Insights into Animal and Plant Lectins with Antimicrobial Activities
Abstract
:1. Introduction
2. Plant Lectins
| Family | Species | Defense against | Reference |
|---|---|---|---|
| Lectin Receptor Kinases (LecRK) | Arabidopsis thaliana | Fungi: Botrytis cinerea, Erysiphe cichoracearum, Erysiphe orontii, Blumeria graminis, Gigaspora rosea; Oomycete: Phytophthora infestans; Virus: Turnip mosaic and Cabbage leaf curl; Bacteria: Pseudomonas syringae | [36,37,40,41] |
| Nicotiana benthamiana | Oomycete: P. infestans | [32] | |
| Oryza sativa | Fungi: Magnaporthe grisea | [34] | |
| Amaranthins | Amaranthus viridis | Fungi: B. cincerea, Fusarium oxysporum | [42] |
| Calreticulin/calnexin | N. benthamiana | Oomycete: P. infestans | [43] |
| A. thaliana | Bacteria: P. syringae | [44] | |
| EUL-related lectins | O. sativa | Bacteria: Xanthomonasoryzae pv. oryzae; Fungi: Magnaporthe oryzae | [45] |
| Jacalin-related lectins (JRLs) | Triticum aestivum | Fungi: Fusarium graminearum, B. graminis f. sp. Tritici, B. cinerea; Oomycete: Phytophthora parasitica var nicotianae; Bacteria: P. syringe pv tabaci; Virus: tobacco mosaic virus (TMV) | [46,47,48,49] |
| A. thaliana | Fungi: F. graminearum, B. graminisf. sp. Tritici, B.cinerea Virus: tobacco etch virus; Plantago asiatica mosaic virus (PlAMV) | [38,39,46,50,51,52] | |
| O. sativa | Fungi: M.grisea | [52] | |
| Nictaba-related | A.thaliana | Fungi: A. flavus, Fusarium moniliforme, Fusarium solani, Rhizoctonia solani and Trichoderma harzianum Virus: Cucurbit aphid-borne yellows virus | [50] [53] |
| Ricin-B | Transgenic N. benthamiana | Virus: tobacco etch virus | [54,55] |
3. Animal Lectins
| Family | Species | Pathogen | Reference |
|---|---|---|---|
| Calnexin/Calreticulin | Marsupenaeus japonicus | Bacteria: Vibrio anguillarum | [96] |
| Branchiostoma japonicum | Bacteria: Escherichia coli; Staphylococcus aureus | [97] | |
| L-type | Ictalurus punctatus | Bacteria: Edwardsiella ictaluri | [98] |
| Marsupenaeus japonicus | Bacteria: Vibrio anguillarum | [92] | |
| Eriocheir sinensis | Bacteria: Staphylococcus aureus; Vibrio parahaemolyticus; Aeromonas hydrophila | [99] | |
| C-type | Homo sapiens | Bacteria: Listeria monocytogenes | [89] |
| Galectin | Homo sapiens | Bacteria: Neisseria gonorrhoeae; | [100] |
| Mus musculus | Fungi: Candida albicans | [88,101] |
4. Lectin Structural Analyses
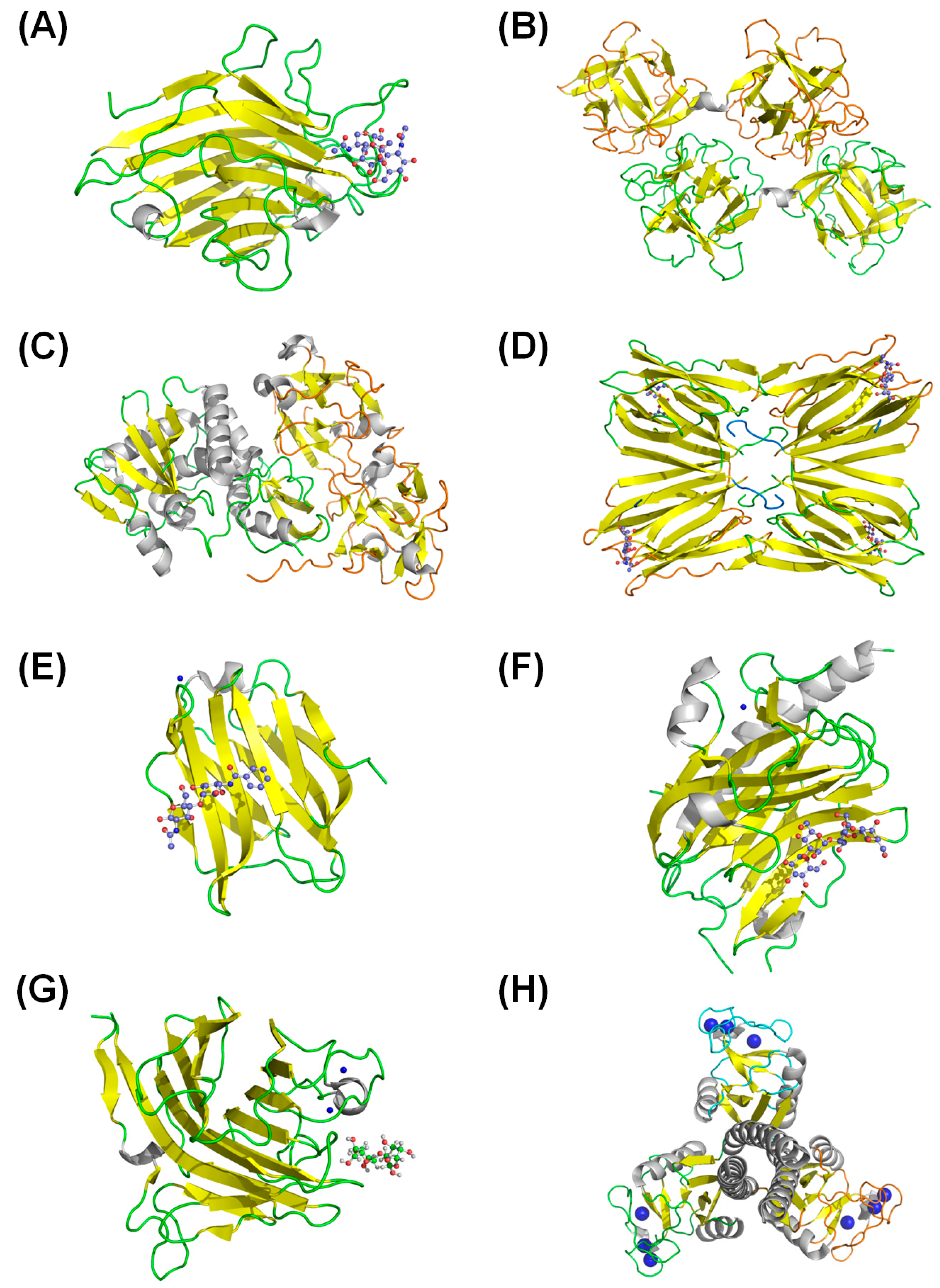
5. Biotechnological Potential
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Peumans, W.J.; Vandamme, E.J.M. Lectins as plant defense proteins. Plant Physiol. 1995, 109, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, E.J.M.; Peumans, W.J.; Barre, A.; Rouge, P. Plant lectins: A composite of several distinct families of structurally and evolutionary related proteins with diverse biological roles. Crit. Rev. Plant Sci. 1998, 17, 575–692. [Google Scholar] [CrossRef]
- Kennedy, J.F.; Palva, P.M.G.; Corella, M.T.S.; Cavalcanti, M.S.M.; Coelho, L. Lectins, versatile proteins of recognition—A review. Carbohydr. Polym. 1995, 26, 219–230. [Google Scholar] [CrossRef]
- Ni, Y.; Tizard, I. Lectin-carbohydrate interaction in the immune system. Vet. Immunol. Immunopathol. 1996, 55, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Surolia, A. Analyses of carbohydrate recognition by legume lectins: Size of the combining site loops and their primary specificity. J. Mol. Biol. 1997, 267, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Weis, W.I.; Drickamer, K. Structural basis of lectin-carbohydrate recognition. Ann. Rev. Biochem. 1996, 65, 441–473. [Google Scholar] [CrossRef] [PubMed]
- Kompella, U.B.; Lee, V.H.L. Delivery systems for penetration enhancement of peptide and protein drugs: Design considerations. Adv. Drug Deliv. Rev. 2001, 46, 211–245. [Google Scholar] [CrossRef] [PubMed]
- Peumans, W.J.; van Damme, E.J.M. Plant lectins: Versatile proteins with important perspectives in biotechnology. Biotechnol. Genet. Eng. Rev. 1998, 15, 199–228. [Google Scholar] [CrossRef]
- VanDamme, E.J.M.; Barre, A.; Rouge, P.; VanLeuven, F.; Balzarini, J.; Peumans, W.J. Molecular cloning of the lectin and a lectin-related protein from common solomon’s seal (polygonatum multiflorum). Plant Mol. Biol. 1996, 31, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.C.; Correia, M.T. Plant lectins and toll-like receptors: Implications for therapy of microbial infections. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef]
- Wang, W.; Owen, S.M.; Rudolph, D.L.; Cole, A.M.; Hong, T.; Waring, A.J.; Lal, R.B.; Lehrer, R.I. Activity of alpha- and theta-defensins against primary isolates of hiv-1. J. Immunol. 2004, 173, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S. First line of defence? Nat. Rev. Microbiol. 2005, 3, 831–831. [Google Scholar] [CrossRef]
- Wang, W.; Cole, A.M.; Hong, T.; Waring, A.J.; Lehrer, R.I. Retrocyclin, an antiretroviral theta-defensin, is a lectin. J. Immunol. 2003, 170, 4708–4716. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, D.C. Animal lectins: A historical introduction and overview. Biochim. Biophys. Acta 2002, 1572, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.M.P.; Harson, R.E.; Zabner, J.; Welsh, M.J. Lectin binding and endocytosis at the apical surface of human airway epithelia. Gene Ther. 2001, 8, 1826–1832. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K. Intracellular lectins involved in folding and transport in the endoplasmic reticulum. Biol. Pharm. Bull. 2009, 32, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Bah, C.S.; Fang, E.F.; Ng, T.B.; Mros, S.; McConnell, M.; Bekhit Ael, D. Purification and characterization of a rhamnose-binding chinook salmon roe lectin with antiproliferative activity toward tumor cells and nitric oxide-inducing activity toward murine macrophages. J. Agric. Food Chem. 2011, 59, 5720–5728. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.F.; Ng, T.B. Antitumor Potential and Other Emerging Medicinal Properties of Natural Compounds; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Kim, M.; Rao, M.V.; Tweardy, D.J.; Prakash, M.; Galili, U.; Gorelik, E. Lectin-induced apoptosis of tumor-cells. Glycobiology 1993, 3, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Chiba, H.; Inokoshi, J.; Kuno, A.; Sugai, T.; Takahashi, A.; Ito, Y.; Tsunoda, M.; Suzuki, K.; Takénaka, A. Mechanism by which the lectin actinohivin blocks hiv infection of target cells. Proc. Natl. Acad. Sci. USA 2009, 106, 15633–15638. [Google Scholar] [CrossRef] [PubMed]
