Enantiomerically Pure Phosphonated Carbocyclic 2'-Oxa-3'-Azanucleosides: Synthesis and Biological Evaluation
Abstract
:1. Introduction
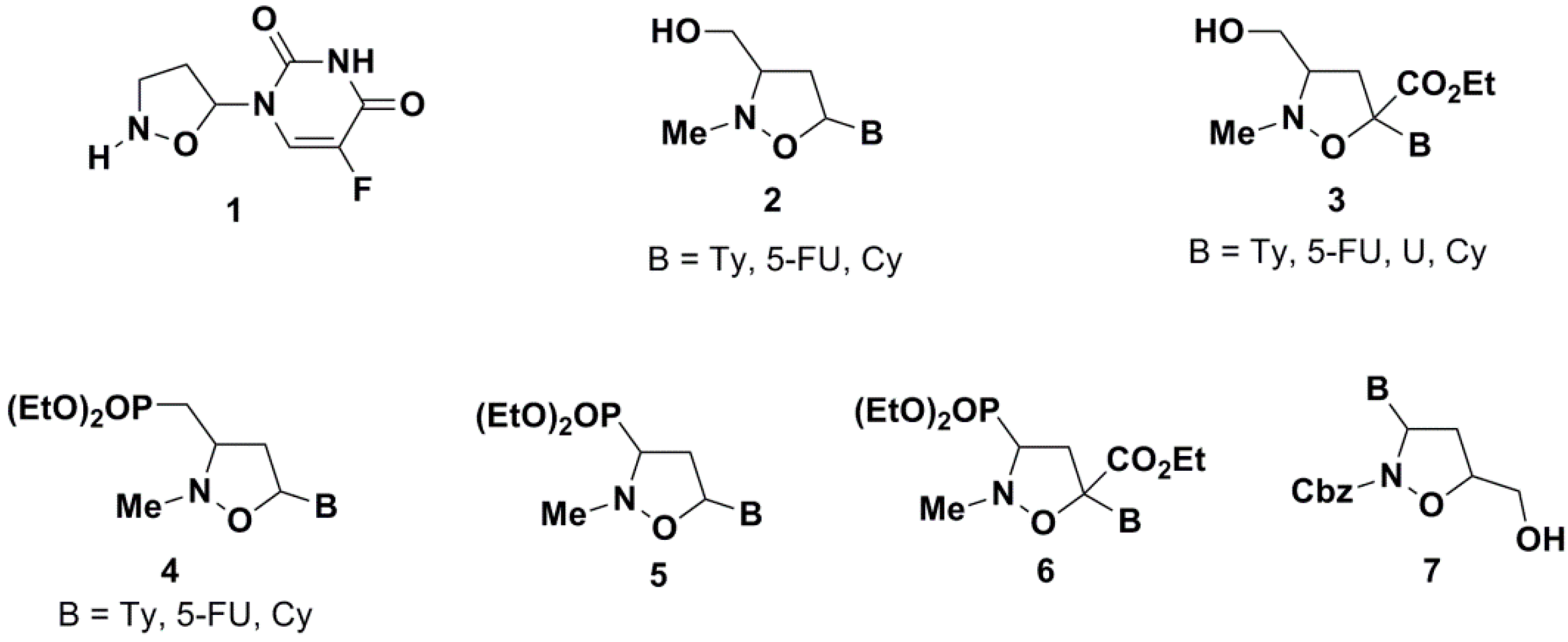
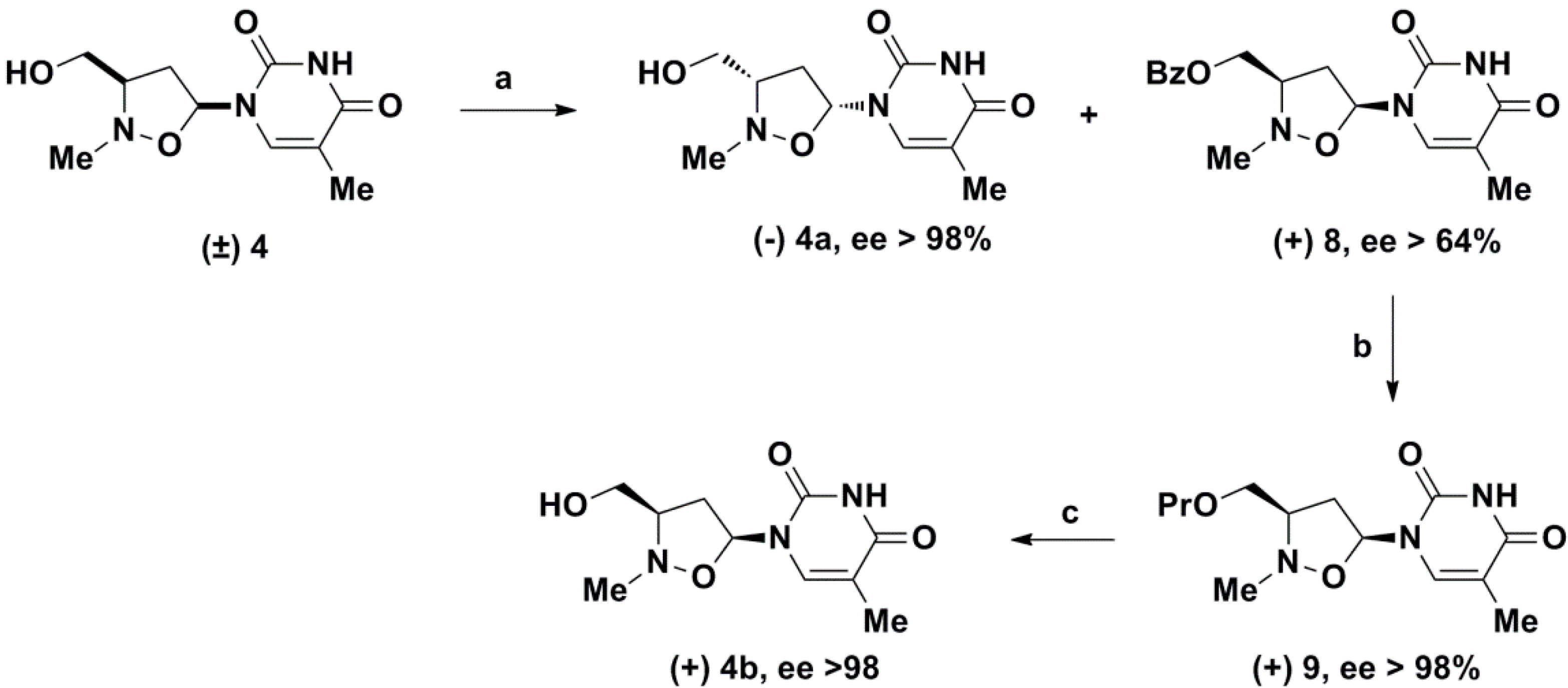
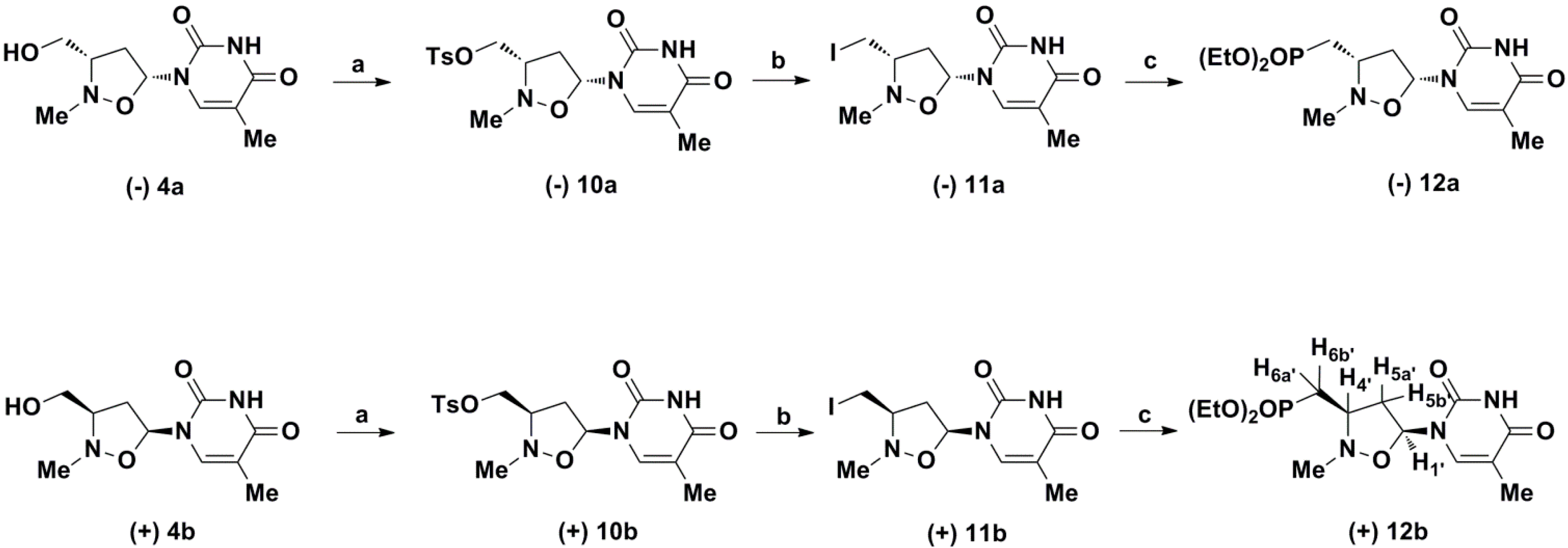
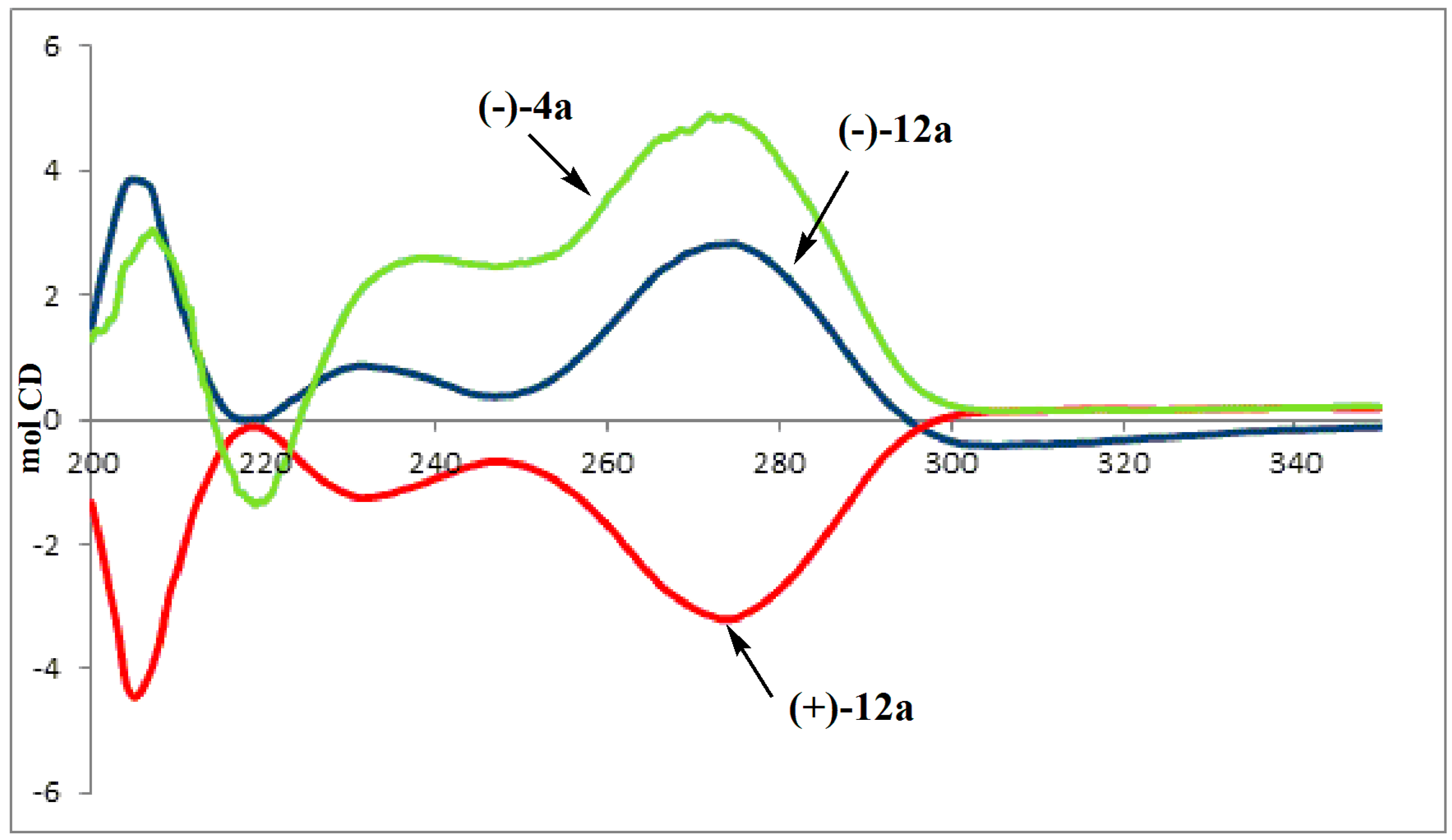
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. Synthesis of ((3S,5R)-5-(3,4-Dihydro-5-methyl-2,4-dioxopyrimidin-1(2H)-yl)-2-methylisoxa-zolidin- 3-yl)methyl 4-methylbenzenesulphonate (−);)10a
 = −84.9 (c 0.25, MeOH). 1H-NMR (CDCl3): δ = 1.77 (s, 3H); 1.93–2.04 (m, 1H), 2.32 (s, 3H); 2.63 (s, 3H); 2.77–2.91 (m, 2H); 3.89–4.07 (m, 2H), 5.94–6.02 (m, 1H), 7.23 (d, J = 8.2 Hz, 2H), 7.46 (s, 1H); 7.63 (d, J = 8.2 Hz, 2H), 9.37 (bs, 1H). 13C-NMR (CDCl3): δ = 8.54, 17.48, 36.53, 40.26, 61.81, 63.64, 89.98, 106.58, 123.67, 125.90, 131.73, 138.66, 144.09, 151.95, 160.09. HRMS: calcd for C17H21N3O6SNa+ 418.1043, found 418.1050.
