Thiosulfoxide (Sulfane) Sulfur: New Chemistry and New Regulatory Roles in Biology
Abstract
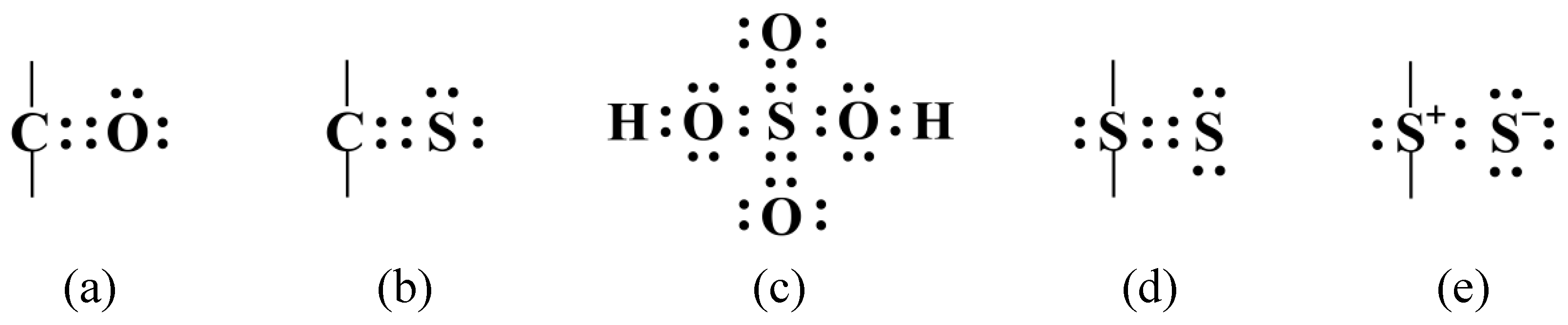
:1. Introduction: Sulfur Bonding

2. Sulfur in Biology
- Elemental sulfur reduction to H2S provides a source of energy in Desulfuromonas and archaea.
- H2S oxidation to elemental sulfur provides a source of energy in Beggiatoa.
- H2S or S0 oxidation to sulfate provides a source of energy in Thiobacillus and archaea.
- Sulfate or sulfite reduction to H2S provides a source of oxygen for Desulfovibrio, archaea.
- H2S splitting during photosynthesis provides a source of hydrogen atoms in purple and green sulfur bacteria.
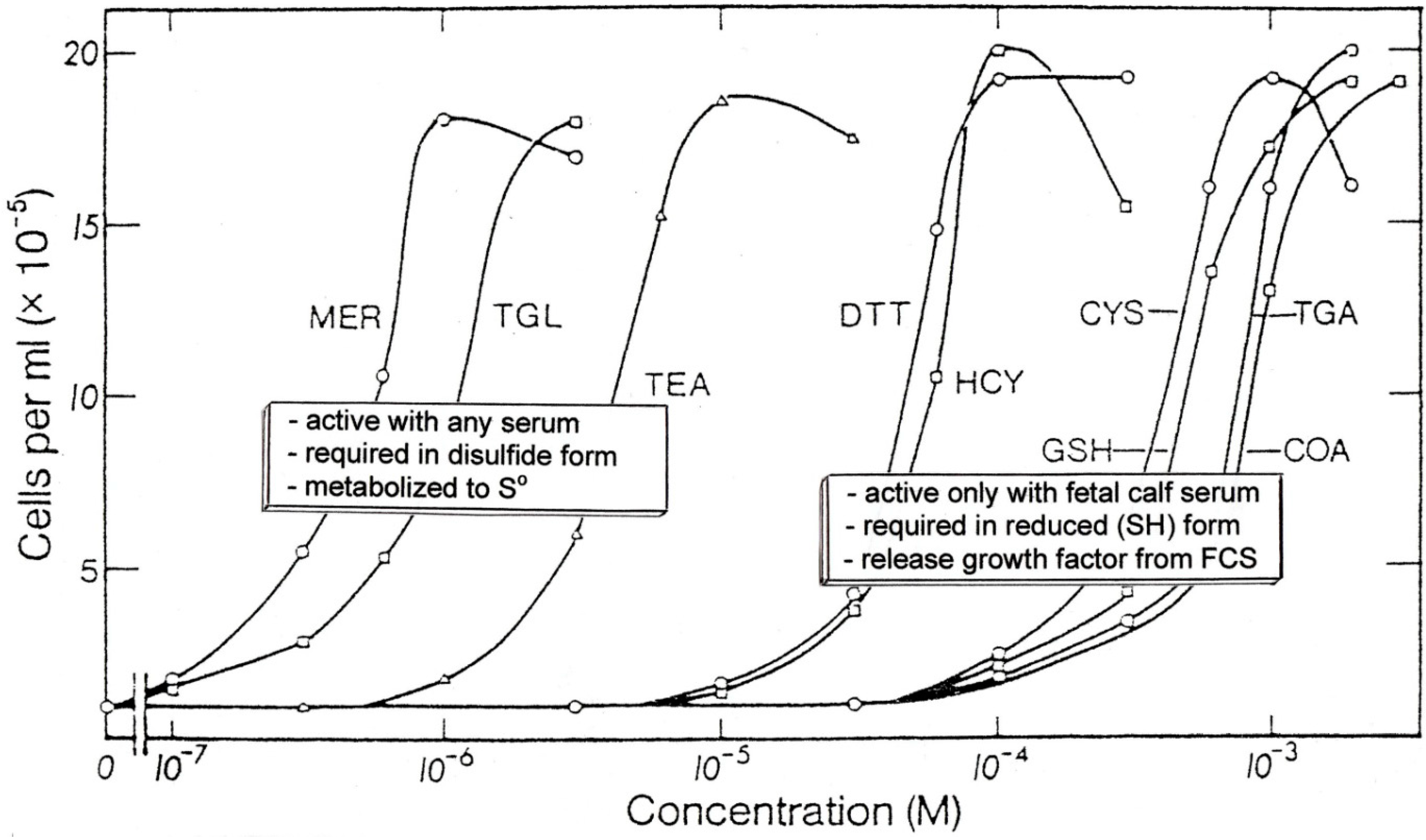
3. Sulfur as a Regulatory Agent
| Substrate | R | X | Y | Catalyst | Refs. |
|---|---|---|---|---|---|
