Synthesis, Leishmanicidal Activity and Theoretical Evaluations of a Series of Substituted bis-2-Hydroxy-1,4-Naphthoquinones
Abstract
:1. Introduction
2. Results and Discussion
| Compounds | Chemical Structure (R=)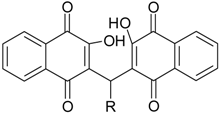 | LC50(µM) a | Maximum Cytotoxicity (%) b |
|---|---|---|---|
| Pentamidine | - | 73.0 ± 6.0 | 78.0 ± 3.8 ** |
| 3a | H | >100 | NT |
| 3b | n-Pentyl | >100 | NT |
| 3c | CH(Et)2 | 67.4 ± 2.1 | 71.7 ± 3.8 ** |
| 3d | -Ph | >100 | NT |
| 3e | -C6H4(4-OMe) | >100 | NT |
| 3f | -C6H4(4-OH) | >100 | NT |
| 3g | -C6H4(4-NO2) | >100 | NT |
| 3h | -C6H4(4-F) | >100 | NT |
| Compounds | Chemical Structure (R=) | L. amazonensis | L. braziliensis | ||
|---|---|---|---|---|---|
| IC50 (µM) a | Maximum Effect (%) b | IC50 (µM) a | MaximumEffect (%) b | ||
| Pentamidine | - | 2.3 ± 0.8 | 85.4 ± 0.4 ** | 0.8 ± 0.06 | 91.0 ± 0.1 ** |
| 3a | H | 71.0 ± 1.1 | 72.8 ± 1.0 ** | 0.9 ± 0.08 | 88.4 ± 0.9 ** |
| 3b | n-Pentyl | 5.2 ± 0.1 | 57.2 ± 3.8 ** | 5.2 ± 0.1 | 93.0 ± 0.1 ** |
| 3c | CH(Et)2 | 0.4 ± 0.1 | 75.3 ± 1.3 ** | 34.7 ± 4.3 | 90.4 ± 0.7 ** |
| 3d | Ph | 0.3 ± 0.1 | 85.0 ± 3.8 ** | >100 | NT |
| 3e | (4-OMe)Ph | >100 | 44.6 ± 2.4 ** | 0.9 ± 0.04 | 61.6 ± 7.5 ** |
| 3f | (4-OH)Ph | 68.7 ± 15.1 | 55.4 ± 4.7 ** | 38.7 ± 2.0 | 91.7 ± 0.3 ** |
| 3g | (4-NO2)Ph | 7.7 ± 1.2 | 55.6 ± 3.2 ** | 2.8 ± 0.1 | 91.7 ± 1.3 ** |
| 3h | (4-F)Ph | 0.6 ± 0.2 | 51.0 ± 5.5 ** | 0.8 ± 0.03 | 88.7 ± 0.3 ** |
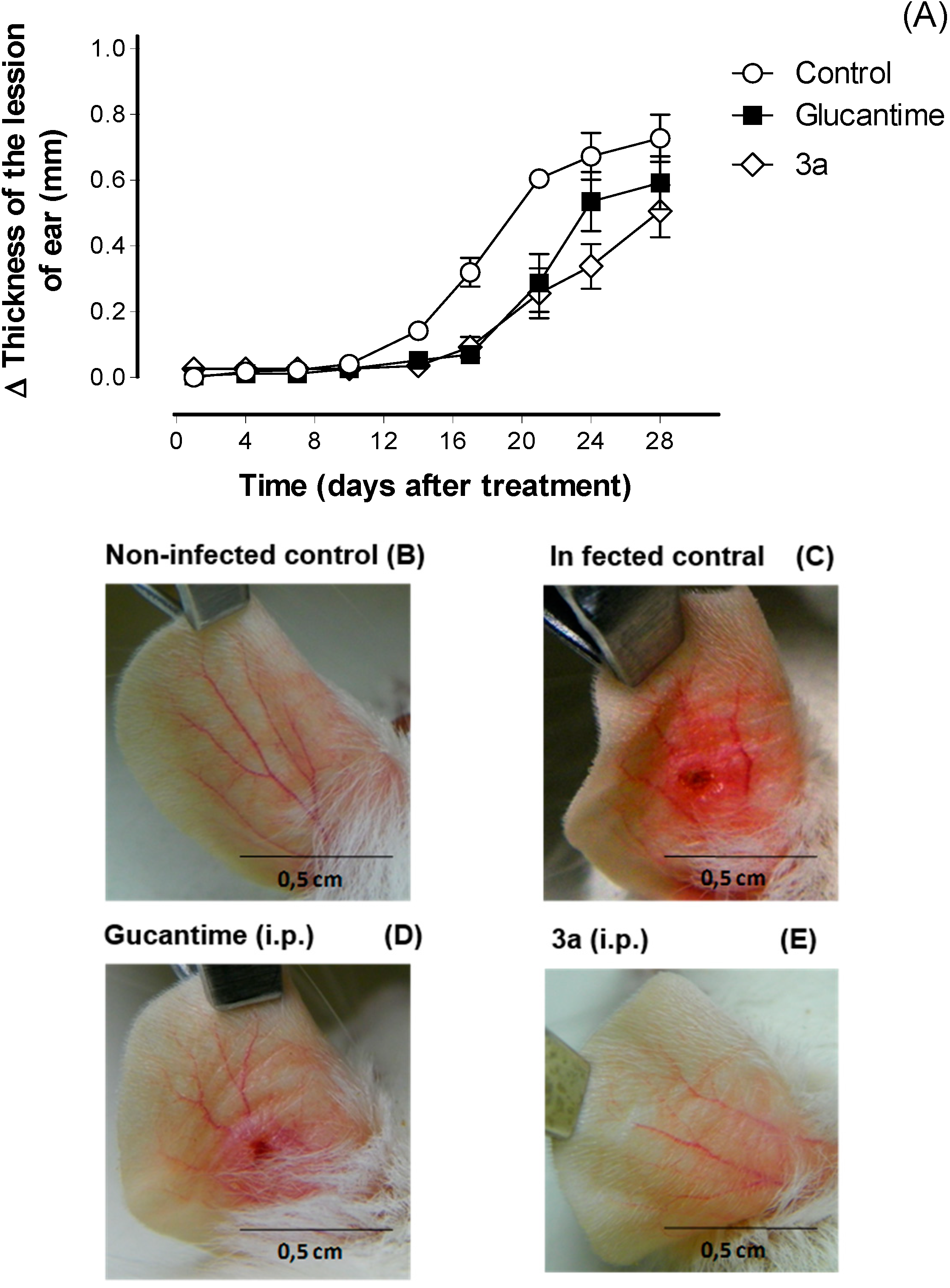
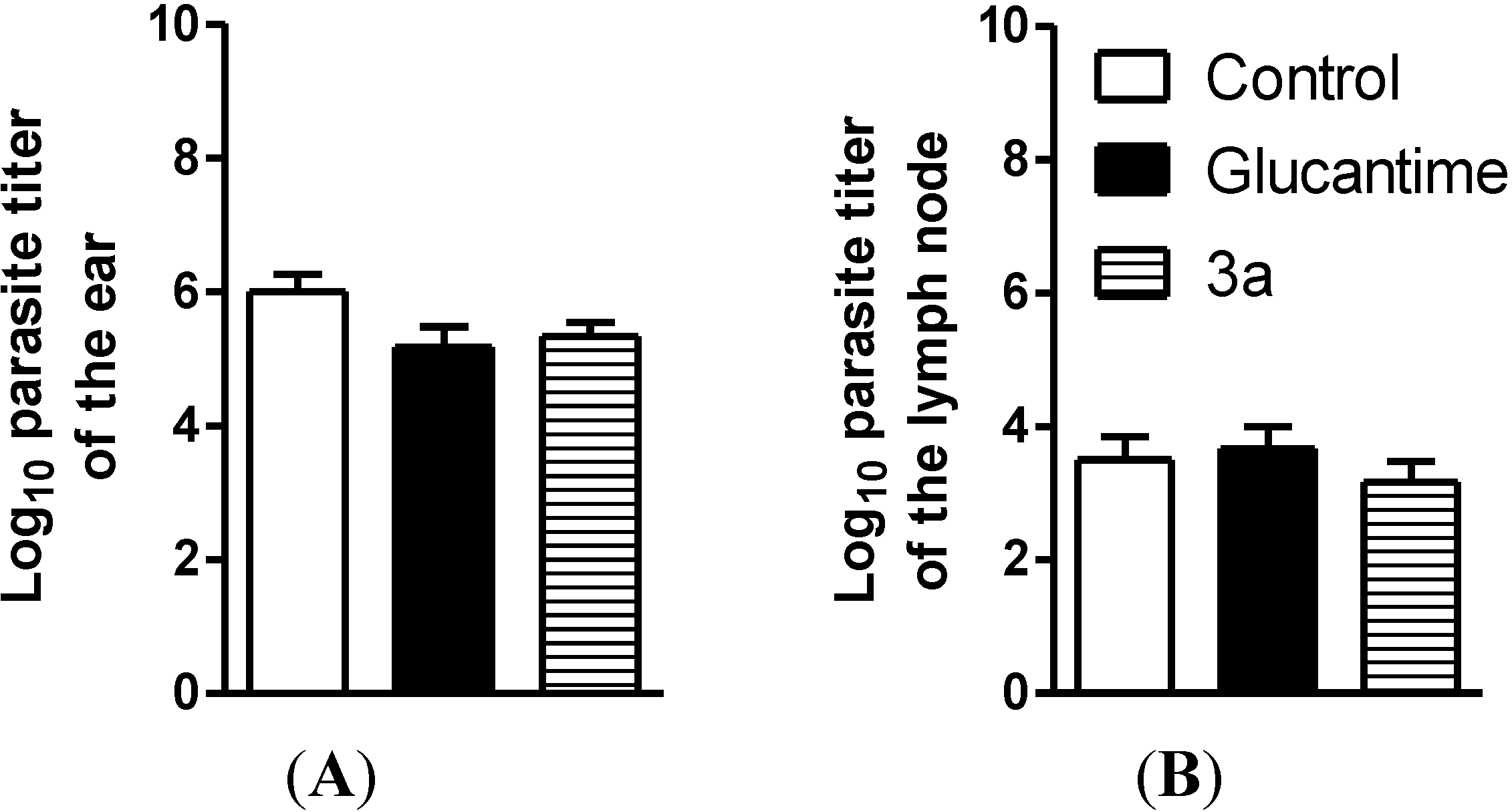
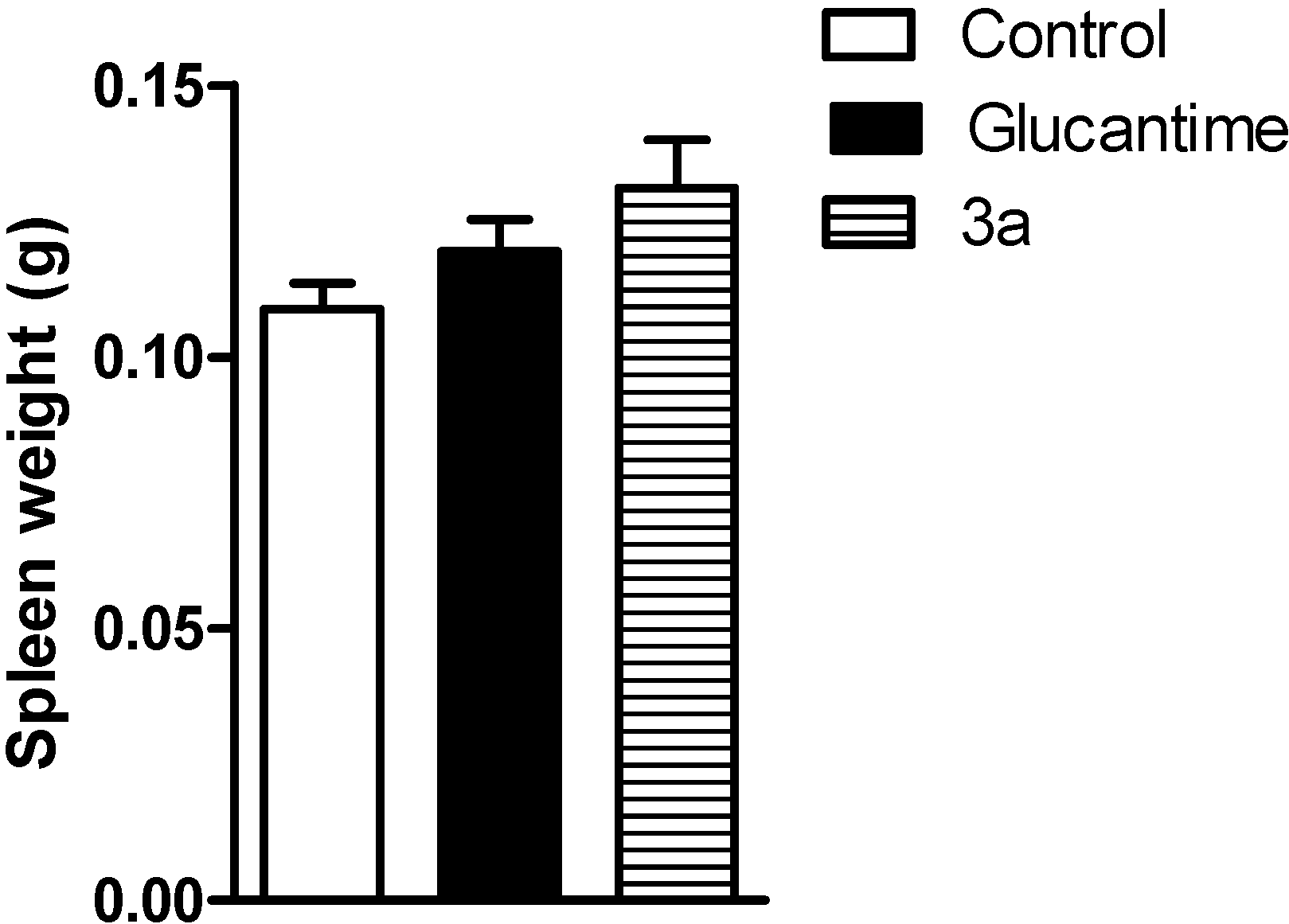
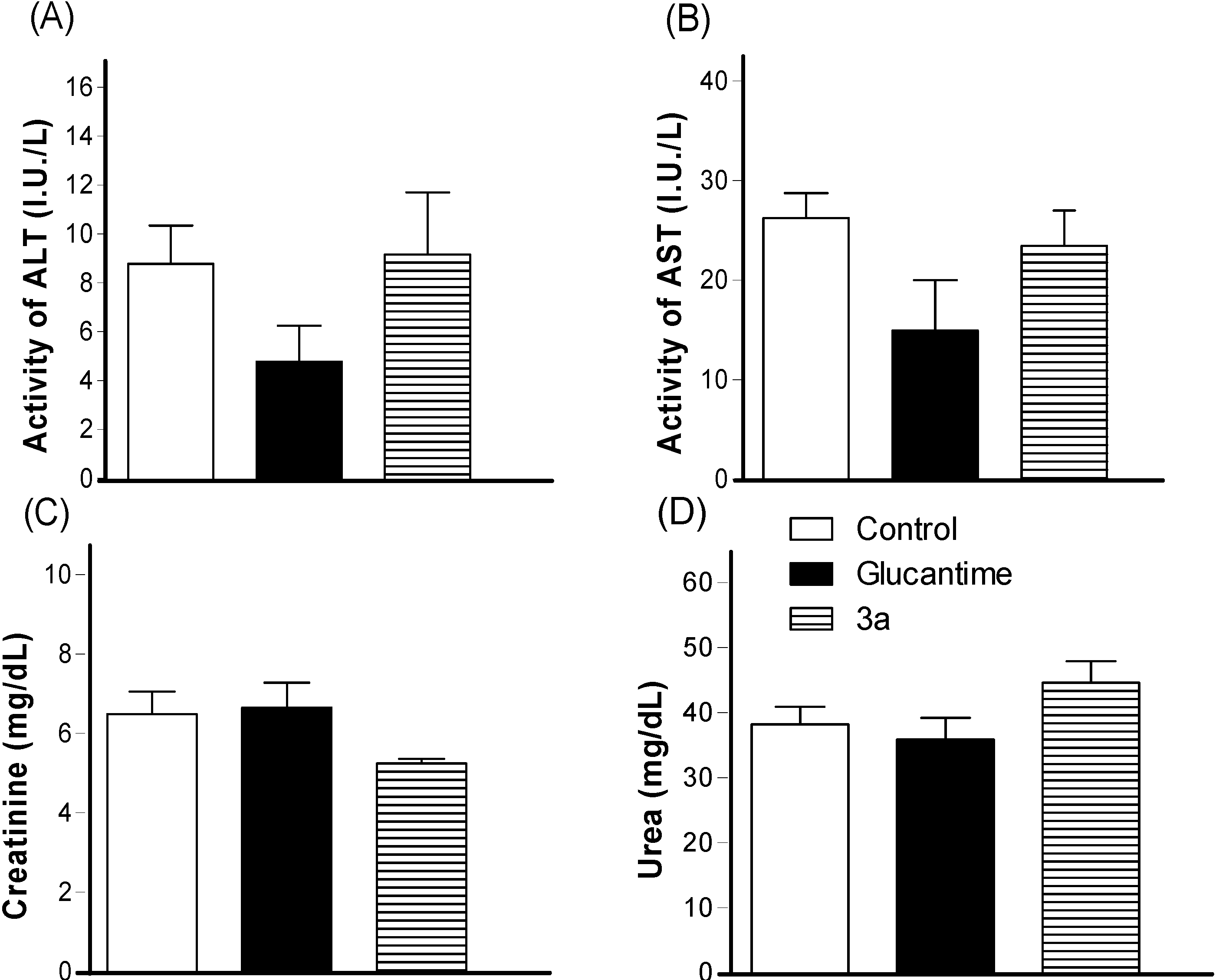
