Identification, Characterization, and Immobilization of an Organic Solvent-Stable Alkaline Hydrolase (PA27) from Pseudomonas aeruginosa MH38
Abstract
:1. Introduction
2. Results and Discussion
2.1. Biochemical Analysis of PA27
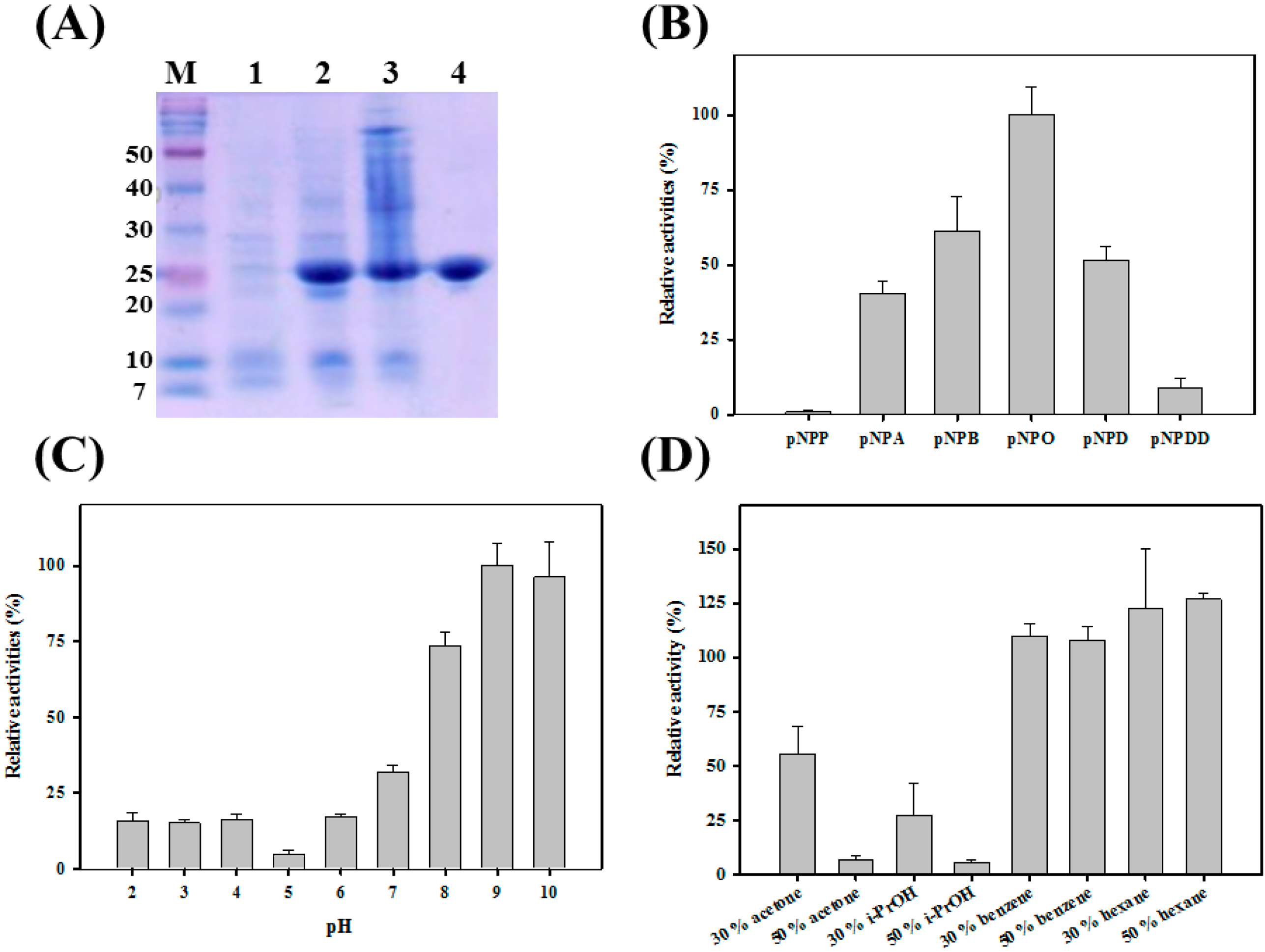

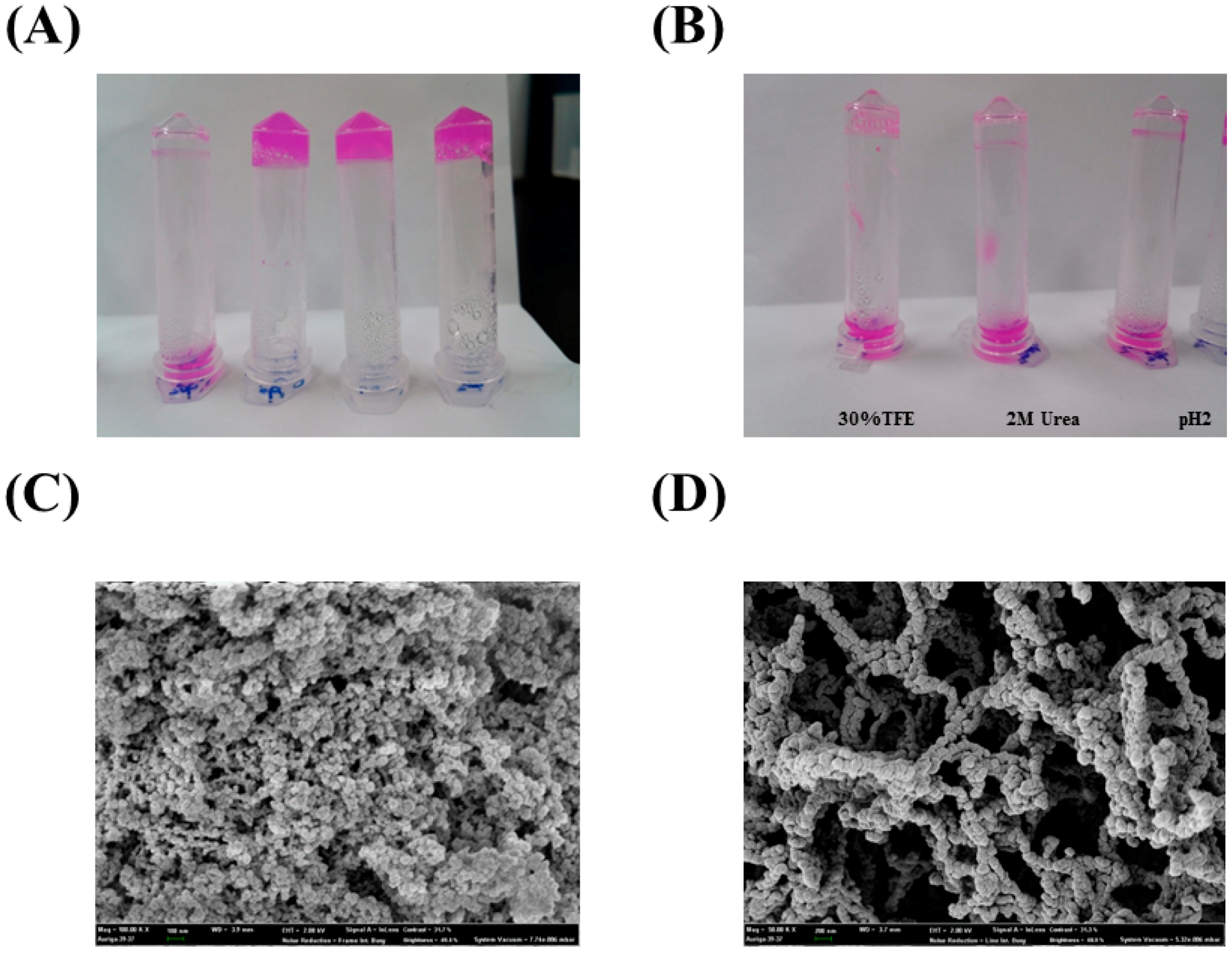
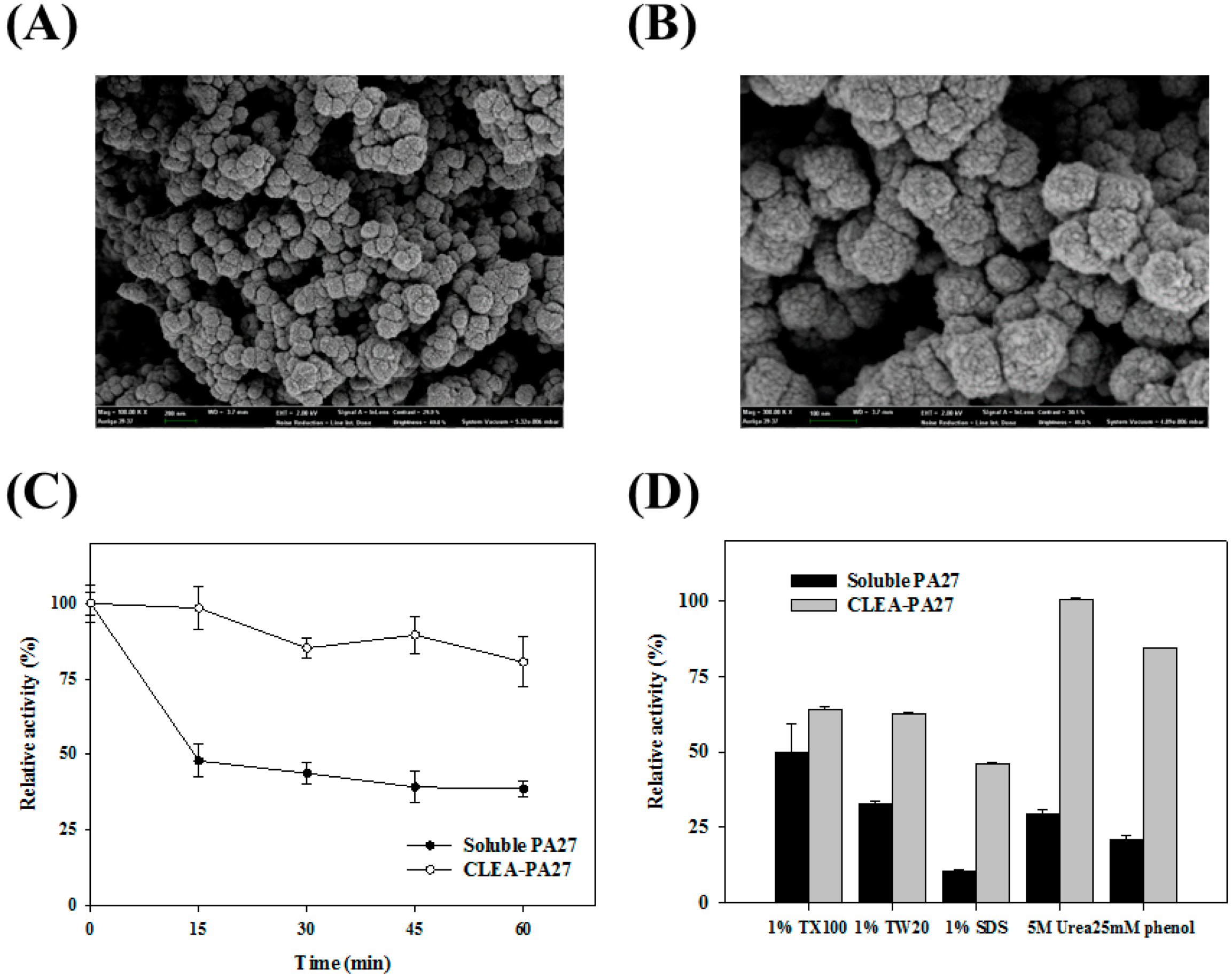
2.2. Immobilization of PA27
| Cycle Number | Relative Activity |
|---|---|
| 10 | 70.2 ± 10.8 |
| 15 | 66.9 ± 8.0 |
| 20 | 60.7 ± 7.1 |
3. Experimental Section
3.1. Bacterial Strains, Reagents, and Chemicals
3.2. Cloning and Purification of PA27
3.3. Enzymatic Assays
3.4. Immobilization of PA27
3.5. Formation of PA27 Hydrogel
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jaeger, K.E.; Reetz, M.T. Microbial lipases form versatile tools for biotechnology. Trends Biotechnol. 1998, 16, 396–403. [Google Scholar]
- Houde, A.; Kademi, A.; Leblanc, D. Lipases and their industrial applications: An overview. Appl. Biochem. Biotechnol. 2004, 118, 155–170. [Google Scholar]
- Straathof, A.J.J.; Panke, S.; Schmid, A. The production of fine chemicals by biotransformations. Curr. Opin. Biotechnol. 2002, 13, 548–556. [Google Scholar]
- Gupta, A.; Khare, S.K. Enzymes from solvent-tolerant microbes: Useful biocatalysts for non-aqueous enzymology. Crit. Rev. Biotechnol. 2009, 29, 448–454. [Google Scholar]
- Torres, S.; Pandey, A.; Castro, G.R. Organic solvent adaptation of Gram positive bacteria: Applications and biotechnological potentials. Biotechnol. Adv. 2011, 29, 442–452. [Google Scholar]