| Cystine | Alanine | NH2 | COOH | Cystathionase | [19] |
| Cystine | Alanine | NH2 | COOH | Pyridoxal | [20] |
| Cysteine-alkyl disulfides | Alkyl | NH2 | COOH | C-S lyases | [21,22] |
| Cystamine | CH2-CH2-NH2 | NH2 | H | Diamine oxidase | [23] |
| Mercaptoethanol disulfide | CH2-CH2-OH | OH | H | Alcohol dehydrogenase | [17] |
4. Sulfane Sulfur from Garlic
5. “Hydrogen Sulfide”
6. Properties of Sulfane Sulfur
- (a)
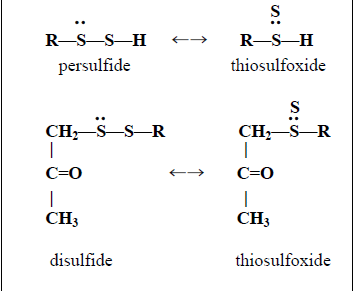
- Thiosulfoxides in the oxidation series ranging from thiosulfenic acid to thiosulfate as shown in the fourth row of Table 1. These compounds should be considered as having two kinds of sulfur, the inner sulfur with a variable oxidation number (e.g., +4 for thiosulfate) and the outer (sulfane) sulfur with an oxidation number of zero.