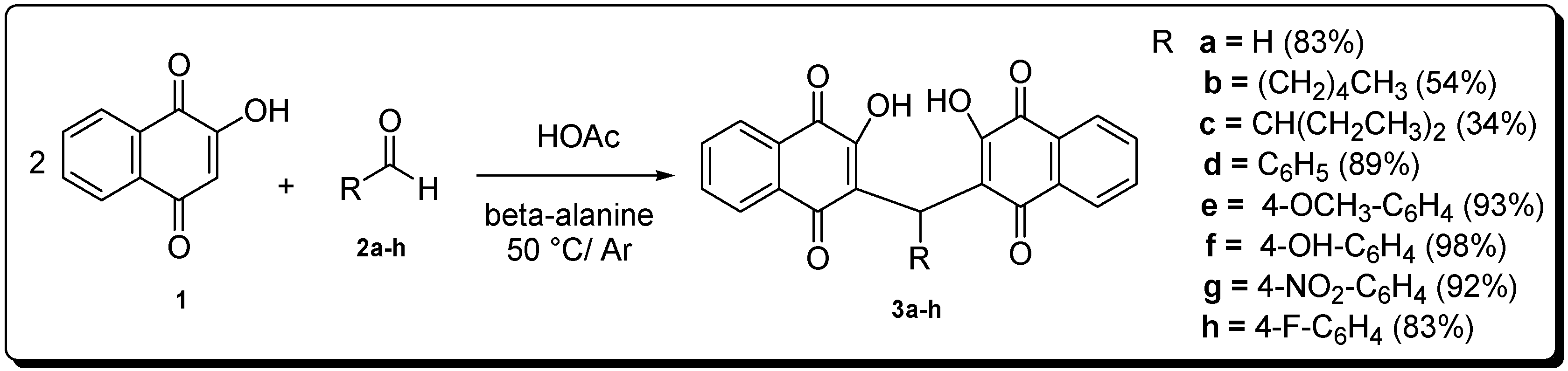
3. Experimental Section
3.1. General Information
3.2. Chemistry
General Procedure for the Synthesis of Compounds 3a–h
3.3. Biological Evaluation of Naphthoquinones
3.3.1. Parasite Culture
3.3.2. J774.A1 Murine Macrophage Culture
3.3.3. Cytotoxicity against Host Cells
3.3.4. In Vitro Activity against L. braziliensis and L. amazonensis
3.3.5. In Vivo Activity against Leishmania amazonensis
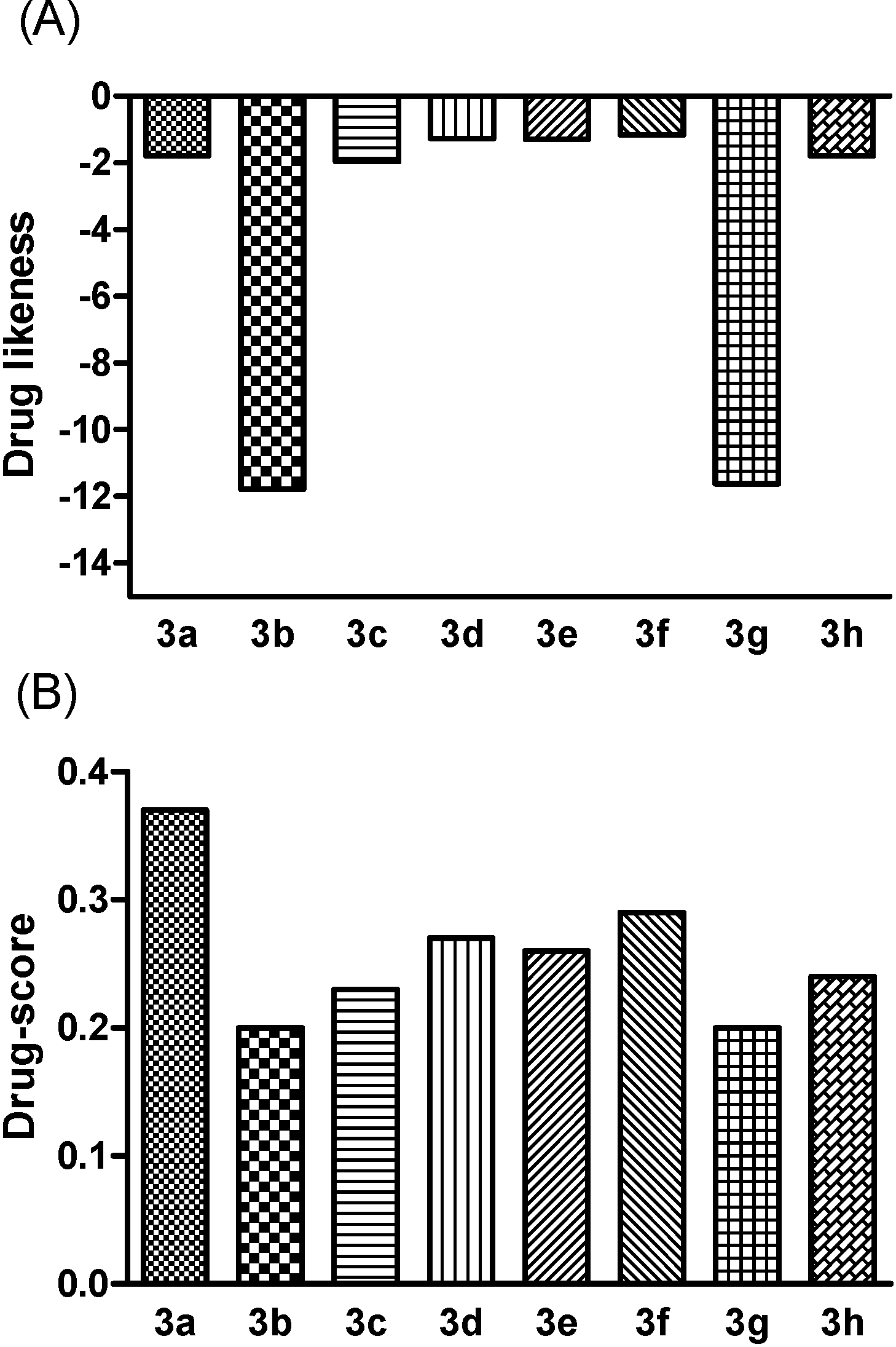
3.3.6. In Silico Screening
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Benítez, J.; Valderrama, J.A.; Rivera, F.; Rojo, L.; Campos, N.; Pedro, M.; Nascimento, M.S.J. Studies on quinones. Part 42: Synthesis of furylquinone and hydroquinones with antiproliferative activity against human tumor cell lines. Bioorg. Med. Chem. 2008, 16, 862–868. [Google Scholar]
- Araújo, A.J.; de Souza, A.A.; da Silva-Júnior, E.N.; Marinho-Filho, J.D.B.; de Moura, M.A.B.F.; Rocha, D.D.; Vasconcellos, M.C.; Costa, C.O.; Pessoa, C.; de Moraes, M.O.; et al. Growth inhibitory effects of 3'-nitro-3-phenylamino nor-beta-lapachone against HL-60: A redox-dependent mechanism. Toxicol. In Vitro 2012, 26, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, T.P.; Camara, C.A.; Silva, T.M.S.; Martins, R.M.; Pinto, A.C.; Vargas, M.D. New 1,2,3,4-tetrahydro-1-aza-anthraquinones and 2-aminoalkyl compounds from nor-lapachol with molluscicidal activity. Bioorg. Med. Chem. 2005, 13, 6464–6469. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.J.; de Almeida, Y.M.; Viana, J.R.; Filha, J.G.H.; Rodrigues, T.P.; Prata, J.R.C., Jr.; Coêlho, I.C.B.; Rao, V.S.; Pompeu, M.M.L. In vitro and in vivo Leishmanicidal activity of 2-hydroxy-3-(3-methyl-2-butenyl)-1,4-naphthoquinone (lapachol). Phytother. Res. 2001, 15, 44–48. [Google Scholar] [CrossRef]