- Tanne, A.; Neyrolles, O. C-type lectins in immune defense against pathogens: The murine dc-sign homologue signr3 confers early protection against mycobacterium tuberculosis infection. Virulence 2010, 1, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Rydz, N.; Swystun, L.L.; Notley, C.; Paterson, A.D.; Riches, J.J.; Sponagle, K.; Boonyawat, B.; Montgomery, R.R.; James, P.D.; Lillicrap, D. The c-type lectin receptor clec4m binds, internalizes, and clears von willebrand factor and contributes to the variation in plasma von willebrand factor levels. Blood 2013, 121, 5228–5237. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, C.; Favery, B.; Lecomte, P.; van Damme, E.; Peumans, W.; Abad, P.; Jouanin, L. Evaluation of the ability of lectin from snowdrop (Galanthus nivalis) to protect plants against root-knot nematodes. Plant Sci. 2003, 164, 517–523. [Google Scholar] [CrossRef]
- Van Parijs, J.; Broekaert, W.F.; Goldstein, I.J.; Peumans, W.J. Hevein: An antifungal protein from rubber-tree (Hevea brasiliensis) latex. Planta 1991, 183, 258–264. [Google Scholar]
- Koo, J.C.; Lee, S.Y.; Chun, H.J.; Cheong, Y.H.; Choi, J.S.; Kawabata, S.; Miyagi, M.; Tsunasawa, S.; Ha, K.S.; Bae, D.W.; et al. Two hevein homologs isolated from the seed of Pharbitis nil L. Exhibit potent antifungal activity. Biochim. Biophys. Acta 1998, 1382, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Liu, Y.; Wang, X.C.; Gu, H.Y.; Chen, Z.L. Purification and characterization of a novel anti-fungal protein from gastrodia elata. Plant Physiol. Biochem. 1998, 36, 899–905. [Google Scholar] [CrossRef]
- Ciopraga, J.; Gozia, O.; Tudor, R.; Brezuica, L.; Doyle, R.J. Fusarium sp. growth inhibition by wheat germ agglutinin. Biochim. Biophys. Acta 1999, 1428, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Freire, M.D.M.; Gomes, V.M.; Corsini, R.E.; Machado, O.L.T.; de Simone, S.G.; Novello, J.C.; Marangoni, S.; Macedo, M.L.R. Isolation and partial characterization of a novel lectin from Talisia esculenta seeds that interferes with fungal growth. Plant Physiol. Biochem. 2002, 40, 61–68. [Google Scholar] [CrossRef]
- Damico, D.C.S.; Freire, M.G.M.; Gomes, V.M.; Toyama, M.H.; Marangoni, S.; Novello, J.C.; Macedo, M.L.R. Isolation and characterization of a lectin from Annona muricata seeds. J. Protein Chem. 2003, 22, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, T.; Hatano, K.-I.; Miyauchi, Y.; Suwa, Y.-I.; Sawano, Y.; Tanokura, M. A secreted protein with plant-specific cysteine-rich motif functions as a mannose-binding lectin that exhibits antifungal activity. Plant Physiol. 2014, 166, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Ang, A.S.W.; Cheung, R.C.F.; Dan, X.; Chan, Y.S.; Pan, W.; Ng, T.B. Purification and characterization of a glucosamine-binding antifungal lectin from Phaseolus vulgaris cv. Chinese pinto beans with antiproliferative activity towards nasopharyngeal carcinoma cells. Appl. Biochem. Biotechnol. 2014, 172, 672–686. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, H.; Saitoh, H.; Takahashi, Y.; Berberich, T.; Ito, A.; Kamoun, S.; Terauchi, R. NbLRK1, a lectin-like receptor kinase protein of Nicotiana benthamiana, interacts with Phytophthora infestans INF1 elicitin and mediates INF1-induced cell death. Planta 2008, 228, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, K.; de Sain, M.; Weide, R.; Gouget, A.; Klamer, S.; Canut, H.; Govers, F. The lectin receptor kinase LecRK-I.9 is a novel Phytophthora resistance component and a potential host target for a RXLR effector. PLoS Pathog. 2011, 7, e1001327. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shang, J.; Chen, D.; Lei, C.; Zou, Y.; Zhai, W.; Liu, G.; Xu, J.; Ling, Z.; Cao, G. A B-lectin receptor kinase gene conferring rice blast resistance. Plant J. 2006, 46, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Ju, H.-W.; Min, J.-H.; Zhang, X.; Kim, S.-H.; Yang, K.-Y.; Kim, C.S. Overexpression of L-type lectin-like protein kinase 1 confers pathogen resistance and regulates salinity response in Arabidopsis thaliana. Plant Sci. 2013, 203, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Chien, C.-C.; Mishra, S.; Tsai, C.-H.; Zimmerli, L. The arabidopsis lectin receptor kinase-VI. 2 is a functional protein kinase and is dispensable for basal resistance to Botrytis cinerea. Plant Signal. Behav. 2012, 8, e22611. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kuo, Y.-C.; Mishra, S.; Tsai, C.-H.; Chien, C.-C.; Chen, C.-W.; Desclos-Theveniau, M.; Chu, P.-W.; Schulze, B.; Chinchilla, D.; et al. The lectin receptor kinase-VI.2 is required for priming and positively regulates arabidopsis pattern-triggered immunity. Plant Cell 2012, 24, 1256–1270. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, S.T.; Mahajan, S.K.; Whitham, S.A.; Yamamoto, M.L.; Carrington, J.C. Cloning of the Arabidopsis RTM1 gene, which controls restriction of long-distance movement of tobacco etch virus. Proc. Natl. Acad. Sci. USA 2000, 97, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, Y.; Maejima, K.; Komatsu, K.; Shiraishi, T.; Okano, Y.; Himeno, M.; Sugawara, K.; Neriya, Y.; Minato, N.; Miura, C.; et al. Lectin-mediated resistance impairs plant virus infection at the cellular level. Plant Cell 2012, 24, 778–793. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, K.; Govers, F. Arabidopsis L-type lectin receptor kinases: Phylogeny, classification, and expression profiles. J. Exp. Bot. 2009, 60, 4383–4396. [Google Scholar] [CrossRef] [PubMed]