- Ogino, H.; Nakagawa, S.; Shinya, K.; Muto, T.; Fujimura, N.; Yasuda, M.; Ishikawa, H. Purification and characterization of organic solvent-stable lipase from organic solvent-tolerant Pseudomonas aeruginosa LST-03. J. Biosci. Bioeng. 2000, 89, 451–457. [Google Scholar] [CrossRef]
- Kim, J.; Jang, S.H.; Lee, C. An organic solvent-tolerant alkaline lipase from cold-adapted Pseudomonas mandelii: Cloning, expression, and characterizatio. Biosci. Biotechnol. Biochem. 2013, 77, 320–323. [Google Scholar]
- Li, M.; Yang, L.R.; Xu, G.; Wu, J.P. Screening, purification and characterization of a novel cold-active and organic solvent-tolerant lipase from Stenotrophomonas maltophilia CGMCC 4254. Bioresour. Technol. 2013, 148, 114–120. [Google Scholar] [CrossRef]
- Yamashiro, Y.; Sakatoku, A.; Tanaka, D.; Nakamura, S. A cold-adapted and organic solvent-tolerant lipase from a psychrotrophic bacterium Pseudomonas sp. strain YY31: Identification, cloning, and characterization. Appl. Biochem. Biotechnol. 2013, 171, 989–1000. [Google Scholar] [CrossRef]
- Cao, Y.; Zhung, Y.; Changin, Y.; Wu, B.; He, B. Purification and characterization of an organic solvent-stable lipase from Pseudomonas stutzeri LC2–8 and its application for efficient resolution of (R,S)-1-phenylethanol. Biochem. Eng. J. 2012, 64, 55–60. [Google Scholar] [CrossRef]