- Ali, A.; Assimopoulou, A.N.; Papageorgiou, V.P.; Kolodziej, H. Structure/antileishmanial activity relationship study of naphthoquinones and dependency of the mode of action on the substitution patterns. Planta Med. 2011, 77, 2003–2012. [Google Scholar] [CrossRef] [PubMed]
- Gafner, S.; Wolfender, J.L.; Nianga, M.; Stoeckli-Evans, H.; Hostettmann, K. Antifungal and antibacterial naphthoquinones from Newbouldia laevis roots. Phytochemistry 1996, 42, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Antunes, R.M.P.; Lima, E.O.; Pereira, M.S.V.; Camara, C.A.; Arruda, T.A.; Catão, R.M.R.; Barbosa, T.P.; Nunes, X.P.; Dias, C.S.; Silva, T.M.S. Atividade antimicrobiana “in vitro” e determinação da concentração inibitória mínina (CIM) de fitoconstituintes e produtos sintéticos sobre bactérias e fungos leveduriformes. Braz. J. Pharmacogn. 2006, 16, 517–524. [Google Scholar]
- Da Silva Júnior, E.N.; de Melo, I.M.M.; Diogo, E.B.T.; Costa, V.A.; Filho, J.D.S.; Valença, W.O.; Camara, C.A.; de Oliveira, R.N.; Araújo, A.S.; Emery, F.S.; et al. On the search for potential anti-Trypanosoma cruzi drugs: synthesis and biological evaluation of 2-hydroxy-3-methylamino and 1,2,3-triazolic naphthoquinoidal compounds obtained by click chemistry reactions. Eur. J. Med. Chem. 2012, 52, 304–312. [Google Scholar]