- Desclos-Theveniau, M.; Arnaud, D.; Huang, T.-Y.; Lin, G.J.-C.; Chen, W.-Y.; Lin, Y.-C.; Zimmerli, L. The arabidopsis lectin receptor kinase LecRK-V.5 represses stomatal immunity induced by Pseudomonas syringae pv. Tomato DC3000. PLoS Pathog. 2012, 8, e1002513. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Dhuna, V.; Kamboj, S.S.; Agrewala, J.N.; Singh, J. A novel antiproliferative and antifungal lectin from amaranthus viridis linn seeds. Protein Pept. Lett. 2006, 13, 895–905. [Google Scholar] [CrossRef]
- Matsukawa, M.; Shibata, Y.; Ohtsu, M.; Mizutani, A.; Mori, H.; Wang, P.; Ojika, M.; Kawakita, K.; Takemoto, D. Nicotiana benthamiana calreticulin 3a is required for the ethylene-mediated production of phytoalexins and disease resistance against oomycete pathogen Phytophthora infestans. Mol. Plant Microbe Interact. 2013, 26, 880–892. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Xi, J.; Du, L.; Roje, S.; Poovaiah, B.W. A dual regulatory role of arabidopsis calreticulin-2 in plant innate immunity. Plant J. 2012, 69, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Atalah, B.A.; De Vleesschauwer, D.; Xu, J.; Fouquaert, E.; Höfte, M.; van Damme, E.J. Transcriptional behavior of EUL-related rice lectins towards important abiotic and biotic stresses. J. Plant Physiol. 2014, 171, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Song, M.; Wei, Z.; Tong, J.; Zhang, L.; Xiao, L.; Ma, Z.; Wang, Y. A jacalin-related lectin-like gene in wheat is a component of the plant defence system. J. Exp. Bot. 2011, 62, 5471–5483. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.-H.; Zhen, W.-B.; Liu, Y.-C. Jacalin domain in wheat jasmonate-regulated protein Ta-JA1 confers agglutinating activity and pathogen resistance. Biochimie 2013, 95, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Gorlach, J.; Volrath, S.; KnaufBeiter, G.; Hengy, G.; Beckhove, U.; Kogel, K.H.; Oostendorp, M.; Staub, T.; Ward, E.; Kessmann, H.; et al. Benzothiadiazole, a novel class of inducers of systemic acquired resistance, activates gene expression and disease resistance in wheat. Plant Cell 1996, 8, 629–643. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.-H.; Tian, B.; Li, Y.-L. Overexpression of a wheat jasmonate-regulated lectin increases pathogen resistance. Biochimie 2010, 92, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.R.; Boltz, K.A.; Lee, S.Y. Molecular chaperone function of arabidopsis thaliana phloem protein 2-a1, encodes a protein similar to phloem lectin. Biochem. Biophys. Res. Commun. 2014, 443, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, S.T.; Parra, M.A.; Anderberg, R.J.; Carrington, J.C. Arabidopsis RTM1 and RTM2 genes function in phloem to restrict long-distance movement of tobacco etch virus. Plant Physiol. 2001, 127, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.M.; Zhang, Q.; Zhao, W.S.; Wang, Y.Y.; Peng, Y.L. Identification of a lectin gene induced in rice in response to magnaporthe grisea infection. Acta Bot. Sin. 2003, 45, 76–81. [Google Scholar]
- Bencharki, B.; Boissinot, S.; Revollon, S.; Ziegler-Graff, V.; Erdinger, M.; Wiss, L.; Dinant, S.; Renard, D.; Beuve, M.; Lemaitre-Guillier, C.; et al. Phloem protein partners of cucurbit aphid borne yellows virus: Possible involvement of phloem proteins in virus transmission by aphids. Mol. Plant Microbe Interact. 2010, 23, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Peumans, W.J.; van Damme, E.J.M. The Sambucus nigra type-2 ribosome-inactivating protein SNA-I’ exhibits in planta antiviral activity in transgenic tobacco. FEBS Lett. 2002, 516, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Vandenbussche, F.; Peumans, W.J.; Desmyter, S.; Proost, P.; Ciani, M.; van Damme, E.J.M. The type-1 and type-2 ribosome-inactivating proteins from iris confer transgenic tobacco plants local but not systemic protection against viruses. Planta 2004, 220, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, E.J.M.; Lannoo, N.; Peumans, W.J. Plant lectins. Adv. Bot. Res. 2008, 48, 107–209. [Google Scholar]
- Lannoo, N.; van Damme, E.J. Lectin domains at the frontiers of plant defense. Front. Plant Sci. 2014, 5, 397. [Google Scholar] [PubMed]
- Singh, P.; Zimmerli, L. Lectin receptor kinases in plant innate immunity. Front. Plant Sci. 2013, 4, 124. [Google Scholar] [CrossRef] [PubMed]
- Vaid, N.; Pandey, P.K.; Tuteja, N. Genome-wide analysis of lectin receptor-like kinase family from arabidopsis and rice. Plant Mol. Biol. 2012, 80, 365–388. [Google Scholar] [CrossRef] [PubMed]
- Rinderle, S.J.; Goldstein, I.J.; Remsen, E.E. Physicochemical properties of amaranthin, the lectin from amaranthus-caudatus seeds. Biochemistry 1990, 29, 10555–10561. [Google Scholar] [CrossRef] [PubMed]
- Rinderle, S.J.; Goldstein, I.J.; Matta, K.L.; Ratcliffe, R.M. Isolation and characterization of amaranthin, a lectin present in the seeds of amaranthus-caudatus, that recognizes the T-antigen (or cryptic-T)-antigen. J. Biol. Chem. 1989, 264, 16123–16131. [Google Scholar] [PubMed]
- Atillasoy, E.O.; Kapetanakis, A.; Itzkowitz, S.H.; Holt, P.R. Amaranthin lectin binding in the rat colon: Response to dietary manipulation. Mt. Sinai J. Med. 1998, 65, 146–153. [Google Scholar] [PubMed]
- Rahbe, Y.; Sauvion, N.; Febvay, G.; Peumans, W.J.; Gatehouse, A.M.R. Toxicity of lectins and processing of ingested proteins in the pea aphid acyrthosiphon-pisum. Entomol. Exp. Appl. 1995, 76, 143–155. [Google Scholar] [CrossRef]
- Wu, J.; Luo, X.; Guo, H.; Xiao, J.; Tian, Y. Transgenic cotton, expressing amaranthus caudatus agglutinin, confers enhanced resistance to aphids. Plant Breed. 2006, 125, 390–394. [Google Scholar] [CrossRef]
- Xin, Y.; Xiangrong, Z.; Mingju, Z.; Wenchao, G.; Yingchuan, T.; Qizhong, X.; Jiahe, W. Transgenic potato overexpressing the agglutinin gene to confer aphid resistance. Crop Sci. 2011, 51, 2119–2124. [Google Scholar] [CrossRef]