- Gaur, R.; Gupta, A.; Khare, S.K. Purification and characterization of lipase from solvent tolerant Pseudomonas aeruginosa PseA. Process Biochem. 2008, 43, 1040–1046. [Google Scholar] [CrossRef]
- Sulong, M.R.; Abdul Rahman, R.N.; Salleh, A.B.; Basri, M. A novel organic solvent tolerant lipase from Bacillus sphaericus 205y: Extracellular expression of a novel OST-lipase gene. Protein Expr. Purif. 2006, 49, 190–195. [Google Scholar] [CrossRef]
- Pesaresi, A.; Lamba, D. Crystallization, X-ray diffraction analysis and phasing of carboxylesterase PA3859 from Pseudomonas aeruginosa in biocatalysis: Why, what and how. Biochim. Biophys. Acta 2005, 1752, 197–201. [Google Scholar] [CrossRef]
- Kim, K.K.; Song, H.K.; Shin, D.H.; Hwang, K.Y.; Choe, S.; Yoo, O.J.; Suh, S.W. Characterization Crystal structure of carboxylesterase from Pseudomonas fluorescens, an alpha/beta hydrolase with broad substrate specificity. Structure 1997, 5, 1571–1584. [Google Scholar]
- Rahman, R.N.; Kamarudin, N.H.; Yunus, J.; Salleh, A.B.; Basri, M. Expression of an organic solvent stable lipase from Staphylococcus epidermidis AT2. Int. J. Mol. Sci. 2010, 11, 3195–3208. [Google Scholar]
- Yan, H.; Saiani, A.; Gough, J.E.; Miller, A.F. Thermoreversible protein hydrogel as cell scaffold. Biomacromolecules 2006, 7, 2776–2782. [Google Scholar]
- Gilbert, E.J.; Cornish, A.; Jones, C.W. Purification and properties of extracellular lipase from Pseudomonas aeruginosa EF2. J. Gen. Microbiol. 1991, 137, 2223–2229. [Google Scholar]