- Stagliano, K.W.; Emadi, A.; Lu, Z.; Malinakova, H.C.; Twenter, B.; Yu, M.; Holland, L.E.; Rom, A.M.; Harwood, J.S.; Amin, R.; et al. Regiocontrolled synthesis and HIV inhibitory activity of unsymmetrical binaphthoquinone and trimeric naphthoquinone derivatives of conocurvone. Bioorg. Med. Chem. 2006, 14, 5651–5665. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, P.; Bastow, K.F. Novel mechanism of cellular DNA topoisomerase II inhibition by the pyranonaphthoquinone derivatives alpha-lapachone and beta-lapachone. Cancer Chemother. Pharmacol. 2001, 47, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.N.; Ferreira, V.F.; de Souza, M.C.B.V. Um panorama atual da química e da farmacologia de naftoquinonas, com ênfase nab-lapachona e derivados. Quim. Nova 2003, 26, 407–416. [Google Scholar] [CrossRef]
- Ferreira, S.B.; Gonzaga, D.T.G.; Santos, W.C.; Araújo, K.G.L.; Ferreira, V.F. β-Lapachona: Sua importância em química medicinal e modificações estruturais. Rev. Virtual Quim. 2010, 2, 140–160. [Google Scholar]
- Patil, R.S.; Patil, M.S.; Kshirsagar, S.S.; Chaudhari, P.S.; Bayas, J.P.; Oswal, R.J. Synthetic and natural products against leishmaniasis: A review. World J. Public Health Sci. 2012, 1, 7–22. [Google Scholar]
- Li, Y.; Du, B.; Xu, X.; Shi, D.; Ji, S. A green and efficient synthesis of 13-Aryl-5,7,12,14-tetrahydrodibenzo[b,i]xanthene-5,7,12,14(13H)-tetraone derivatives in ionic liquid. Chin. J. Chem. 2009, 27, 1563–1568. [Google Scholar] [CrossRef]
- Tisseh, Z.N.; Azimi, S.C.; Mirzaei, P.; Bazgir, A. The efficient synthesis of aryl-5H-dibenzo[b,i]xanthene-5,7,12,14(13H)-tetraone leuco-dye derivatives. Dyes Pigm. 2008, 79, 273–275. [Google Scholar] [CrossRef]