- Helenius, A.; Trombetta, E.S.; Hebert, D.N.; Simons, J.F. Calnexin, calreticulin and the folding of glycoproteins. Trends Cell Biol. 1997, 7, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Persson, S.; Wyatt, S.E.; Love, J.; Thompson, W.F.; Robertson, D.; Boss, W.F. The Ca2+ status of the endoplasmic reticulum is altered by induction of calreticulin expression in transgenic plants. Plant Physiol. 2001, 126, 1092–1104. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Nguyen Hoai, N.; Ngoc Trinh, N.; Hong, S.-W.; Lee, H. Loss of all three calreticulins, CRT1, CRT2 and CRT3, causes enhanced sensitivity to water stress in arabidopsis. Plant Cell Rep. 2013, 32, 1843–1853. [Google Scholar] [CrossRef] [PubMed]
- An, Y.Q.; Lin, R.M.; Wang, F.T.; Feng, J.; Xu, Y.F.; Xu, S.C. Molecular cloning of a new wheat calreticulin gene TaCRT1 and expression analysis in plant defense responses and abiotic stress resistance. Genet. Mol. Res. 2011, 10, 3576–3585. [Google Scholar] [CrossRef] [PubMed]
- Jaouannet, M.; Magliano, M.; Arguel, M.J.; Gourgues, M.; Evangelisti, E.; Abad, P.; Rosso, M.N. The root-knot nematode calreticulin Mi-CRT is a key effector in plant defense suppression. Mol. Plant Microbe Interact. 2013, 26, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Fouquaert, E.; Peumans, W.J.; Smith, D.F.; Proost, P.; Savvides, S.N.; van Damme, E.J.M. The “old” euonymus europaeus agglutinin represents a novel family of ubiquitous plant proteins. Plant Physiol. 2008, 147, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Fouquaert, E.; Peumans, W.J.; Vandekerckhove, T.T.M.; Ongenaert, M.; van Damme, E.J.M. Proteins with an euonymus lectin-like domain are ubiquitous in embryophyta. BMC Plant Biol. 2009, 9, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hove, J.; Fouquaert, E.; Smith, D.F.; Proost, P.; van Damme, E.J.M. Lectin activity of the nucleocytoplasmic EUL protein from Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2011, 414, 101–105. [Google Scholar]
- Pacak, F.; Kocourek, J. Studies on phytohemagglutinins: xxv. Isolation and characterization of hemagglutinins of the spindle tree seeds (Evonymus europaea L.). Biochim. Biophys. Acta 1975, 400, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Petryniak, J.; Pereira, M.E.; Kabat, E.A. The lectin of Euonymus europeus: Purification, characterization, and an immunochemical study of its combining site. Arch. Biochem. Biophys. 1977, 178, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Petryniak, J.; Goldstein, I.J. Evonymus-europaea lectin. Methods Enzymol. 1987, 138, 552–561. [Google Scholar] [PubMed]
- Fouquaert, E.; Van Damme, E.J. Promiscuity of the euonymus carbohydrate-binding domain. Biomolecules 2012, 2, 415–434. [Google Scholar] [CrossRef] [PubMed]
- Peumans, W.J.; Zhang, W.L.; Barre, A.; Astoul, C.H.; Balint-Kurti, P.J.; Rovira, P.; Rouge, P.; May, G.D.; van Leven, F.; Truffa-Bachi, P.; et al. Fruit-specific lectins from banana and plantain. Planta 2000, 211, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Xu, W.; Xiang, Y.; Jia, H.; Zhang, L.; Ma, Z. Association of jacalin-related lectins with wheat responses to stresses revealed by transcriptional profiling. Plant Mol. Biol. 2014, 84, 95–110. [Google Scholar] [CrossRef]
- Chen, Y.; Peumans, W.J.; Hause, B.; Bras, J.; Kumar, M.; Proost, P.; Barre, A.; Rougé, P.; van Damme, E.J. Jasmonic acid methyl ester induces the synthesis of a cytoplasmic/nuclear chito-oligosaccharide binding lectin in tobacco leaves. FASEB J. 2002, 16, 905–907. [Google Scholar] [PubMed]
- Lannoo, N.; Peumans, W.J.; van Pamel, E.; Alvarez, R.; Xiong, T.-C.; Hause, G.; Mazars, C.; van Damme, E.J.M. Localization and in vitro binding studies suggest that the cytoplasmic/nuclear tobacco lectin can interact in situ with high-mannose and complex N-glycans. FEBS Lett. 2006, 580, 6329–6337. [Google Scholar] [CrossRef] [PubMed]
- Lannoo, N.; Vandenborre, G.; Miersch, O.; Smagghe, G.; Wasternack, C.; Peumans, W.J.; Van Damme, E.J.M. The jasmonate-induced expression of the nicotiana tabacum leaf lectin. Plant Cell Physiol. 2007, 48, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Stenzel, I.; Hause, B.; Hause, G.; Kutter, C.; Maucher, H.; Neumerkel, J.; Feussner, I.; Miersch, O. The wound response in tomato - role of jasmonic acid. J. Plant Physiol. 2006, 163, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Vandenborre, G.; Miersch, O.; Hause, B.; Smagghe, G.; Wasternack, C.; Van Damme, E.J.M. Spodoptera littoralis-induced lectin expression in tobacco. Plant Cell Physiol. 2009, 50, 1142–1155. [Google Scholar] [CrossRef] [PubMed]
- Vandenbussche, F.; Desmyter, S.; Ciani, M.; Proost, P.; Peumans, W.J.; Van Damme, E.J.M. Analysis of the in planta antiviral activity of elderberry ribosome-inactivating proteins. Eur. J. Biochem. 2004, 271, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.Q.; Liu, R.S.; Wang, Q.O.; Liu, W.Y. Toxicity of two type II ribosome-inactivating proteins (cinnamomin and ricin) to domestic silkworm larvae. Arch. Insect Biochem. Physiol. 2004, 57, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Shahidi-Noghabi, S.; van Damme, E.J.M.; Smagghe, G. Expression of sambucus nigra agglutinin (SNA-I’) from elderberry bark in transgenic tobacco plants results in enhanced resistance to different insect species. Transgenic Res. 2009, 18, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Jawhara, S.; Thuru, X.; Standaert-Vitse, A.; Jouault, T.; Mordon, S.; Sendid, B.; Desreumaux, P.; Poulain, D. Colonization of mice by candida albicans is promoted by chemically induced colitis and augments inflammatory responses through galectin-3. J. Infect. Dis. 2008, 197, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Zheng, H.; Derebe, M.G.; Callenberg, K.M.; Partch, C.L.; Rollins, D.; Propheter, D.C.; Rizo, J.; Grabe, M.; Jiang, Q.-X.; et al. Antibacterial membrane attack by a pore-forming intestinal c-type lectin. Nature 2014, 505, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Thurston, T.L.M.; Wandel, M.P.; von Muhlinen, N.; Foeglein, A.; Randow, F. Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature 2012, 482, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Paz, I.; Sachse, M.; Dupont, N.; Mounier, J.; Cederfur, C.; Enninga, J.; Leffler, H.; Poirier, F.; Prevost, M.-C.; Lafont, F.; et al. Galectin-3, a marker for vacuole lysis by invasive pathogens. Cell. Microbiol. 2010, 12, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-H.; Bi, W.-J.; Wang, X.-W.; Zhao, Y.-R.; Zhao, X.-F.; Wang, J.-X. Two novel c-type lectins with a low-density lipoprotein receptor class a domain have antiviral function in the shrimp marsupenaeus japonicus. Dev. Comp. Immunol. 2014, 42, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-W.; Xu, Y.-H.; Xu, J.-D.; Zhao, X.-F.; Wang, J.-X. Collaboration between a soluble c-type lectin and calreticulin facilitates white spot syndrome virus infection in shrimp. J. Immunol. 2014, 193, 2106–2117. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Ogawa, Y.; Aoki, R.; Shimada, S. Innate and intrinsic antiviral immunity in skin. J. Dermatol. Sci. 2014, 75, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Shimojima, M.; Takenouchi, A.; Shimoda, H.; Kimura, N.; Maeda, K. Distinct usage of three C-type lectins by Japanese encephalitis virus: DC-sign, Dc-SIGNR, and LSECtin. Arch. Virol. 2014, 159, 2023–2031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, X.-Q.; Jiang, H.-S.; Jia, W.-M.; Zhao, X.-F.; Wang, J.-X. Calnexin functions in antibacterial immunity of Marsupenaeus japonicus. Dev. Comp. Immunol. 2014, 46, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, N.; Zhang, S. Calreticulin is a microbial-binding molecule with phagocytosis-enhancing capacity. Fish Shellfish Immunol. 2013, 35, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Peatman, E.; Liu, H.; Feng, T.; Chen, L.; Liu, Z. Molecular characterization of three L-type lectin genes from channel catfish, Ictalurus punctatus and their responses to Edwardsiella ictaluri challenge. Fish Shellfish Immunol. 2012, 32, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Tan, J.-M.; Wang, Z.; Yin, S.-W.; Huang, X.; Wang, W.; Ren, Q. Cloning and characterization of two different L-type lectin genes from the Chinese mitten crab Eriocheir sinensis. Dev. Comp. Immunol. 2014, 46, 255–266. [Google Scholar] [CrossRef] [PubMed]
- John, C.M.; Jarvis, G.A.; Swanson, K.V.; Leffler, H.; Cooper, M.D.; Huflejt, M.E.; Griffiss, J.M. Galectin-3 binds lactosaminylated lipooligosaccharides from Neisseria gonorrhoeae and is selectively expressed by mucosal epithelial cells that are infected. Cell. Microbiol. 2002, 4, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Fradin, C.; Poulain, D.; Jouault, T. Beta-1,2-linked oligomannosides from candida albicans bind to a 32-kilodalton macrophage membrane protein homologous to the mammalian lectin galectin-3. Infect. Immun. 2000, 68, 4391–4398. [Google Scholar] [CrossRef] [PubMed]
- Drickamer, K. Two distinct classes of carbohydrate-recognition domains in animal lectins. J. Biol. Chem. 1988, 263, 9557–9560. [Google Scholar] [PubMed]
- Gabius, H.-J. Glycans: Bioactive signals decoded by lectins. Biochem. Soc. Trans. 2008, 36, 1491–1496. [Google Scholar] [CrossRef] [PubMed]
- Parodi, A.J. Protein glucosylation and its role in protein folding. Ann. Rev. Biochem. 2000, 69, 69–93. [Google Scholar] [CrossRef] [PubMed]
- Muller-Taubenberger, A.; Lupas, A.N.; Li, H.W.; Ecke, M.; Simmeth, E.; Gerisch, G. Calreticulin and calnexin in the endoplasmic reticulum are important for phagocytosis. EMBO J. 2001, 20, 6772–6782. [Google Scholar] [CrossRef] [PubMed]
- Guerin, R.; Beauregard, P.B.; Leroux, A.; Rokeach, L.A. Calnexin regulates apoptosis induced by inositol starvation in fission yeast. PLoS One 2009, 4, e6244. [Google Scholar] [CrossRef] [PubMed]
- Luana, W.; Li, F.; Wang, B.; Zhang, X.; Liu, Y.; Xiang, J. Molecular characteristics and expression analysis of calreticulin in chinese shrimp fenneropenaeus chinensis. Comp. Biochem. Physiol. B-Biochem. Mol. Biol. 2007, 147, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Watthanasurorot, A.; Jiravanichpaisal, P.; Soderhall, K.; Soderhall, I. A calreticulin/gC1qR complex prevents cells from dying: A conserved mechanism from arthropods to humans. J. Mol. Cell Biol. 2013, 5, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hinson, E.R.; Cresswell, P. The interferon-inducible protein viperin inhibits influenza virus release by perturbing lipid rafts. Cell Host Microbe 2007, 2, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Sharon, N.; Lis, H. History of lectins: From hemagglutinins to biological recognition molecules. Glycobiology 2004, 14, 53R–62R. [Google Scholar] [CrossRef] [PubMed]
- Itin, C.; Schindler, R.; Hauri, H.P. Targeting of protein ERGIC-53 to the ER/ERGIC/cis-Golgi recycling pathway. J. Cell Biol. 1995, 131, 57–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neve, E.P.A.; Svensson, K.; Fuxe, J.; Pettersson, R.F. VIPL, a VIP36-like membrane protein with a putative function in the export of glycoproteins from the endoplasmic reticulum. Exp. Cell Res. 2003, 288, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Shimada, O.; Hara-Kuge, S.; Yamashita, K.; Tosaka-Shimada, H.; Li, Y.C.; Li, E.N.; Atsumi, S.; Ishikawa, H. Localization of VIP36 in the post-Golgi secretory pathway also of rat parotid acinar cells. J. Histochem. Cytochem. 2003, 51, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Yerushalmi, N.; Keppler-Hafkemeyer, A.; Vasmatzis, G.; Liu, X.F.; Olsson, P.; Bera, T.K.; Duray, P.; Lee, B.; Pastan, I. ERGL, a novel gene related to ERGIC-53 that is highly expressed in normal and neoplastic prostate and several other tissues. Gene 2001, 265, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Hauri, H.P.; Appenzeller, C.; Kuhn, F.; Nufer, O. Lectins and traffic in the secretory pathway. FEBS Lett. 2000, 476, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Rini, J.M. Lectin structure. Ann. Rev. Biophys. Biomol. Struct. 1995, 24, 551–577. [Google Scholar] [CrossRef]