- (b)
- Chains of sulfur atoms in which one sulfur atom can move to the thiosulfoxide position on one of the other sulfur atoms. This includes elemental sulfur, persulfides (RSSH), polysulfides (R-Sn-R) where n is 3 or greater, and polythionates (−SO3-Sn-SO3−) where n is 3 or greater. Disulfides (R-S-S-R) are not in this category unless one C-S bond is activated.
- (c)
- Disulfides in which one sulfur is activated by a C=O, C=C, or C=N group adjacent to a C-S bond. The activating effect of these unsaturated groups has been documented for the β-ene group in allyl disulfides [1,67] (Equation (4)), the β-keto group created de novo during oxidation of cystamine and mercaptoethanol disulfide as referenced in Table 2 (Equation (5)), and the β-ketimine intermediates in pyridoxal 5'-phosphate (PLP)-catalyzed reactions, such as the desulfuration of cysteine to alanine by the desulfurases discussed below (Equation (6)).
- (d)
- α or β-keto thiols. The sulfur of these compounds behaves as sulfane sulfur although it cannot form a thiosufoxide. The classical example is mercptopyruvate [68]. An analogous weakening of the C-S bond is seen when alanine-3-sulfinate is transaminated to sulfinyl pyruvate during the biodegradation of cysteine [69]. For the keto group in the β position, the mechanism may involve keto-enol tautomerization resulting in C=C group adjacent to the C-S bond [70].


7. Sulfane Sulfur Transport
7.1. The Rhodanese Homology Domain
7.2. Non-RHOD S0 Binding to Proteins
8. Sulfane Sulfur Generation
8.1. Cysteine Deamination (Generation of a C=O Group α to a C-S Bond)
8.2. Homocysteine Deamination (Generation of a C=O or C=N Group β to a C-S Bond)
8.3. Cysteine Desulfurases (C=N in the α Position)
8.4. Cysteine S-conjugate Lyases (C-S Lyases) (β Elimination)
8.5. Cystathionine γ-Lyase (CTH)
8.6. Allyl Disufides (C=C Group in the α Position)
8.7. The Polyamine Pathway
9. The “Antioxidant” Properties Attributed to Sulfur Compounds
9.1. Superoxide Dismutase Requires S0
9.2. The Sulfur in the Iron-sulfur Clusters Originates as S0
9.3. The Reducing Capacity Is Increased in S0-stimulated Cells
9.4. Glutathione Persulfide (G-S-SH) Is a Powerful Reductant
10. Sulfur Compounds and Elemental Sulfur in Plant Defense
11. Is There a Selenium Analog of S0?
12. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kutney, G.W.; Turnbull, K. Compounds containing the S=S bond. Chem. Rev. 1982, 82, 333–357. [Google Scholar] [CrossRef]
- Noury, S.; Silvi, B.; Gillespie, R.J. Chemical bonding in hypervalent molecules: Is the octet rule relevant? Inorg. Chem. 2002, 41, 2164–2172. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, A.; Yang, D.Y. Hypervalency in sulfur? Ab initio and DFT studies of the structures of thiosulfate and related sulfur oxyanions. Sulfur Lett. 2003, 26, 171–180. [Google Scholar] [CrossRef]
- Schmøkel, M.S.; Cenedese, S.; Overgaard, J.; Jørgensen, M.R.; Chen, Y.S.; Gatti, C.; Stalke, D.; Iversen, B.B. Testing the concept of hypervalency: Charge density analysis of K2SO4. Inorg. Chem. 2012, 51, 8607–8616. [Google Scholar]
- Miaskiewicz, K.; Steudel, R. Sulfur compounds. Part 140. Structures and relative stabilities of seven isomeric forms of H2S2O2. J. Chem. Soc. Dalton Trans. 1991, 2395–2398. [Google Scholar]