= −84.9 (c 0.25, MeOH). 1H-NMR (CDCl3): δ = 1.77 (s, 3H); 1.93–2.04 (m, 1H), 2.32 (s, 3H); 2.63 (s, 3H); 2.77–2.91 (m, 2H); 3.89–4.07 (m, 2H), 5.94–6.02 (m, 1H), 7.23 (d, J = 8.2 Hz, 2H), 7.46 (s, 1H); 7.63 (d, J = 8.2 Hz, 2H), 9.37 (bs, 1H). 13C-NMR (CDCl3): δ = 8.54, 17.48, 36.53, 40.26, 61.81, 63.64, 89.98, 106.58, 123.67, 125.90, 131.73, 138.66, 144.09, 151.95, 160.09. HRMS: calcd for C17H21N3O6SNa+ 418.1043, found 418.1050.3.3. Synthesis of (3R,5S)-1-(3-(Iodomethyl)-2-methylisoxazolidin-5-yl)-5-methylpyrimidin-2,4-(1H,3H) dione (−)11a
 = −88.50 (c 0.5, MeOH); 1H-NMR (CDCl3): δ = 1.96 (s, 3H), 2.20 (ddd, J = 13.7, 9.2, 4.1 Hz, 1H), 2.51–2.64 (m, 1H), 2.70 (s, 3H), 3.06 (m, 1H), 3.11 (dd, J = 10.7, 4.1Hz, 1H), 3.25 (dd, J = 10.7, 3.2 Hz, 1H), 6.16 (dd, J = 7.8, 4.1 Hz, 1H), 7.76 (s, 1H), 8.54 (bs, 1H). 13C-NMR (CDCl3): δ = 8.44; 25.45, 38.99, 41.18, 63.28, 77.44, 110.20, 132.30, 150.20, 163.4. HRMS: calcd for C10H14N3O3INa+ 373.9972, found 373.9981
= −88.50 (c 0.5, MeOH); 1H-NMR (CDCl3): δ = 1.96 (s, 3H), 2.20 (ddd, J = 13.7, 9.2, 4.1 Hz, 1H), 2.51–2.64 (m, 1H), 2.70 (s, 3H), 3.06 (m, 1H), 3.11 (dd, J = 10.7, 4.1Hz, 1H), 3.25 (dd, J = 10.7, 3.2 Hz, 1H), 6.16 (dd, J = 7.8, 4.1 Hz, 1H), 7.76 (s, 1H), 8.54 (bs, 1H). 13C-NMR (CDCl3): δ = 8.44; 25.45, 38.99, 41.18, 63.28, 77.44, 110.20, 132.30, 150.20, 163.4. HRMS: calcd for C10H14N3O3INa+ 373.9972, found 373.99813.4. Synthesis of Diethyl{(1'R,4'S)-1'-[[(5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl]-3'-methyl-2'-oxa-3'-aza-cyclopent-4'-yl]}methylphosphonate (−)12a
 = −83.4 (c 0.50, MeOH); 1H-NMR (CDCl3): δ= 1.30 (dt, 6H, J = 3.6 and 7.1 Hz), 1.89 (ddd, 1H, J = 10.2, 15.0 and 18.3 Hz, H5'a), 1.93 (d, 3H, J = 1.3Hz), 2.08 (ddd, 1H, J = 3.2, 15.0 and 20.6 Hz, H5'b), 2.23 (ddd, 1H,J = 4.6, 10.1 and 13.8 Hz, H6'a), 2.75 (s, 3H, N-CH3), 2.98 (dddd, 1H, J = 3.2, 7.0, 10.1 and 10.2 Hz, H4'), 3.18 (ddd, 1H, J = 7.0, 7.9 and 13.8 Hz, , H6'b), 4.10–4.17 (m, 4H), 6.20 (dd, 1H, J = 4.6 and 7.9 Hz, H1'), 7.66 (q, 1H, J = 1.3 Hz, H6), 9.56 (bs, 1H, NH); 13C-NMR (CDCl3); δ: 12.57, 16.33, 16.38, 27.88 (d, J = 143.2 Hz), 42.68, 44.35, 61.93, 61.98, 63.37, 81.94, 110.66, 135.96, 150.56, 164.11 CD: λext 200 (Δε +1.49), 206 (Δε +3.82), 219 (Δε −0.11), 233(Δε +0.84), 249 (Δε +0.38), 275 (Δε +2.82), 290 (Δε +0.67), 307 (Δε −0.42), 360 (Δε −0.08).