- Klumperman, J.; Schweizer, A.; Clausen, H.; Tang, B.L.; Hong, W.J.; Oorschot, V.; Hauri, H.P. The recycling pathway of protein ERGIC-53 and dynamics of the ER-Golgi intermediate compartment. J. Cell Sci. 1998, 111, 3411–3425. [Google Scholar] [PubMed]
- Vollenweider, F.; Kappeler, F.; Itin, C.; Hauri, H.P. Mistargeting of the lectin ERGIC-53 to the endoplasmic reticulum of Hela cells impairs the secretion of a lysosomal enzyme. J. Cell Biol. 1998, 142, 377–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appenzeller, C.; Andersson, H.; Kappeler, F.; Hauri, H.P. The lectin ERGIC-53 is a cargo transport receptor for glycoproteins. Nat. Cell Biol. 1999, 1, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Moussalli, M.; Pipe, S.W.; Hauri, H.P.; Nichols, W.C.; Ginsburg, D.; Kaufman, R.J. Mannose-dependent endoplasmic reticulum (ER)-Golgi intermediate compartment-53-mediated ER to Golgi trafficking of coagulation factors V and VIII. J. Biol. Chem. 1999, 274, 32539–32542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Kaufman, R.J.; Ginsburg, D. LMAN1 and MCFD2 form a cargo receptor complex and interact with coagulation factor VIII in the early secretory pathway. J. Biol. Chem. 2005, 280, 25881–25886. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, L.; Wang, X.-W.; Zhao, Y.-R.; Bi, W.-J.; Zhao, X.-F.; Wang, J.-X. L-type lectin from the kuruma shrimp marsupenaeus japonicus promotes hemocyte phagocytosis. Dev. Comp. Immunol. 2014, 44, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Zelensky, A.N.; Gready, J.E. The c-type lectin-like domain superfamily. FEBS J. 2005, 272, 6179–6217. [Google Scholar] [CrossRef] [PubMed]
- Yabe, R.; Iwakura, Y.; Saijo, S. The role of c-type lectin receptors c-type lectin receptors in the host defense against microbial pathogens pathogens. In Glycoscience: Biology and Medicine; Springer: Tokyo, Japan, 2014; pp. 1–10. [Google Scholar]
- Vasta, G.R. Roles of galectins in infection. Nat. Rev. Microbiol. 2009, 7, 424–438. [Google Scholar] [CrossRef] [PubMed]
- Varki, A.; Angata, T. Siglecs—The major subfamily of i-type lectins. Glycobiology 2006, 16, 1R–27R. [Google Scholar] [CrossRef] [PubMed]
- Crocker, P.R. Siglecs: Sialic-acid-binding immunoglobulin-like lectins in cell-cell interactions and signalling. Curr. Opin. Struct. Biol. 2002, 12, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Jandus, C.; Simon, H.-U.; von Gunten, S. Targeting siglecs—a novel pharmacological strategy for immuno- and glycotherapy. Biochem. Pharmacol. 2011, 82, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Avril, T.; Wagner, E.R.; Willison, H.J.; Crocker, P.R. Sialic acid-binding immunoglobulin-like lectin 7 mediates selective recognition of sialylated glycans expressed on campylobacter jejuni lipooligosaccharides. Infect. Immun. 2006, 74, 4133–4141. [Google Scholar] [CrossRef] [PubMed]
- Klaas, M.; Oetke, C.; Lewis, L.E.; Erwig, L.P.; Heikema, A.P.; Easton, A.; Willison, H.J.; Crocker, P.R. Sialoadhesin promotes rapid proinflammatory and type I IFN responses to a sialylated pathogen, Campylobacter jejuni. J. Immunol. 2012, 189, 2414–2422. [Google Scholar] [CrossRef] [PubMed]
- Crocker, P.R.; Redelinghuys, P. Siglecs as positive and negative regulators of the immune system. Biochem. Soc. Trans. 2008, 36, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Sumner, J.B.; Howell, S.F. Identification of hemagglutinin of jack bean with concanavalin a. J. Bacteriol. 1936, 32, 227–237. [Google Scholar] [PubMed]
- Oliveira, M.L.D.; Beltramini, L.M.; Simone, S.G.D.; Brumano, M.H.N.; Silva-Lucca, R.A.; Nakaema, M.K.K.; Pires, C.V.; Oliveira, M.G.D.A. Purification and partial characterization of a lectin from Caesalpinia tinctoria Domb, ex Dc fruits. Braz. J. Plant Physiol. 2003, 15, 119–122. [Google Scholar] [CrossRef]
- Elgavish, S.; Shaanan, B. Lectin-carbohydrate interactions: Different folds, common recognition principles. Trends Biochem. Sci. 1997, 22, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.J.; Hori, K. Marine algal lectins—New developments. Hydrobiologia 1993, 261, 589–593. [Google Scholar] [CrossRef]
- Soedjanaatmadja, U.M.S.; Subroto, T.; Beintema, J.J. Processed products of the hevein precursor in the latex of the rubber tree (heven brasiliensis). FEBS Lett. 1995, 363, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Loris, R. Principles of structures of animal and plant lectins. Biochim. Biophys. Acta 2002, 1572, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Transue, T.R.; Smith, A.K.; Mo, H.Q.; Goldstein, I.J.; Saper, M.A. Structure of benzyl T-antigen disaccharide bound to amaranthus caudatus agglutinin. Nat. Struct. Biol. 1997, 4, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, L.; Roberts, L.M. The enemy within: Ricin and plant cells. J. Exp. Bot. 1998, 49, 1473–1480. [Google Scholar] [CrossRef]
- Monzingo, A.F.; Robertus, J.D. X-ray-analysis of substrate-analogs in the ricin A-chain active-site. J. Mol. Biol. 1992, 227, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, A.H.; Rogers, D.J.; Barwell, C.J. A galactose-specific lectin from the red marine alga ptilota filicina. Phytochemistry 1998, 48, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Jeyaprakash, A.A.; Rani, P.G.; Reddy, G.B.; Banumathi, S.; Betzel, C.; Sekar, K.; Surolia, A.; Vijayan, M. Crystal structure of the jacalin-T-antigen complex and a comparative study of lectin-T-antigen complexes. J. Mol. Biol. 2002, 321, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Etzler, M.E.; Surolia, A.; Cummings, R.D. L-type lectins. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor Laboratory Press, The Consortium of Glycobiology Editors: La Jolla, CA, USA, 2009. [Google Scholar]
- Kabir, S.R.; Nabi, M.M.; Haque, A.; Zaman, R.U.; Mahmud, Z.H.; Abu Reza, M. Pea lectin inhibits growth of ehrlich ascites carcinoma cells by inducing apoptosis and g(2)/m cell cycle arrest in vivo in mice. Phytomedicine 2013, 20, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Diehl, C.; Engstrom, O.; Delaine, T.; Hakansson, M.; Genheden, S.; Modig, K.; Leffler, H.; Ryde, U.; Nilsson, U.J.; Akke, M. Protein flexibility and conformational entropy in ligand design targeting the carbohydrate recognition domain of galectin-3. J. Am. Chem. Soc. 2010, 132, 14577–14589. [Google Scholar] [CrossRef] [PubMed]