- Sheldon, R.A. Characteristic features and biotechnological applications of cross-linked enzyme aggregates (CLEAs). Appl. Microbiol. Biotechnol. 2011, 92, 467–477. [Google Scholar]
- Bae, S.Y.; Ryu, B.H.; Jang, E.; Kim, S.; Kim, T.D. Characterization and immobilization of a novel SGNH hydrolase (Est24) from Sinorhizobium meliloti. Appl. Microbiol. Biotechnol. 2013, 97, 1637–1647. [Google Scholar]
- Ju, H.; Jang, E.; Ryu, B.H.; Kim, T.D. Characterization and preparation of highly stable aggregates of a novel type of hydrolase (BL28) from Bacillus licheniformis. Bioresour. Technol. 2013, 128, 81–86. [Google Scholar] [CrossRef]
- Ngo, T.D.; Ryu, B.H.; Ju, H.; Jang, E.; Park, K.; Kim, K.K.; Kim, T.D. Structural and functional analyses of a bacterial homologue of hormone-sensitive lipase from a metagenomic library. Acta Crystallogr. D Biol. Crystallogr. 2013, 69, 1726–1737. [Google Scholar]
- Ngo, T.D.; Ryu, B.H.; Ju, H.; Jang, E.; Kim, K.K.; Kim, T.D. Crystallographic analysis and biochemical applications of a novel penicillin-binding protein/β-lactamase homologue from a metagenomic library. Acta Crystallogr. D Biol. Crystallogr. 2014, 70, 12455–2466. [Google Scholar]
- Ju, H.; Ryu, B.H.; Kim, T.D. Identification, characterization, immobilization of a novel type hydrolase (LmH) from Listeria monocytogene. Int. J. Biol. Macomol. 2015, 72, 63–70. [Google Scholar] [CrossRef]