- Steudel, R.; Drozdova, Y.; Miaskiewicz, K.; Hertwig, R.H.; Koch, W. How unstable are thiosulfoxides? An ab initio MO study of various disulfanes RSSR (R=H, Me, Pr, All), their branched isomers R2SS, and the related transition states. J. Am. Chem. Soc. 1997, 119, 1990–1996. [Google Scholar] [CrossRef]
- Roos, G.; Messens, J. Protein sulfenic acid formation: From cellular damage to redox regulation. Free Radic. Biol. Med. 2011, 51, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Laxman, S.; Sutter, B.M.; Wu, X.; Kumar, S.; Guo, X.; Trudgian, D.C.; Mirzaei, H.; Tu, B.P. Sulfur amino acids regulate translational capacity and metabolic homeostasis through modulation of tRNA thiolation. Cell 2013, 154, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Mendel, R.R. The molybdenum cofactor. J. Biol. Chem. 2013, 288, 13165–13172. [Google Scholar] [CrossRef] [PubMed]
- L’vov, N.P.; Nosikov, A.N.; Antipov, A.N. Tungsten-containing enzymes. Biochemistry (Mosc.) 2002, 67, 196–200. [Google Scholar]
- Fanger, M.W.; Hart, D.A.; Wells, J.V.; Nisonoff, A. Enhancement by reducing agents of the transformation of human and rabbit peripheral lymphocytes. J. Immunol. 1970, 105, 1043–1045. [Google Scholar] [PubMed]
- Click, R.E.; Benck, L.; Alter, B.J. Enhancement of antibody synthesis in vitro by mercaptoethanol. Cell. Immunol. 1972, 3, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Heber-Katz, E.; Click, R.E. Immune responses in vitro. Role of mercaptoethanol in the mixed leukocyte reaction. Cell. Immunol. 1972, 5, 410–418. [Google Scholar]
- Goodman, M.G.; Weigle, W.O. Nonspecific activation of murine lymphocytes. 1. Proliferation and polyclonal activation induced by 2-mercaptoethanol and α-thioglycerol. J. Exp. Med. 1977, 145, 473–488. [Google Scholar]
- Broome, J.D.; Jeng, M.W. Promotion of replication in lymphoid cells by specific thiols and disulfides. J. Exp. Med. 1973, 138, 574–592. [Google Scholar] [CrossRef] [PubMed]
- Toohey, J.I. Sulfhydryl dependence in primary explant hematopoietic cells; inhibition of growth in vitro with vitamin B12 compounds. Proc. Nat. Acad. Sci. USA 1975, 72, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Toohey, J.I. Persulfide sulfur is a growth factor for cells defective in sulfur metabolism. Biochem. Cell Biol. 1986, 64, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Toohey, J.I. Macrophages and methylthio groups in lymphocyte proliferation. J. Supramol. Struct. 1981, 17, 11–25. [Google Scholar] [CrossRef]
- Costa, M.T.; Wolf, A.M.; Giarnieri, D. Cleavage of cystine by cystathionase. Enzymologia 1972, 43, 271–279. [Google Scholar] [PubMed]
- Cavallini, D.; de Marco, C.; Mondovi, B. Cleavage of cystine by a pyridoxal model. Arch. Biochem. Biophys. 1960, 87, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.J.L.; Pinto, J.T. Aminotransferase, L-amino acid oxidase and β-lyase reactions involving l-cysteine S-conjugates found in allium extracts: Relevance to biological activity? Biochem. Pharmacol. 2005, 69, 209–220. [Google Scholar]
- Cooper, A.J.L.; Pinto, J.T. Cysteine S-conjugate β-lyases. Amino Acids 2006, 30, 1–15. [Google Scholar] [CrossRef] [PubMed]
- De Marco, C.; Coletta, M.; Mondovi, B. Transulfuration reactions coupled to enzymic oxidation of cystamine. Ital. J. Biochem. 1960, 9, 77–84. [Google Scholar]