= −83.4 (c 0.50, MeOH); 1H-NMR (CDCl3): δ= 1.30 (dt, 6H, J = 3.6 and 7.1 Hz), 1.89 (ddd, 1H, J = 10.2, 15.0 and 18.3 Hz, H5'a), 1.93 (d, 3H, J = 1.3Hz), 2.08 (ddd, 1H, J = 3.2, 15.0 and 20.6 Hz, H5'b), 2.23 (ddd, 1H,J = 4.6, 10.1 and 13.8 Hz, H6'a), 2.75 (s, 3H, N-CH3), 2.98 (dddd, 1H, J = 3.2, 7.0, 10.1 and 10.2 Hz, H4'), 3.18 (ddd, 1H, J = 7.0, 7.9 and 13.8 Hz, , H6'b), 4.10–4.17 (m, 4H), 6.20 (dd, 1H, J = 4.6 and 7.9 Hz, H1'), 7.66 (q, 1H, J = 1.3 Hz, H6), 9.56 (bs, 1H, NH); 13C-NMR (CDCl3); δ: 12.57, 16.33, 16.38, 27.88 (d, J = 143.2 Hz), 42.68, 44.35, 61.93, 61.98, 63.37, 81.94, 110.66, 135.96, 150.56, 164.11 CD: λext 200 (Δε +1.49), 206 (Δε +3.82), 219 (Δε −0.11), 233(Δε +0.84), 249 (Δε +0.38), 275 (Δε +2.82), 290 (Δε +0.67), 307 (Δε −0.42), 360 (Δε −0.08).3.5. Biological Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, X.; Zhan, P.; de Clercq, E.; Liu, X. The HIV-1 non-nucleoside reverse transcriptase inhibitors (Part 5): Capravirine and its analogues. Curr. Med. Chem. 2012, 19, 6138–6149. [Google Scholar] [CrossRef]
- Saag, M.S. New and investigational antiretroviral drugs for HIV infection: Mechanisms of action and early research findings. Top. Antivir. Med. 2012, 20, 162–167. [Google Scholar]
- De Clercq, E. Highlights in the discovery of antiviral drugs: A personal retrospective. J. Med. Chem. 2010, 53, 1438–1450. [Google Scholar] [CrossRef]
- Mehellou, Y.; de Clercq, E. Twenty-six years of anti-HIV drug discovery: Where do we stand and where do we go. J. Med. Chem. 2010, 53, 521–538. [Google Scholar] [CrossRef]
- Stambasky, J.; Hocek, M.; Kocovsky, P. C-nucleosides: Synthetic strategies and biological applications. Chem. Rev. 2009, 109, 6729–6764. [Google Scholar] [CrossRef]
- Galmarini, C.M.; Popowycz, F.; Joseph, B. Cytotoxic nucleoside analogues: Different strategies to improve their clinical efficacy. Curr. Med. Chem. 2008, 15, 1072–1082. [Google Scholar] [CrossRef]
- Kool, E.T. Modified DNA bases: Probing base-pair recognition by polymerases. Modified nucleosides. In Biochemistry,Biotechnology and Medicine; Herdewijn, P., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008. [Google Scholar]
- Balestrieri, E.; Matteucci, C.; Ascolani, A.; Piperno, A.; Romeo, R.; Romeo, G.; Chiacchio, U.; Mastino, A.; Macchi, B. Effect of phosphonated carbocyclic 2'-oxa-3'-aza-nucleoside on human T-cell leukemia virus type 1 infection in vitro. Antimicrob. Agents Chemother. 2008, 52, 54–64. [Google Scholar] [CrossRef] [Green Version]
- De Clercq, E. New approaches toward anti-HIV chemotherapy. J. Med. Chem. 2005, 48, 1297–1313. [Google Scholar] [CrossRef]
- Sari, O.; Roy, V.; Agrofoglio, L.A. Nucleosides modified at the base moiety. In Chemical Synthesis of Nucleoside Analogues; 1st ed.; Merino, P., Ed.; Wiley-VCH: Hoboken, NJ, USA, 2013; p. 49. [Google Scholar]
- Romeo, R.; Carnovale, C.; Rescifina, A.; Chiacchio, M.A. Phosphonated nucleoside analogues. In Chemical Synthesis of Nucleoside Analogues; 1st ed.; Merino, P., Ed.; Wiley-VCH: Hoboken, NJ, USA, 2013; p. 163. [Google Scholar]
- Chiacchio, U.; Corsaro, A.; Giofrè, S.V.; Romeo, G. Isoxazolidinyl nucleosides. In Chemical Synthesis of Nucleoside Analogues; 1st ed.; Merino, P., Ed.; Wiley-VCH: Hoboken, NJ, USA, 2013; p. 781. [Google Scholar]
- Peyrottes, S.; Perigaud, C. Mononucleotide prodrug synthetic strategies. In Chemical Synthesis of Nucleoside Analogues; 1st ed.; Merino, P., Ed.; Wiley-VCH: Hoboken, NJ, USA, 2013; p. 229. [Google Scholar]
