Pharmacological Effects of “Jutsu” (Atractylodis rhizome and Atractylodis lanceae rhizome) on 1-(2,5-Dimethoxy-4-iodophenyl)-2-aminopropane (DOI)-Induced Head Twitch Response in Mice (I)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of Byaku-Jutsu and So-jutsu Extracts on DOI-Induced HTR in Mice
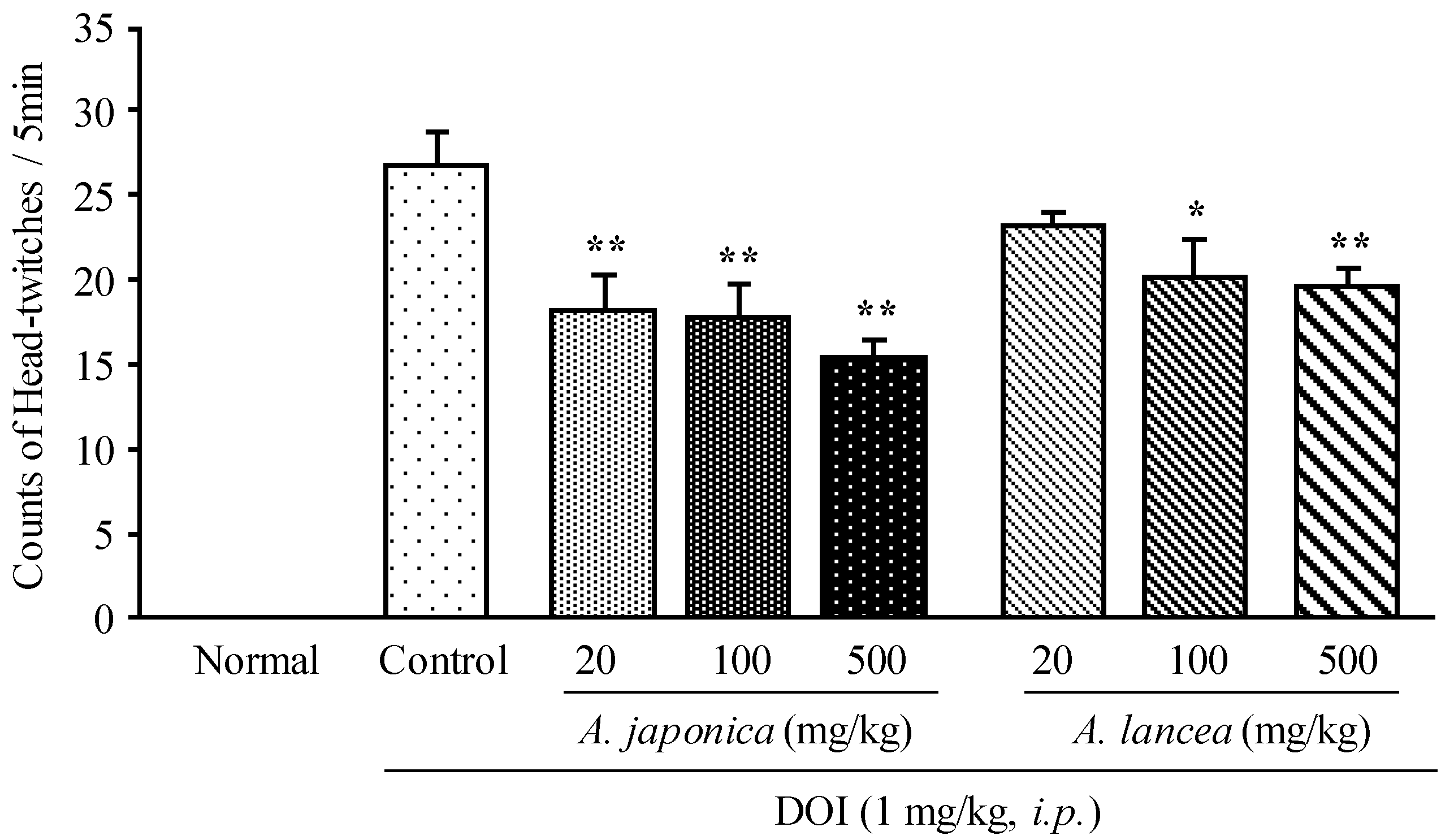
2.2. Effects of Atractylenolide III and β-Eudesmol on DOI-Induced HTR in Mice
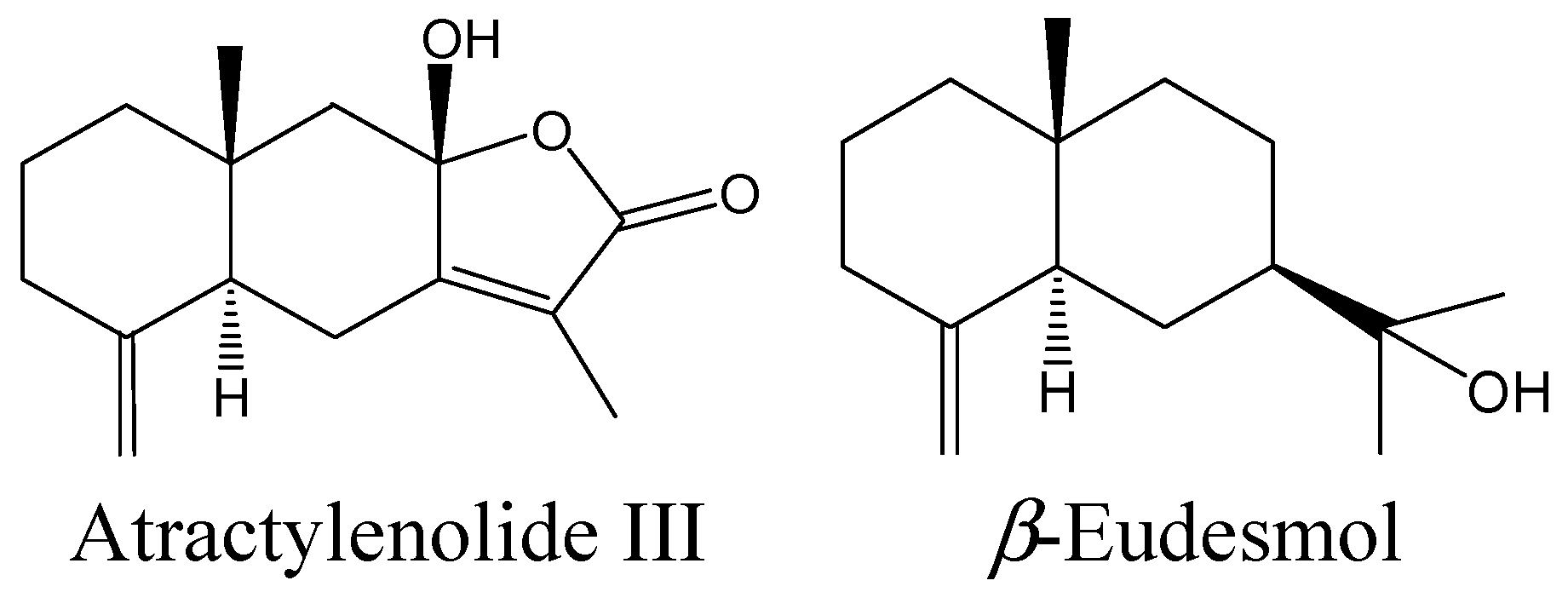
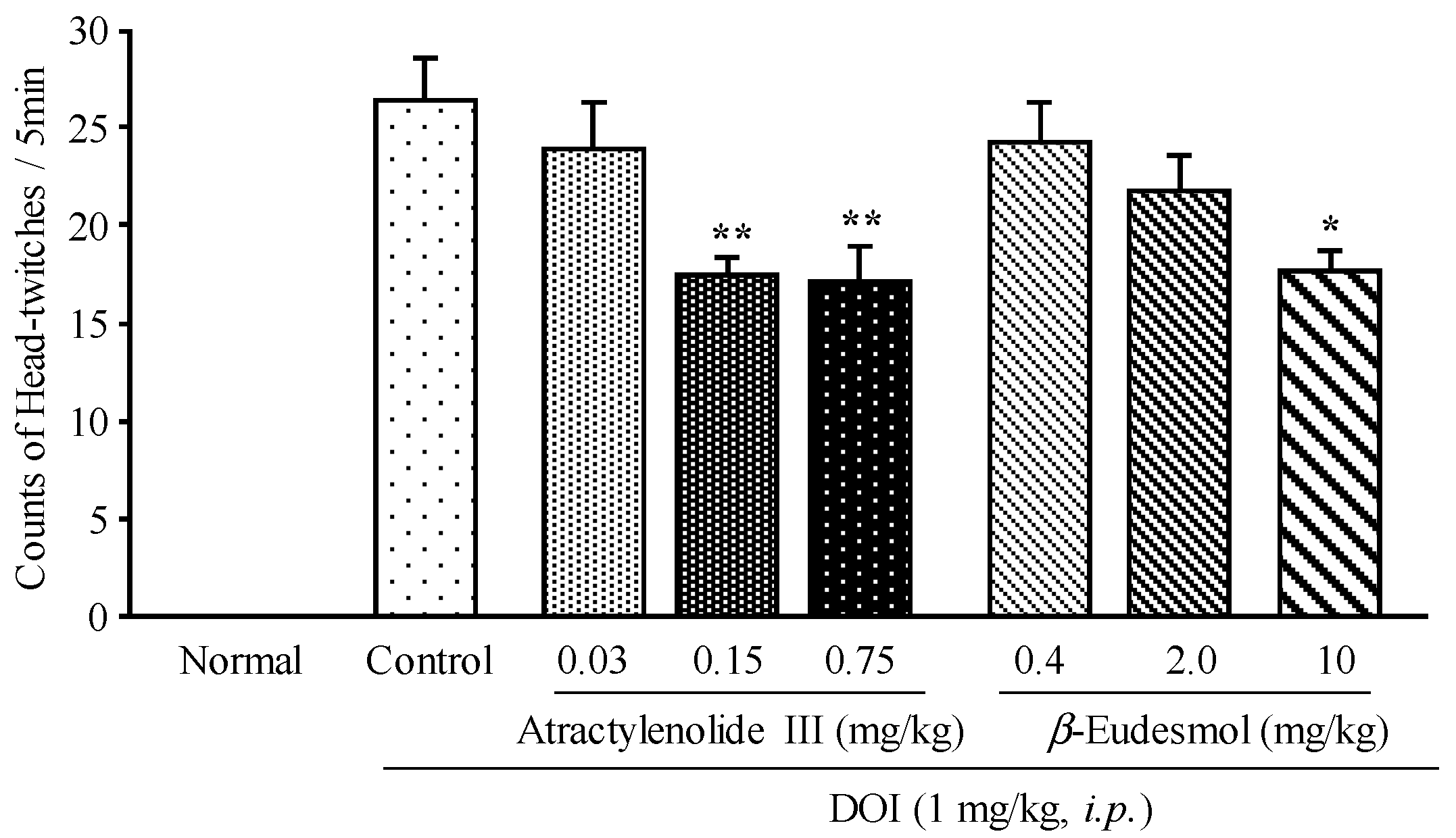
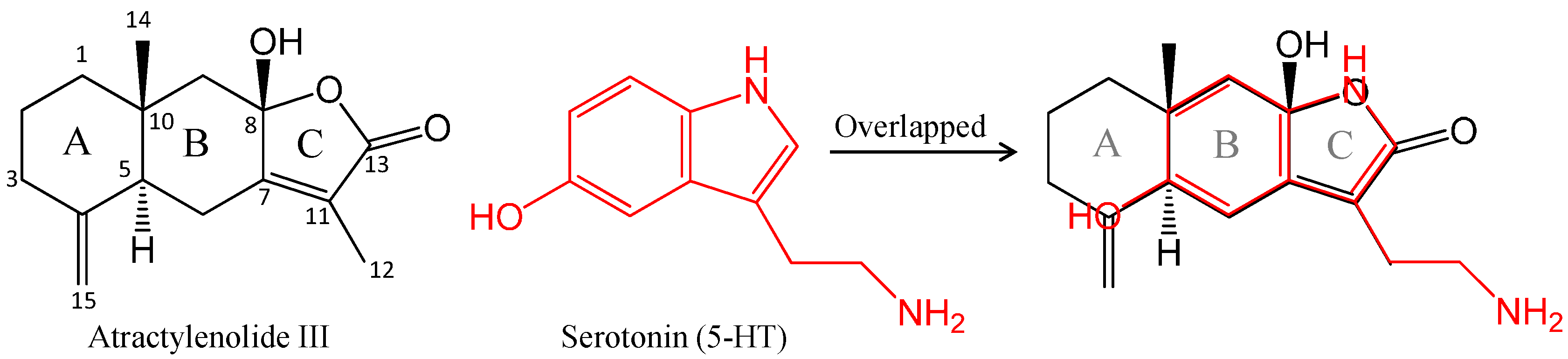
3. Experimental Section
3.1. Experimental Animals
3.2. Chemicals and Extract Preparation of Byaku-Jutsu and So-Jutsu
3.3. DOI-Induced Head Twitch Response Experiments