- Chouquet, A.; Paidassi, H.; Ling, W.L.; Frachet, P.; Houen, G.; Arlaud, G.J.; Gaboriaud, C. X-ray structure of the human calreticulin globular domain reveals a peptide-binding area and suggests a multi-molecular mechanism. PLoS One 2011, 6, e17886. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, G.; Bastos-Aristizabal, S.; Maeaettaenen, P.; Rosenauer, A.; Zheng, F.; Killikelly, A.; Trempe, J.-F.; Thomas, D.Y.; Gehring, K. Structural basis of cyclophilin B binding by the calnexin/calreticulin P-domain. J. Biol. Chem. 2010, 285, 35551–35557. [Google Scholar] [CrossRef] [PubMed]
- Schrag, J.D.; Bergeron, J.J.M.; Li, Y.G.; Borisova, S.; Hahn, M.; Thomas, D.Y.; Cygler, M. The structure of calnexin, an ER chaperone involved in quality control of protein folding. Mol. Cell 2001, 8, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Sharon, N.; Lis, H. Legume lectins—A large family of homologous proteins. FASEB J. 1990, 4, 3198–3208. [Google Scholar] [PubMed]
- Zheng, C.; Page, R.C.; Das, V.; Nix, J.C.; Wigren, E.; Misra, S.; Zhang, B. Structural characterization of carbohydrate binding by LMAN1 protein provides new insight into the endoplasmic reticulum export of factors V (FV) and viii (FVIII). J. Biol. Chem. 2013, 288, 20499–20509. [Google Scholar] [CrossRef] [PubMed]
- Shrive, A.K.; Tharia, H.A.; Strong, P.; Kishore, U.; Burns, I.; Rizkallah, P.J.; Reid, K.B.M.; Greenhough, T.J. High-resolution structural insights into ligand binding and immune cell recognition by human lung surfactant protein D. J. Mol. Biol. 2003, 331, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Hamelryck, T.W.; Poortmans, F.; Goossens, A.; Angenon, G.; van Montagu, M.; Wyns, L.; Loris, R. Crystal structure of arcelin-5, a lectin-like defense protein from phaseolus vulgaris. J. Biol. Chem. 1996, 271, 32796–32802. [Google Scholar] [CrossRef] [PubMed]
- Riera, A.S.; Daud, A.; Gallo, A.; Genta, S.; Aybar, M.; Sanchez, S. Antibacterial activity of lactose-binding lectins from Bufo arenarum skin. BIOCELL 2003, 27, 37–46. [Google Scholar] [PubMed]
- Konozy, E.H.E.; Bernardes, E.S.; Rosa, C.; Faca, V.; Greene, L.J.; Ward, R.J. Isolation, purification, and physicochemical characterization of a d-galactose-binding lectin from seeds of Erythrina speciosa. Arch. Biochem. Biophys. 2003, 410, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, Y.K.; Green, D.F. Carbohydrate recognition by the antiviral lectin cyanovirin-N. J. Am. Chem. Soc. 2012, 134, 19639–19651. [Google Scholar] [CrossRef] [PubMed]
- Barre, A.; Herve, C.; Lescure, B.; Rouge, P. Lectin receptor kinases in plants. Crit. Rev. Plant Sci. 2002, 21, 379–399. [Google Scholar] [CrossRef]
- Canado Viana, J.F.; Dias, S.C.; Franco, O.L.; Lacorte, C. Heterologous production of peptides in plants: Fusion proteins and beyond. Curr. Protein Pept. Sci. 2013, 14, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Bebber, D.P.; Ramotowski, M.A.T.; Gurr, S.J. Crop pests and pathogens move polewards in a warming world. Nat. Clim. Chang. 2013, 3, 985–988. [Google Scholar] [CrossRef]
- Boyd, L.A.; Ridout, C.; O’Sullivan, D.M.; Leach, J.E.; Leung, H. Plant-pathogen interactions: Disease resistance in modern agriculture. Trends Genet. 2013, 29, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Burrows, P.R.; Barker, A.D.P.; Newell, C.A.; Hamilton, W.D.O. Plant-derived enzyme inhibitors and lectins for resistance against plant-parasitic nematodes in transgenic crops. Pestic. Sci. 1998, 52, 176–183. [Google Scholar] [CrossRef]
- Davis, E.L.; Kaplan, D.T. Lectin binding to aqueous-soluble and body wall proteins from infective juveniles of meloidogyne species. Fundam. Appl. Nematol. 1992, 15, 243–250. [Google Scholar]
- Marban, N.; Bess, M.; Bert, M. Evaluation of control of meloidogyne incognita and nacobbus aberrans on tomato by two leguminous plants. Rev. Nématol. 1989, 12, 409–412. [Google Scholar]
- Guo, P.; Wang, Y.; Zhou, X.; Xie, Y.; Wu, H.; Gao, X. Expression of soybean lectin in transgenic tobacco results in enhanced resistance to pathogens and pests. Plant Sci. 2013, 211, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Eisemann, C.H.; Donaldson, R.A.; Pearson, R.D.; Cadogan, L.C.; Vuocolo, T.; Tellam, R.L. Larvicidal activity of lectins on lucilia-cuprina—Mechanism of action. Entomol. Exp. Appl. 1994, 72, 1–10. [Google Scholar] [CrossRef]
- Machuka, J.S.; Okeola, O.G.; Chrispeels, M.J.; Jackai, L.E.N. The african yam bean seed lectin affects the development of the cowpea weevil but does not affect the development of larvae of the legume pod borer. Phytochemistry 2000, 53, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Foissac, X.; Loc, N.T.; Christou, P.; Gatehouse, A.M.R.; Gatehouse, J.A. Resistance to green leafhopper (Nephotettix virescens) and brown planthopper (Nilaparvata lugens) in transgenic rice expressing snowdrop lectin (Galanthus nivalis agglutinin; GNA). J. Insect Physiol. 2000, 46, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wu, A.; Tang, K. Transgenic rice lines with enhanced resistance to the small brown planthopper. Crop Prot. 2002, 21, 511–514. [Google Scholar] [CrossRef]