- Mitsuya, H.; Weinhold, K.J.; Furman, P.A.; St. Clair, M.H.; Lehrman, S.N.; Gallo, R.C.; Bolognesi, D.; Barry, D.W.; Broder, S. 3'-Azido-3'-deoxythymidine (BW A509U): An antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc. Natl. Acad. Sci. USA 1985, 82, 7096–7100. [Google Scholar] [CrossRef]
- Mitsuya, H.; Broder, S. Inhibition of the in vitro infectivity and cytopathic effect of human T-lymphotrophic virus type III/lymphadenopathy-associated virus (HTLV-III/LAV) by 2',3'-dideoxynucleosides. Proc. Natl. Acad. Sci. USA 1986, 83, 1911–1915. [Google Scholar] [CrossRef]
- Romeo, G.; Chiacchio, U.; Corsaro, A.; Merino, P. Chemical synthesis of heterocyclic-sugar nucleoside analogues. Chem. Rev. 2010, 110, 3337–3370. [Google Scholar] [CrossRef]
- Chiacchio, U.; Padwa, A.; Romeo, G. Cycloaddition methodology: A useful entry towards biologically active heterocycle. Curr. Org. Chem. 2009, 13, 422–443. [Google Scholar] [CrossRef]
- Piperno, A.; Chiacchio, M.A.; Iannazzo, D.; Romeo, R. Synthesis and biological activity of phosphonated nucleosides: Part 1. Furanose, carbocyclic and heterocyclic analogues. Curr. Med. Chem. 2006, 13, 3675–3695. [Google Scholar] [CrossRef]
- Merino, P.; Tejero, T.; Unzurrunzaga, F.J.; Franco, S.; Chiacchio, U.; Saita, M.G.; Iannazzo, D.; Piperno, A.; Romeo, G. An efficient approach to enantiomeric isoxazolidinyl analogues of tiazofurin based on nitrone cycloadditions. Tetrahedron Asymmetry 2005, 16, 3865–3876. [Google Scholar] [CrossRef]
- Chiacchio, U.; Genovese, F.; Iannazzo, D.; Librando, V.; Merino, P.; Rescifina, A.; Romeo, R.; Procopio, A.; Romeo, G. Diastereoselective synthesis of homo-N,O-nucleosides. Tetrahedron 2004, 60, 441–448. [Google Scholar] [CrossRef]
- Chiacchio, U.; Corsaro, A.; Iannazzo, D.; Piperno, A.; Pistarà, V.; Rescifina, A.; Romeo, R.; Sindona, G.; Romeo, G. Diastereo- and enantioselective synthesis of N,O-nucleosides. Tetrahedron Asymmetry 2003, 14, 2717–2723. [Google Scholar] [CrossRef]
- Chiacchio, U.; Corsaro, A.; Mates, J.; Merino, P.; Piperno, A.; Rescifina, A.; Romeo, G.; Tejero, T. Isoxazolidine analoguese of pseudouridine: A new class of modified nucleosides. Tetrahedron 2003, 59, 4733–4738. [Google Scholar] [CrossRef]
- Chiacchio, U.; Corsaro, A.; Pistarà, V.; Rescifina, A.; Iannazzo, D.; Piperno, A.; Romeo, G.; Romeo, R.; Grassi, G. Diastereoselective synthesis of N,O-psiconucleosides, a new class of modified nucleosides. Eur. J. Org. 2002, 7, 1206–1212. [Google Scholar]
- Chiacchio, U.; Corsaro, A.; Iannazzo, D.; Piperno, A.; Procopio, A.; Rescifina, A.; Romeo, G.; Romeo, R. A stereoselective approach to isoxazolidinyl nucleosides. Eur. J. Org. Chem. 2001, 10, 1893–1898. [Google Scholar]
- Chiacchio, U.; Gumina, G.; Rescifina, A.; Romeo, R.; Uccella, N.; Casuscelli, F.; Piperno, A.; Romeo, G. Modified dideoxynucleosides: Synthesis of 2'-N-alkyl-3'-hydroxyalkyl-1',2'-isoxazolidinyl thymidine and 5-fluorouridine derivatives. Tetrahedron 1996, 52, 8889–8898. [Google Scholar] [CrossRef]
- Romeo, R.; Giofrè, S.V.; Macchi, B.; Balestrieri, E.; Mastino, A.; Merino, P.; Carnovale, C.; Romeo, G.; Chiacchio, U. Truncated reverse isoxazolidinyl nucleosides: A new class of allosteric HIV-1 reverse transcriptase inhibitors. ChemMedChem 2012, 7, 565–569. [Google Scholar] [CrossRef]
- Romeo, R.; Carnovale, C.; Giofrè, S.V.; Romeo, G.; Macchi, B.; Frezza, C.; Marino-Merlo, F.; Pistarà, V.; Chiacchio, U. Truncated phosphonated C-1'-branched N,O-nucleosides: A new class of antiviral agents. Bioorg. Med. Chem. 2012, 20, 3652–3657. [Google Scholar] [CrossRef] [Green Version]
- Piperno, A.; Giofrè, S.V.; Iannazzo, D.; Romeo, R.; Romeo, G.; Chiacchio, U.; Rescifina, A.; Piotrowska, D.G. Synthesis of C-4'-truncated phosphonated carbocyclic 2'-oxa-3'-azanucleosides as antiviral agents. J. Org. Chem. 2010, 75, 2798–2805. [Google Scholar] [CrossRef]