- Tisseh, Z.N.; Bazgir, A. An efficient, clean synthesis of 3,3'-(arylmethylene)bis(2-hydroxynaphthalene-1,4-dione) derivatives. Dyes Pigm. 2009, 83, 258–261. [Google Scholar] [CrossRef]
- Mazumder, A.; Wang, S.; Neamati, N.; Nicklaus, M.; Sunder, S.; Chen, J.; Milne, G.W.A.; Rice, W.G.; Burke, T.R., Jr.; Pommier, Y. Antiretroviral agents as inhibitors of both human immunodeficiency virus type 1 integrase and protease. J. Med. Chem. 1996, 39, 2472–2481. [Google Scholar] [CrossRef] [PubMed]
- OSIRIS Property Explorer. Available online: http://www.organic-chemistry.org/prog/peo/ (accessed on 14 April 2014).
- Sander, T.; Freyss, J.; von Korff, M.; Reich, J.R.; Rufener, C. OSIRIS, an entirely in-house developed drug discovery informatics system. J. Chem. Inf. Model. 2009, 49, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Moore, H. Bioactivation as a model for drug design bioreductive alkylation. Science 1977, 197, 527–532. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Leishmaniasis. Available online: http://www.who.int/leishmaniasis/en/ (accessed on 19 July 2013).
- Marr, A.K.; Mc Gwire, B.S.; Mc Master, W.R. Modes of action of Leishmanicidal antimicrobial peptides. Future Microbiol. 2012, 7, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Lima, N.M.F.; Correia, C.S.; Leon, L.L.; Machado, G.M.C.; Madeira, M.F.; Santana, A.E.G.; Goulart, M.O.F. Antileishmanial activity of lapachol analogues. Mem. Inst. Oswaldo Cruz 2004, 99, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Kayser, O.; Kiderlen, A.F.; Laatsch, H.; Croft, S.L. In vitro leishmanicidal activity