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Onofrj, M.; Taylor, J.P.; Monaco, D.; Franciotti, R.; Anzellotti, F.; Bonanni, L.; Onofrj, V.; Thomas, A. Visual hallucinations in PD and Lewy body dmentias: Old and new hypotheses. Behav. Neurol. 2013, 27, 479–493. [Google Scholar]
- Bertram, G.K. Drugs of Abuse. Basic & Clinical Pharmacology, 9th ed.; Bernstein, J., Ransom, J., Foltin, J., Holton, B., Eds.; The McGraw-Hill Companies, Inc.: New York, NY, USA, 2004; pp. 523–525. [Google Scholar]
- Kawanabe, T.; Yoritaka, A.; Shimura, H.; Oizumi, H.; Tanaka, S.; Hattori, N. Successful treatment with Yokukansan for behavioral and psychological symptoms of Parkinsonian dementia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 284–287. [Google Scholar]
- Iwasaki, K.; Kosaka, K.; Mori, H.; Okitsu, R.; Furukawa, K.; Manabe, Y.; Yoshita, M.; Kanamori, A.; Ito, N.; Wada, K.; et al. Improvement in delusions and hallucinations in patients with dementia with Lewy bodies upon administration of yokukansan, a traditional Japanese medicine. Psychogeriatrics 2012, 12, 235–241. [Google Scholar]
- Magome, A. Effect of Yokukansankachimpihange on dementia–Including the point of view of oriental medicine. Psychiatry 2011, 18, 108–114. (In Japanese) [Google Scholar]
- Miyazawa, J. Study of the clinical efficacy of Yokukansankachimpihange on Alzheimer’s disease. Psychiatry 2009, 14, 535–542. (In Japanese) [Google Scholar]
- Corne, S.J.; Pickering, R.W. A possible correlation between drug-induced hallucinations in man and a behavioural response in mice. Psychopharmacologia 1967, 11, 65–78. [Google Scholar]
- Egashira, N.; Iwasaki, K.; Ishibashi, A.; Hayakawa, K.; Okuno, R.; Abe, M.; Uchida, N.; Mishima, K.; Takasaki, K.; Nishimura, R.; et al. Repeated administration of Yokukansan inhibits DOI-induced head-twitch response and decreases expression of 5-hydroxytryptamine (5-HT)2A receptors in the prefrontal cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1516–1520. [Google Scholar]
- Murayama, C.; Michihara, S.; Okubo, T.; Norimoto, H. Effect of Yokukansankachinpihange on DOI-induced Head-twitch Response in Mice. In Proceedings of the 134th Annual Meeting of the Pharmaceutical Society of Japan, Kumamoto, Japan, 28–30 March 2014.