- Jang, E.; Ryu, B.H.; Shim, H.W.; Ju, H.; Kim, D.W.; Kim, T.D. Identification, characterization, immobilization of a novel type hydrolase (LmH) Adsorption of microbial esterases on Bacillus subtilis-templated cobalt oxide nanoparticles. Int. J. Biol. Macomol. 2014, 65, 188–192. [Google Scholar] [CrossRef]
- Sample Availability: Samples (cDNA, plasmid, and proteins) are available from authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jang, E.; Ryu, B.H.; Kim, T.D. Identification, Characterization, and Immobilization of an Organic Solvent-Stable Alkaline Hydrolase (PA27) from Pseudomonas aeruginosa MH38. Molecules 2014, 19, 14396-14405. https://doi.org/10.3390/molecules190914396
Jang E, Ryu BH, Kim TD. Identification, Characterization, and Immobilization of an Organic Solvent-Stable Alkaline Hydrolase (PA27) from Pseudomonas aeruginosa MH38. Molecules. 2014; 19(9):14396-14405. https://doi.org/10.3390/molecules190914396
Chicago/Turabian StyleJang, Eunjin, Bum Han Ryu, and Thomas Doohun Kim. 2014. "Identification, Characterization, and Immobilization of an Organic Solvent-Stable Alkaline Hydrolase (PA27) from Pseudomonas aeruginosa MH38" Molecules 19, no. 9: 14396-14405. https://doi.org/10.3390/molecules190914396
APA StyleJang, E., Ryu, B. H., & Kim, T. D. (2014). Identification, Characterization, and Immobilization of an Organic Solvent-Stable Alkaline Hydrolase (PA27) from Pseudomonas aeruginosa MH38. Molecules, 19(9), 14396-14405. https://doi.org/10.3390/molecules190914396