- Roy, A.B.; Trudinger, P.A. The Biochemistry of Inorganic Compounds of Sulphur; Cambridge University Press: Cambridge, UK, 1970; pp. 20–34. [Google Scholar]
- Toohey, J.I. Methylthioadenosine nucleoside phosphorylase deficiency in methylthio-dependent murine cells. Biochem. Biophys. Res. Commun. 1978, 83, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Livingston, D.M.; Fergson, C.; Gollogly, R.; Lazarus, H. Accumulation of cystine auxotrophic thymocytes accompanying type C viral leukemogenesis in the mouse. Cell 1976, 7, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Bertino, J.R.; Waud, W.R.; Parker, W.B.; Lubin, M. Targeting tumors that lack methythioadenosine phosphorylase (MTAP) activity. Current strategies. Cancer Biol. Ther. 2011, 11, 1–6. [Google Scholar] [CrossRef]
- Della Ragione, F.; Oliva, A.; Palumbo, R.; Russo, G.; Gragnaniello, V.; Zappia, V. Deficiency of 5'-deoxy-5'-methylthioadenosine phosphorylase activity in malignancy; Absence of the protein in human enzyme-deficient cell lines. Biochem. J. 1992, 281, 533–538. [Google Scholar]
- Click, R.E. A Review: Alteration of in vitro reproduction processes by thiols—Emphasis on 2-mercaptoethanol. J. Reprod. Dev. 2014. [Google Scholar] [CrossRef]
- Okada, M.; Oka, M.; Yoneda, Y. Effective culture conditions for the induction of pluripotent stem cells. Biochim. Biophys. Acta 2010, 1800, 956–963. [Google Scholar]
- Click, R.E. Review: 2-mercaptoethanol alteration of in vitro immune functions of species other than murine. J. Immunol. Methods 2014, 402, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Toohey, J.I. Sulfur metabolism in AIDS: Cystamine as an anti-HIV agent. AIDS Res. Hum. Retrov. 2009, 25, 1057–1060. [Google Scholar] [CrossRef]
- Bergamini, A.; Capozzi, M.; Ghibelli, L.; Dini, L.; Salanitro, A.; Milanese, G.; Wagner, T.; Beninati, S.; Pesce, C.D.; Amici, C.; et al. Cystamine potently suppresses in vitro HIV replication in acutely and chronically infected human cells. J. Clin. Investig. 1994, 93, 2251–2257. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.; Zhu, X.; Song, L.; Lee, H.; Cutilli, J.R.; Douglas, S.D. Cystamine inhibits HIV type I replication in cells of macrocyte/macrophage and T cell lineages. AIDS Res. Hum. Retrov. 1995, 11, 451–459. [Google Scholar]
- Gibrat, C.; Cicchetti, F. Potential of cystamine and cysteamine in the treatment of neurodegenerative diseases. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, T.H.; Brizel, D.M. The role of amifostine as a radioprotector. Oncology (Williston Park) 2001, 15, 1349–1354. [Google Scholar]
- Murley, J.S.; Kataoka, Y.; Baker, K.L.; Diamond, A.M.; Morgan, W.F.; Grdina, D.J. Manganese superoxide dismutase (SOD2)-mediated delayed radioprotection induced by the free thiol form of amifostine and tumor necrosis factor α. Radiat. Res. 2007, 167, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Click, R.E. Obesity, longevity, quality of life: Alteration by dietary 2-mercaptoethanol. Virulence 2010, 1, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Heidrick, M.; Hendricks, L.C.; Cook, D.E. Effect of dietary 2-mercaptoethanol on the life span, immune system, tumor incidence and lipid peroxidation damage in spleen lymphocytes of ageing BC3F1 mice. Mech. Ageing Dev. 1984, 31, 341–356. [Google Scholar] [CrossRef]