of monomeric and dimeric naphthoquinones. Acta Trop. 2000, 77, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Fry, M.; Pudney, M. Site of action of the antimalarial hydroxynaphthoquinone, 2-[trans-4-(4'-chlorophenyl) cyclohexyl]-3-hydroxy-1,4-naphthoquinone (566C80). Biochem. Pharmacol. 1992, 43, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.E. Coenzyme Q homologs in parasitic protozoa as targets for chemotherapeutic attack. Parasitol. Today 1994, 10, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Croft, S.L.; Evans, A.T.; Neal, R.A. The activity of plumbagin and other electron carriers against Leishmania donovani and Leishmania mexicana amazonensis. Ann. Trop. Med. Parasitol. 1985, 79, 651–653. [Google Scholar] [PubMed]
- Croft, S.L.; Hogg, J.; Gutteridge, W.E.; Hudson, A.T.; Randall, A.W.J. The activity of hydroxynaphthoquinones against Leishmania donovani. Antimicrob. Chemother. 1992, 30, 827–832. [Google Scholar] [CrossRef]
- Sharma, G.; Chowdhury, S.; Sinha, S.; Majumder, H.K.; Kumar, V.J. Antileishmanial activity evaluation of bis-lawsone analogs and DNA topoisomerase-I inhibition studies. Enzym. Inhib. Med. Chem. 2004, 2, 185–189. [Google Scholar]
- Plyta, Z.; Li, T.; Papageorgiou, V.; Mellidis, A.; Assimopoulou, A.; Pitsinos, E.; Culadouros, E. Inhibition of topoisomerase I by naphthoquinone derivatives. Bioorg. Med. Chem. Lett. 1998, 8, 3385–3390. [Google Scholar] [CrossRef]
- Panichayupakaranant, P.; Noguchi, H.; De-Eknamkul, W.; Sankawa, U. Naphthoquinones and coumarins from Impatiens balsamina root cultures. Phytochemistry 1995, 40, 1141–1143. [Google Scholar] [CrossRef]
