Google Scholar]
- Balakian, S.; Berg, H. The role of polyphenols in the behavior of potassium bitartrate in red wines. Am. J. Enol. Vitic. 1968, 19, 91–100. [Google Scholar]
- Boulange-Petermann, L.; Vernhet, A.; Dupre, K.; Moutounet, M. Kht cold stabilization: A scanning electron microscopy study of the formation of surface deposits on stainless steel in model wines. Vitis 1999, 38, 43–45. [Google Scholar]
- Konja, G.; Lovric, T. Berry fruit juices. In Fruit Juice Processing Technology; Nagy, S., Chen, C.S., Shaw, P.E., Eds.; Agscience: Auburndale, FL, USA, 1993; pp. 436–514. [Google Scholar]
- Dunsford, P.; Boulton, R. The kinetics of potassium bitartrate crystallization from table wines. I. Effect of particle size, particle surface area and agitation. Am. J. Enol. Vitic. 1981, 32, 100–105. [Google Scholar]
- Dunsford, P.; Boulton, R. The kinetics of potassium bitartrate crystallization from table wines. II. Effect of temperature and cultivar. Am. J. Enol. Vitic. 1981, 32, 106–110. [Google Scholar]
- Gerbaud, V.; Gabas, N.; Blouin, J.; Laguerie, C. Nucleation studies of potassium hydrogen tartrate in model solutions and wines. J. Cryst. Growth 1996, 166, 172–178. [Google Scholar] [CrossRef]
- Buchert, J.; Koponen, J.M.; Suutarinen, M.; Mustranta, A.; Lille, M.; Törrönen, R.; Poutanen, K. Effect of enzyme-aided pressing on anthocyanin yield and profiles in bilberry and blackcurrant juices. J. Sci. Food Agric. 2005, 85, 2548–2556. [Google Scholar] [CrossRef]
- Montgomery, M.W.; Reyes, F.G.R.; Cornwell, C.; Beavers, D.V. Sugars and acid analysis and effect of heating on color stability of northwest Concord grape juice. J. Food Sci. 1982, 47, 1883–1885. [Google Scholar] [CrossRef]
- Celotti, E.; Bornia, L.; Zoccolan, E. Evaluation of the electrical properties of some products used in the tartaric stabilization of wines. Am. J. Enol. Vitic. 1999, 50, 343–350. [Google Scholar]
- Rodriguez-Clemente, R.; Correa-Gorospe, I. Structural, morphological, and kinetic aspects of potassium hydrogen tartrate precipitation from wines and ethanolic solutions. Am. J. Enol. Vitic. 1988, 39, 169–179. [Google Scholar]
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. Principles and Practices of Winemaking; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1999. [Google Scholar]
- Slimestad, R.; Solheim, H. Anthocyanins from black currants (Ribes nigrum L.). J. Agric. Food Chem. 2002, 50, 3228–3231. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.W. Random and cooperative sequential adsorption. Rev. Mod. Phys. 1993, 65, 1281–1329. [Google Scholar] [CrossRef]
- Wrolstad, R.E. Symposium 12: Interaction of natural colors with other ingredients—Anthocyanin pigments—Bioactivity and coloring properties. J. Food Sci. 2004, 69, C419–C421. [Google Scholar] [CrossRef]
- Ohta, H.; Tonohara, K.; Yoza, K.I.; Nogata, Y. Tartar stabilization of Concord grape juice by means of ion-exchange resins—(Studies on quality of grape juice.12). J. Jpn. Soc. Food Sci. Technol.-Nippon Shokuhin Kagaku Kogaku Kaishi 1992, 39, 1105–1111. [Google Scholar]
- Harvey, D. Modern Analytical Chemistry; McGraw-Hill: Boston, MA, USA, 2000. [Google Scholar]
- Côté, J.; Caillet, S.; Doyon, G.; Sylvain, J.F.; Lacroix, M. Analyzing cranberry bioactive compounds. Crit. Rev. Food Sci. Nutr. 2010, 50, 872–888. [Google Scholar] [CrossRef] [PubMed]
- Kraemer Schafhalter, A.; Fuchs, H.; Pfannhauser, W. Solid phase extraction (SPE)—A comparison of 16 materials for the purification of anthocyanins from Aronia melanocarpa var nero. J. Sci. Food Agric. 1998, 78, 435–440. [Google Scholar]
- Castellari, M.; Versari, A.; Spinabelli, U.; Galassi, S.; Amati, A. An improved hplc method for the analysis of organic acids, carbohydrates, and alcohols in grape musts and wines. J. Liquid Chromatogr. Rel. Technol. 2000, 23, 2047–2056. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Price, S.F.; McCord, J.D. Reversed-phase high-performance liquid chromatography methods for analysis of wine polyphenols. Methods Enzymol. 1999, 299, 113–121. [Google Scholar]
- Manns, D.C.; Mansfield, A.K. A core-shell column approach to a comprehensive high-performance liquid chromatography phenolic analysis of Vitis vinifera L. and interspecific hybrid grape juices, wines, and other matrices following either solid phase extraction or direct injection. J. Chromatogr. A 2012, 1251, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manns, D.C.; Siricururatana, P.; Padilla-Zakour, O.I.; Sacks, G.L. Decreasing pH Results in a Reduction of Anthocyanin Coprecipitation during Cold Stabilization of Purple Grape Juice. Molecules 2015, 20, 556-572. https://doi.org/10.3390/molecules20010556
Manns DC, Siricururatana P, Padilla-Zakour OI, Sacks GL. Decreasing pH Results in a Reduction of Anthocyanin Coprecipitation during Cold Stabilization of Purple Grape Juice. Molecules. 2015; 20(1):556-572. https://doi.org/10.3390/molecules20010556
Chicago/Turabian StyleManns, David C., Passaporn Siricururatana, Olga I. Padilla-Zakour, and Gavin L. Sacks. 2015. "Decreasing pH Results in a Reduction of Anthocyanin Coprecipitation during Cold Stabilization of Purple Grape Juice" Molecules 20, no. 1: 556-572. https://doi.org/10.3390/molecules20010556