- Chiacchio, U.; Rescifina, A.; Iannazzo, D.; Piperno, A.; Romeo, R.; Borrello, L.; Sciortino, M.T.; Balestrieri, E.; Macchi, B.; Mastino, A.; et al. Phosphonated carbocyclic 2'-Oxa-3'-azanucleosides as new antiretroviral agents. J. Med. Chem. 2007, 50, 3747–3750. [Google Scholar] [CrossRef] [Green Version]
- Chiacchio, U.; Iannazzo, D.; Piperno, A.; Romeo, R.; Romeo, G.; Rescifina, A.; Saglimbeni, M. Synthesis and biological evaluation of phosphonated carbocyclic 2'-oxa-3'-aza-nucleosides. Bioorg. Med. Chem. 2006, 14, 955–959. [Google Scholar] [CrossRef]
- Chiacchio, U.; Balestrieri, E.; Macchi, B.; Iannazzo, D.; Piperno, A.; Rescifina, A.; Romeo, R.; Saglimbeni, M.; Sciortino, M.T.; Valveri, V.; et al. Synthesis of phosphonated carbocyclic 2'-Oxa-3'-aza-nucleosides: Novel inhibitors of reverse transcriptase. J. Med. Chem. 2005, 48, 1389–1394. [Google Scholar] [CrossRef] [Green Version]
- Chiacchio, U.; Corsaro, A.; Iannazzo, D.; Piperno, A.; Pistarà, V.; Rescifina, A.; Romeo, R.; Valveri, V.; Mastino, A.; Romeo, G. Enantioselective syntheses and cytotoxicity of N,O-nucleosides. J. Med. Chem. 2003, 46, 3696–3702. [Google Scholar] [CrossRef]
- Hecker, S.J.; Erion, M.D. Prodrugs of phosphates and phosphonates. J. Med.Chem. 2008, 51, 2328–2345. [Google Scholar] [CrossRef]
- McGuigan, C.; Derudes, M.; Bugert, J.J.; Andrei, G.; Snoecke, R.; Balzarini, J. Successful kinase bypass with new acyclovir phosphoramidate prodrugs. Bioorg. Med. Chem. Lett. 2008, 18, 4364–4367. [Google Scholar] [CrossRef]
- He, G.-X.; Krise, J.P.; Oliyai, R. Prodrugs: Challenges and Rewards; Springer: New York, NY, USA, 2007; pp. 223–264. [Google Scholar]
- Perrone, P.; Luoni, G.M.; Kelleher, M.R.; Daverio, F.; Angell, A.; Mulready, S.; Congiatu, C.; Rajyageru, S.; Martin, J.A.; Leveque, V.; et al. Application of the phosphoramidate protide approach to 4'-azidouridine confers sub-micromolar potency versus hepatitis C virus on an inactive nucleoside. J. Med. Chem. 2007, 50, 1840–1849. [Google Scholar] [CrossRef]
- Ariza, M.E. Current prodrug strategies for the delivery of nucleotides into cells. Drug Del. Rev. 2005, 2, 273–387. [Google Scholar]
- Congiatu, C.; McGuigan, C.; Jiang, W.G.; Davies, G.; Mason, M.D. Naphthyl phosphoramidate derivatives of BVdU as potential anticancer agents: Design, synthesis and biological evaluation. Nucleosides Nucleotides Nucleic Acids 2005, 24, 485–489. [Google Scholar] [CrossRef]
- McGuigan, C.; Harris, S.A.; Daluge, S.M.; Gudmundsson, K.S.; McLean, E.W.; Burnette, T.C.; Marr, H.; Hazen, R.; Condreay, L.D.; Johnson, L.; et al. Application of phosphoramidate pronucleotide technology to abacavir leads to a significant enhancement of antiviral potency. J. Med. Chem. 2005, 48, 3504–3515. [Google Scholar] [CrossRef]
- Mackman, R.L.; Cihlar, T. Prodrug strategies in the design of nucleoside and nucleotide antiviral therapeutics. Annu. Rep. Med. Chem. 2004, 39, 305–321. [Google Scholar] [CrossRef]
- Schultz, C. Prodrugs of biologically active phosphate esters. Bioorg. Med. Chem. 2003, 11, 885–898. [Google Scholar] [CrossRef]
- De Clercq, E. The clinical potential of the acyclic (and cyclic) nucleoside phosphonates.The magic of the phosphonate bond. Biochem. Pharmacol. 2011, 82, 99–109. [Google Scholar] [CrossRef]
- De Clercq, E.; Holy, A. Acyclic nucleoside phosphonates: A key class of antiviral drugs. Nat. Res. Drug Discov. 2005, 4, 928–940. [Google Scholar] [CrossRef]