- Nagabhushana, K.S.; Ameer, F.; Green, I.R. Condensation products between caproaldehyde and 2-hydroxy-1,4-naphthoquinone. Syn. Commun. 2001, 31, 719–724. [Google Scholar] [CrossRef]
- Bock, K.; Jacobsen, N.; Terem, B. Reaction of 2-hydroxy-1,4-naphthoquinone with aldehydes. Synthesis of 2-hydroxy-3-(1-alkenyl)naphthoquinones. J. Chem. Soc. Perkin Trans. 1 1986, 1986, 659–664. [Google Scholar]
- Hussain, R.F.; Nouri, A.M.; Oliver, R.T. A new approach for measurement of cytotoxicity using colorimetric assay. J. Immunol. Methods 1993, 160, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Rangel, H.; Dagger, F.; Hernandez, A.; Liendo, A.; Urbina, J.A. Naturally azole-resistant Leishmania braziliensis promastigotes are rendered susceptible in the presence of terbinafine: comparative study with azole-susceptible Leishmania mexicana promastigotes. Antimicrob. Agents Chemother. 1996, 40, 2785–2791. [Google Scholar] [PubMed]
- Pereira, J.C.; Carregaro, V.; Costa, D.L.; da Silva, J.S.; Cunha, F.Q.; Franco, D.W. Antileishmanial activity of ruthenium(II)tetraammine nitrosyl complexes. Eur. J. Med. Chem. 2010, 45, 4180–4187. [Google Scholar] [CrossRef] [PubMed]
- Taswell, C. Limiting dilution assays for the separation, characterization and quantification of biologically active particles and their clonal progeny. In Cell Separation: Methods and Selected Applications; Pretlow, T.C., Pretlow, T.P., Eds.; Academic Press: New York, NY, USA, 1986; pp. 109–145. [Google Scholar]
- Sample Availability: Samples of the compounds 3a–h are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