- Click, R.E. Dietary supplemented 2-mercaptoethanol prevents spontaneous and delays virally-induced murine mammary tumorigenesis. Cancer Biol. Ther. 2013, 14, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Prolongation of the normal lifespan and inhibition of spontaneous cancer by antioxidants. J. Gerontol. 1961, 16, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Toohey, J.I. Sulphane sulphur in biological systems: A possible regulatory role. Biochem. J. 1989, 264, 625–632. [Google Scholar] [PubMed]
- Mueller, E.G. Trafficking in persulfides: Delivering sulfur in biosynthetic pathways. Nat. Chem. Biol. 2006, 2, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Kessler, D. Enzymatic activation of sulfur for incorporation into biomolecules in prokaryotes. FEMS Microbiol. Rev. 2006, 30, 825–840. [Google Scholar] [CrossRef] [PubMed]
- Bayan, L.; Koulivand, P.H.; Gorji, A. Garlic: A review of potential therapeutic effects. Avicenna J. Phytomed. 2014, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cerella, C.; Dicato, M.; Jacob, C.; Diederich, M. Chemical properties and mechanisms determining the anti-cancer action of garlic-derived organic sulfur compounds. Anticancer Agents Med. Chem. 2011, 11, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Thomson, M.; Ali, M. Garlic [Allium sativum]: A Review of its potential use as an anti-cancer agent. Curr. Cancer Drug Targets 2003, 3, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Ray, B.; Chauhan, N.B.; Lahiri, D.K. The “aged garlic extract:” (AGE) and one of its active ingredients S-allyl-l-cysteine (SAC) as potential preventive and therapeutic agents for Alzheimer’s disease. Curr. Med. Chem. 2011, 18, 3306–3313. [Google Scholar] [CrossRef] [PubMed]
- Padiya, R.; Banerjee, S.K. Garlic as an anti-diabetic agent: Recent progress and patent reviews. Recent Pat. Food Nutr. Agric. 2013, 5, 105–127. [Google Scholar] [CrossRef] [PubMed]
- Ried, K.; Toben, C.; Fakler, P. Effect of garlic on serum lipids: An updated meta-analysis. Nutr. Rev. 2013, 71, 282–299. [Google Scholar] [CrossRef] [PubMed]
- Borek, C. Garlic reduces dementia and heart-disease risk. J. Nutr. 2006, 136, S810–S812. [Google Scholar]
- Amagase, H. Clarifying the real bioactive constituents of garlic. J. Nutr. 2006, 136, S716–S725. [Google Scholar]
- Li, L.; Sun, T.; Tian, J.; Yang, K.; Yi, K.; Zhang, P. Garlic in clinical practice: An evidence-based overview. Crit. Rev. Food Sci. Nutr. 2013, 53, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Martelli, A.; Testai, L.; Breschi, M.C.; Blandizzi, C.; Virdis, A.; Taddei, S.; Calderone, V. Hydrogen sulphide: Novel opportunity for drug discovery. Med. Res. Rev. 2012, 32, 1093–1130. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Rajapakse, A.; Shen, X.; Gates, K.S. Generation of DNA-damaging reactive oxygen species via the autoxidation of hydrogen sulfide under physiologically-relevant conditions: Chemistry relevant to both the genotoxic and cell signaling properties of H2S. Chem. Res. Toxicol. 2012, 25, 1609–1615. [Google Scholar] [CrossRef] [PubMed]
- Toohey, J.I. Sulfur signaling; is the agent sulfide or sulfane? Anal. Biochem. 2011, 413, 1–7. [Google Scholar]
- Greiner, R.; Pálinkás, Z.; Bäsell, K.; Becher, D.; Antelmann, H.; Nagy, P.; Dick, T.P. Polysulfides link H2S to protein thiol oxidation. Antioxid. Redox. Signal. 2013, 19, 1749–1765. [Google Scholar] [CrossRef] [PubMed]