- Deville-Bonne, D.; El Amri, C.; Meyer, P.; Chen, Y.X.; Agrofoglio, L.A. Human and viral nucleoside/nucleotide kinases involved in antiviral drug activation: Structural and catalytic properties. Antivir. Res. 2010, 86, 101–120. [Google Scholar] [CrossRef]
- De Clercq, E. In search of a selective therapy of viral infections. Antivir. Res. 2010, 85, 19–24. [Google Scholar] [CrossRef]
- De Clercq, E. Antiviral drug discovery: Ten more compounds, and ten more stories (part B). Med. Res. Rev. 2009, 29, 571–610. [Google Scholar] [CrossRef]
- Gallier, F.; Alexandre, J.A.C.; El Amri, C.; Deville-Bonne, D.; Peyrotts, S.; Perigaud, C. 5',6'-Nucleoside phosphonate analogues architecture: Synthesis and comparative evaluation towards metabolic enzymes. ChemMedChem. 2011, 6, 1094–1106. [Google Scholar] [CrossRef]
- Meurillon, M.; Gallier, F.; Peyrottes, S.; Périgaud, C. Developing an efficient route to the synthesis of nucleoside1-alkynylphosphonates. Tetrahedron 2009, 65, 6039–6046. [Google Scholar] [CrossRef]
- Gallier, F.; Peyrottes, S.; Perigaud, C. Ex-chiral-pool synthesis of β-hydroxyphosphonate nucleoside analogues. Eur. J. Org. Chem. 2007, 925–933. [Google Scholar] [CrossRef]
- Chiacchio, U.; Corsaro, A.; Iannazzo, D.; Piperno, A.; Procopio, A.; Rescifina, A.; Romeo, G.; Romeo, R. Intramolecular cycloadditions of α-allyloxycarbonylnitrones: Stereoselective synthesis of 3-amino-2(5H)furanones. J. Org. Chem. 2002, 67, 4380–4383. [Google Scholar] [CrossRef]
- Chiacchio, U.; Corsaro, A.; Pistarà, V.; Rescifina, A.; Romeo, G.; Romeo, R. An asymmetric approach to pyrrolidinone and pyrrolizidinone systems by intramolecular oxime-olefin cycloaddition. Tetrahedron 1996, 52, 7875–7884. [Google Scholar] [CrossRef]
- Hutt, A.J. Drug chirality and its pharmacological consequences. In Introduction to the Principles of Drug Design and Action; Smith, H.J., Williams, H., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 117–183. [Google Scholar]
- Carnovale, C.; Iannazzo, D.; Nicolosi, G.; Piperno, A.; Sanfilippo, C. Preparation of isoxazolidinyl nucleoside enantiomers by lipase-catalysed kinetic resolution. Tetrahedron Asymmetry 2009, 20, 425–430. [Google Scholar] [CrossRef]
- Gervaix, A.; West, D.; Leoni, L.M.; Richman, D.D.; Wong-Staal, F.; Corbeil, J. A new reporter cell line to monitor HIV infection and drug susceptibility in vitro. Proc. Natl. Acad. Sci. USA 1997, 94, 4653–4658. [Google Scholar]
- Balestrieri, E.; Pizzimenti, F.; Ferlazzo, A.; Giofrè, S.V.; Iannazzo, D.; Piperno, A.; Romeo, R.; Chiacchio, M.; Macchi, B. Antiviral activity of seed extract from Citrus bergamia towards human retroviruses. Bioorg. Med. Chem. 2011, 19, 2084–2089. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Romeo, R.; Carnovale, C.; Giofrè, S.V.; Monciino, G.; Chiacchio, M.A.; Sanfilippo, C.; Macchi, B. Enantiomerically Pure Phosphonated Carbocyclic 2'-Oxa-3'-Azanucleosides: Synthesis and Biological Evaluation. Molecules 2014, 19, 14406-14416. https://doi.org/10.3390/molecules190914406
Romeo R, Carnovale C, Giofrè SV, Monciino G, Chiacchio MA, Sanfilippo C, Macchi B. Enantiomerically Pure Phosphonated Carbocyclic 2'-Oxa-3'-Azanucleosides: Synthesis and Biological Evaluation. Molecules. 2014; 19(9):14406-14416. https://doi.org/10.3390/molecules190914406
Chicago/Turabian StyleRomeo, Roberto, Caterina Carnovale, Salvatore V. Giofrè, Giulia Monciino, Maria A. Chiacchio, Claudia Sanfilippo, and Beatrice Macchi. 2014. "Enantiomerically Pure Phosphonated Carbocyclic 2'-Oxa-3'-Azanucleosides: Synthesis and Biological Evaluation" Molecules 19, no. 9: 14406-14416. https://doi.org/10.3390/molecules190914406