De Araújo, M.V.; De Souza, P.S.O.; De Queiroz, A.C.; Da Matta, C.B.B.; Leite, A.B.; Da Silva, A.E.; De França, J.A.A.; Silva, T.M.S.; Camara, C.A.; Alexandre-Moreira, M.S. Synthesis, Leishmanicidal Activity and Theoretical Evaluations of a Series of Substituted bis-2-Hydroxy-1,4-Naphthoquinones. Molecules 2014, 19, 15180-15195. https://doi.org/10.3390/molecules190915180
De Araújo MV, De Souza PSO, De Queiroz AC, Da Matta CBB, Leite AB, Da Silva AE, De França JAA, Silva TMS, Camara CA, Alexandre-Moreira MS. Synthesis, Leishmanicidal Activity and Theoretical Evaluations of a Series of Substituted bis-2-Hydroxy-1,4-Naphthoquinones. Molecules. 2014; 19(9):15180-15195. https://doi.org/10.3390/molecules190915180
Chicago/Turabian StyleDe Araújo, Morgana V., Patricia S. O. De Souza, Aline C. De Queiroz, Carolina B. B. Da Matta, Anderson Brandão Leite, Amanda Evelyn Da Silva, José A. A. De França, Tania M. S. Silva, Celso A. Camara, and Magna S. Alexandre-Moreira. 2014. "Synthesis, Leishmanicidal Activity and Theoretical Evaluations of a Series of Substituted bis-2-Hydroxy-1,4-Naphthoquinones" Molecules 19, no. 9: 15180-15195. https://doi.org/10.3390/molecules190915180
APA StyleDe Araújo, M. V., De Souza, P. S. O., De Queiroz, A. C., Da Matta, C. B. B., Leite, A. B., Da Silva, A. E., De França, J. A. A., Silva, T. M. S., Camara, C. A., & Alexandre-Moreira, M. S. (2014). Synthesis, Leishmanicidal Activity and Theoretical Evaluations of a Series of Substituted bis-2-Hydroxy-1,4-Naphthoquinones. Molecules, 19(9), 15180-15195. https://doi.org/10.3390/molecules190915180