- Ida, T.; Sawa, T.; Ihara, H.; Tsuchiya, Y.; Watanabe, Y.; Kugamai, Y.; Suematsu, M.; Motohashi, H.; Fujii, S.; Matsunaga, T.; et al. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 7606–7611. [Google Scholar] [CrossRef] [PubMed]
- Koenitzer, J.R.; Isbell, T.S.; Patel, H.D.; Benavides, G.A.; Dickenson, D.A.; Patel, R.P.; Darley-Usmar, V.M.; Lancaster, J.R.; Doeller, J.E.; Kraus, D.W. Hydrogen sulfide mediates vasoactivity in an O2-dependent manner. Am. J. Heart Circ. Physiol. 2007, 292, H1963–H1960. [Google Scholar]
- Olson, K.R.; Forgan, L.G.; Dombkowski, R.A.; Forster, M.E. Oxygen dependency of hydrogen sulfide-mediated vasoconstriction in cyclostome aortas. J. Exp. Biol. 2008, 211, 2205–2213. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Snyder, S.H. H2S signaling through protein sulfhydration and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Toohey, J.I. Conversion of H2S to sulfane sulfur. Nat. Rev. Mol. Cell Biol. 2013, 13, 803. [Google Scholar] [CrossRef]
- Sparatore, A.; Santus, G.; Giustarini, D.; Rossi, R.; del Soldato, P. Therapeutic potential of new hydrogen sulfide-releasing hybrids. Expert Rev. Clin. Pharmacol. 2011, 4, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Cava, M.P.; Levinson, M.I. Thionation reactions of Lawesson’s reagents. Tetrahedron 1985, 41, 5061–5087. [Google Scholar] [CrossRef]
- Zhang, Y.; Munday, M. Dithiolethiones for cancer chemoprevention: Where do we stand? Mol. Cancer Ther. 2008, 7, 3470–3479. [Google Scholar]
- Kimura, Y.; Mikami, Y.; Osumi, K.; Tsugane, M.; Oka, J.; Kimura, H. Polysulfides are possible H2S-derived signaling molecules in rat brain. FASEB J. 2013, 27, 2451–2457. [Google Scholar] [CrossRef] [PubMed]
- Höfle, G.; Baldwin, J.E. Thiosufoxides. The intermediates in rearrangement and reduction of allylic disulfides. J. Am. Chem. Soc. 1971, 93, 6307–6308. [Google Scholar]
- Meister, A.; Fraser, P.; Tice, S.V. Enzymatic desulfuration of β-mercaptopyruvate to pyruvate. J. Biol. Chem. 1954, 206, 561–575. [Google Scholar] [PubMed]
- Kearney, E.B.; Singer, T.P. Enzymic transformation of l-cysteinesulfinic acid. Biochim. Biophys. Acta 1953, 11, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Nicolet, B.H. The mechanism of sulfur lability in cysteine and its derivatives. Some thioethers readily split by alkali. J. Am. Chem. Soc. 1931, 53, 3066–3072. [Google Scholar] [CrossRef]
- Hofmann, K.; Bucher, P.; Kajava, A.V. A model of Cdc25 phosphatase catalytic domain and Cdk-interaction surface based on the presence of a rhodanese homology domain. J. Mol. Biol. 1998, 282, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Yamanishi, T.; Tuboi, S. The mechanism of the l-cystine cleavage reaction catalyzed by rat liver γ-cystathionase. J. Biochem. 1981, 89, 1913–1921. [Google Scholar] [PubMed]
- Jarabak, R.; Westley, J. Serum albumin and cyanide detoxication. Kinetic characterization of a reactive albumin-sulfur complex. J. Biol. Chem. 1986, 261, 10793–10796. [Google Scholar]
- Cipollone, R.; Acsenzi, P.; Visca, P. Common themes and variations in the rhodanese superfamily. IUBMB Life 2007, 59, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Bordo, D.; Bork, P. The rhodanese/Cdc25 phosphatase superfamily. Sequence-structure-function relations. EMBO Rep. 2002, 3, 741–746. [Google Scholar]