- Fitches, E.; Gatehouse, A.M.R.; Gatehouse, J.A. Effects of snowdrop lectin (GNA) delivered via artificial diet and transgenic plants on the development of tomato moth (Lacanobia oleracea) larvae in laboratory and glasshouse trials. J. Insect Physiol. 1997, 43, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.V.; Rathore, K.S.; Hodges, T.K.; Fu, X.; Stoger, E.; Sudhakar, D.; Williams, S.; Christou, P.; Bharathi, M.; Bown, D.P.; et al. Expression of snowdrop lectin (GNA) in transgenic rice plants confers resistance to rice brown planthopper. Plant J. 1998, 15, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.D.; Layne, D.R.; Scorza, R.; Schnabel, G. Gastrodia anti-fungal protein from the orchid gastrodia elata confers disease resistance to root pathogens in transgenic tobacco. Planta 2006, 224, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Zhang, X.; Daniell, H. Pinellia ternata agglutinin expression in chloroplasts confers broad spectrum resistance against aphid, whitefly, lepidopteran insects, bacterial and viral pathogens. Plant Biotechnol. J. 2012, 10, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Ueno, K.; Lim, D.J. Glycoconjugates in the chinchilla tubotympanum: A lectin histochemical study. Nihon Jibiinkoka Gakkai kaiho 1991, 94, 1826–1833. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.; Majumder, G.C.; Chatterjee, T. Goat sperm membrane: Lectin-binding sites of sperm surface and lectin affinity chromatography of the mature sperm membrane antigens. Biochim. Biophys. Acta 1991, 1070, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, F.; Kawauchi, H.; Takayanagi, G. Blood group B-specific lectin of Plecoglossus altivelis (Ayu fish) eggs. Biochim. Biophys. Acta 1985, 841, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Khang, N.Q.; Jeanluc, G.; Johan, H. A blood group A specific lectin from the seeds of Crotalaria striata. Biochim. Biophys. Acta 1990, 1033, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Monte, L.G.; Santi-Gadelha, T.; Reis, L.B.; Braganhol, E.; Prietsch, R.F.; Dellagostin, O.A.; Rodrigues e Lacerda, R.; Gadelha, C.A.A.; Conceicao, F.R.; Pinto, L.S. Lectin of Abelmoschus esculentus (okra) promotes selective antitumor effects in human breast cancer cells. Biotechnol. Lett. 2014, 36, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Chavarria, I.; Cerro, R.P.; Parra, N.P.; Sandoval, F.A.; Zuniga, F.A.; Omazabal, V.A.; Lamperti, L.I.; Jimenez, S.P.; Fernandez, E.A.; Gutierrez, N.A.; et al. Lectin-like oxidized LDL receptor-1 is an enhancer of tumor angiogenesis in human prostate cancer cells. PLoS One 2014, 9, e106219. [Google Scholar] [CrossRef] [PubMed]
- Ryder, S.D.; Smith, J.A.; Rhodes, J.M. Peanut lectin—A mitogen for normal human colonic epithelium and human HT29 colorectal-cancer cells. J. Natl Cancer Inst. 1992, 84, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Kawano, J.I.; Oinuma, T.; Haraguchi, Y.; Eto, T.; Suganuma, T. Human colorectal carcinoma-specific glycoconjugates detected by pokeweed mitogen lectin. J. Histochem. Cytochem. 1993, 41, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.H.; Kobayashi, R.; Hirabayashi, Y.; Fujisue-Sakai, M.; Mizuguchi, S.; Nomura, K. Involvement of blood-group-B-active trisaccharides in Ca2+-dependent cell-cell adhesion in the Xenopus blastula. Dev. Genes Evol. 1998, 208, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.A.; Hawkins, R.A.; Miller, W.R. Lectin binding and steroid-receptors in human-breast carcinomas. J. Pathol. 1985, 147, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S. The isolation and characterization of jacalin [Artocarpus heterophyllus (jackfruit) lectin] based on its charge properties. Int. J. Biochem. Cell Biol. 1995, 27, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.; Zhou, X.; White, M.; Hartshorn, K.; Takahashi, K.; Kinane, T.B.; Anandaiah, A.; Koziel, H. Recombinant human mannose-binding lectin dampens human alveolar macrophage inflammatory responses to influenza a virus in vitro. J. Leukoc. Biol. 2014, 95, 715–722. [Google Scholar] [CrossRef]
- Rajoriya, N.; Fergusson, J.R.; Leithead, J.A.; Klenerman, P. Gamma delta T-lymphocytes in hepatitis c and chronic liver disease. Front. Immunol. 2014, 5, 400. [Google Scholar] [CrossRef] [PubMed]
- Adamek, M.; Heyder, J.; Heinold, A.; Fiedler, G.; Opelz, G.; Tran, T.H. Characterization of mannose-binding lectin (MBL) variants by allele-specific sequencing of MBL2 and determination of serum MBL protein levels. Tissue Antigens 2013, 82, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Yazgan, I.; Noah, N.M.; Toure, O.; Zhang, S.; Sadik, O.A. Biosensor for selective detection of E. coli in spinach using the strong affinity of derivatized mannose with fimbrial lectin. Biosens. Bioelectron. 2014, 61, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Delanghe, J.R.; Debuyzere, M.L.; Descheerder, I.K.; Faust, U.; Wieme, R.J. Activation-energy and lectin affinity-chromatography of gamma-glutamyltransferase as a marker for enzyme heterogeneity. Clin. Biochem. 1989, 22, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Lescar, J.; Sanchez, J.-F.; Audfray, A.; Coll, J.-L.; Breton, C.; Mitchell, E.P.; Imberty, A. Structural basis for recognition of breast and colon cancer epitopes Tn antigen and forssman disaccharide by Helix pomatia lectin. Glycobiology 2007, 17, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- La, M.; Cao, T.V.; Cerchiaro, G.; Chilton, K.; Hirabayashi, J.; Kasao, K.; Oliani, S.M.; Chernajovsky, Y.; Perretti, M. A novel biological activity for galectin-1: Inhibition of leukocyte-endothelial cell interactions in experimental inflammation. Am. J. Pathol. 2003, 163, 1505–1515. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, R.D.O.; Machado, L.D.S.; Migliolo, L.; Franco, O.L. Insights into Animal and Plant Lectins with Antimicrobial Activities. Molecules 2015, 20, 519-541. https://doi.org/10.3390/molecules20010519
Dias RDO, Machado LDS, Migliolo L, Franco OL. Insights into Animal and Plant Lectins with Antimicrobial Activities. Molecules. 2015; 20(1):519-541. https://doi.org/10.3390/molecules20010519
Chicago/Turabian StyleDias, Renata De Oliveira, Leandro Dos Santos Machado, Ludovico Migliolo, and Octavio Luiz Franco. 2015. "Insights into Animal and Plant Lectins with Antimicrobial Activities" Molecules 20, no. 1: 519-541. https://doi.org/10.3390/molecules20010519
APA StyleDias, R. D. O., Machado, L. D. S., Migliolo, L., & Franco, O. L. (2015). Insights into Animal and Plant Lectins with Antimicrobial Activities. Molecules, 20(1), 519-541. https://doi.org/10.3390/molecules20010519