- Japanese Pharmacopoeia Editorial Committee. The Japanese Pharmacopoeia, 16th ed.; The Ministry of Health, Labor and Welfare: Tokyo, Japan, 2006; pp. 1604–1605. [Google Scholar]
- Hikino, H.; Hikino, Y.; Yoshioka, I. Studies on the constituents of Atractylodes. IX. Structure and Autoxidation of Atractylon. Chem. Pharm. Bull. 1964, 12, 755–760. [Google Scholar]
- Wang, K.T.; Chen, L.G.; Yang, L.L.; Ke, W.M.; Chang, H.C.; Wang, C.C. Analysis of the sesquiterpenoids in processed Atractylodis Rhizoma. Chem. Pharm. Bull. 2007, 55, 50–56. [Google Scholar]
- Darmani, N.A.; Shaddy, J.; Gerdes, C.F. Differential ontogenesis of the DOI-induced behavior in mice. Physiol. Behav. 1996, 60, 1495–1500. [Google Scholar]
- Feng, X.; Wang, Z.L.; Lin, Y.C.; Zhou, Y.; Liu, Y.Z.; Yang, H.Z. Effects of biatractylolide on the AD rats induced by Aβ1–40. Chin. Pharmacol. Bull. 2009, 25, 951–954. [Google Scholar]
- Liu, Y.; Liao, C.M. Effects of bitatractylolide on the AD rats Induced by ALCl3. J. Hunan Norm. Univ. (Med. Sci.) 2006, 3, 25–26. [Google Scholar]
- Barnes, N.M.; Sharp, T. A review of central 5-HT receptors and their function. Neuropharmacology 1999, 38, 1083–1152. [Google Scholar]
- Kobayashi, Y.; Bhatt, I.D. Antidpressant effect of the Atactylodes japonica essential oil in mice. Aroma Res. 2005, 6, 356–361. (In Japanese) [Google Scholar]
- Nichols, D.E. Strucrure-activity relationships of serotonin 5-HT2A agonists. WIREs Membr. Transp. Signal. 2012, 1, 559–579. [Google Scholar]
- Sample Availability: Samples of the compounds of atractylenolide III and β-eudesmol are not available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Murayama, C.; Wang, C.-C.; Michihara, S.; Norimoto, H. Pharmacological Effects of “Jutsu” (Atractylodis rhizome and Atractylodis lanceae rhizome) on 1-(2,5-Dimethoxy-4-iodophenyl)-2-aminopropane (DOI)-Induced Head Twitch Response in Mice (I). Molecules 2014, 19, 14979-14986. https://doi.org/10.3390/molecules190914979
Murayama C, Wang C-C, Michihara S, Norimoto H. Pharmacological Effects of “Jutsu” (Atractylodis rhizome and Atractylodis lanceae rhizome) on 1-(2,5-Dimethoxy-4-iodophenyl)-2-aminopropane (DOI)-Induced Head Twitch Response in Mice (I). Molecules. 2014; 19(9):14979-14986. https://doi.org/10.3390/molecules190914979
Chicago/Turabian StyleMurayama, Chiaki, Ching-Chiung Wang, Seiwa Michihara, and Hisayoshi Norimoto. 2014. "Pharmacological Effects of “Jutsu” (Atractylodis rhizome and Atractylodis lanceae rhizome) on 1-(2,5-Dimethoxy-4-iodophenyl)-2-aminopropane (DOI)-Induced Head Twitch Response in Mice (I)" Molecules 19, no. 9: 14979-14986. https://doi.org/10.3390/molecules190914979