- You, Z.; Cao, X.; Taylor, A.B.; Hart, P.J.; Levine, R.L. Characterization of a covalent polysulfane bridge in Cu-Zn superoxide dismutase. Biochemistry 2010, 49, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, R.W.; Tachibana, C.; Hansen, N.E.; Winther, J.R. Trisulfides in proteins. Antiox. Redox. Signal. 2011, 15, 67–75. [Google Scholar] [CrossRef]
- Gu, S.; Wen, D.; Weinreb, P.H.; Sun, Y.; Zhang, L.; Foley, S.F.; Kshirsagar, R.; Evans, D.; Mi, S.; Meier, W.; et al. Characterization of trisulfide modifications in antibodies. Anal. Biochem. 2010, 400, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Knowles, L.M.; Milner, J.A. Diallyl disulfide inhibits p34(Cdc2) kinase activity through changes in complex formation and phosphorylation. Carcinogenesis 2000, 21, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Fu, C.; Pappin, D.; Tonks, N.K. H2S-induced sulfhydration of PTP1B and its role in the endoplasmic reticulum stress response. Sci. Signal. 2011, 4, ra86. [Google Scholar] [PubMed]
- Chen, S.S.; Walgate, J.H.; Duerre, J.A. Oxidative deamination of sulfur amino acids by bacterial and snake venom l-amino acid oxidase. Arch. Biochem. Biophys. 1971, 146, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.J.L.; Meister, A. Enzymatic oxidation of l-homocysteine. Arch. Biochem. Biophys. 1985, 239, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; White, R.H.; Cash, V.L.; Dean, D.R. Mechanism for the desulfuration of l-cysteine by the nifS gene product. Biochemistry 1994, 33, 4714–4720. [Google Scholar] [CrossRef] [PubMed]
- Marelja, Z.; Mullick Chowdhury, M.; Dosche, C.; Hille, C.; Baumann, O.; Löhmannsröben, H.G.; Leimkuhler, S. The l-cysteine desulfurase NFS1 is localized in the cytosol where it provides the sulfur for molybdenum cofactor biosynthesis in humans. PLoS One 2013, 8, e60869. [Google Scholar] [CrossRef] [PubMed]
- Hidese, R.; Mihara, H.; Esaki, N. Bacterial cysteine desulfurases; versatile key players in biosynthetic pathways of sulfur-containing biofactors. Appl. Microbiol. Biotechnol. 2011, 91, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.J.L.; Krasnikov, B.F.; Niatsetskaya, Z.V.; Pinto, J.T.; Callery, P.S.; Villar, M.T.; Artigues, A.; Bruschi, S.A. Cysteine S-conjugate β-lyases: Important roles in the metabolism of naturally occurring sulfur and selenium-containing compounds, xenobiotics and anticancer agents. Amino Acids 2011, 41, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Buckberry, L.D.; Patel, R.; Hollingworth, L.; Teesdale-Spittle, P.H. Cysteine conjugate β-lyase activity of amino acid decarboxylases. Biochem. Soc. Trans. 1998, 26, S269. [Google Scholar] [PubMed]
- Blom, H.J.; Boers, G.H.J.; van den Elzen, J.P.; Gahl, W.A.; Tangerman, A. Transamination of methionine in humans. Clin. Sci. 1989, 76, 43–49. [Google Scholar] [PubMed]
- Tomisawa, H.; Ichimoto, N.; Ichihara, S.; Fukazawa, H. Involvement of cystathionase in the formation of alkane-thiols from corresponding cysteine conjugates. Xenobiotica 1988, 18, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, M.; Ubuka, T.; Yao, W.B.; Abe, T. l-Cysteine metabolism in guinea pig and rat tissues. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1997, 116, 223–226. [Google Scholar]
- Nagahara, N.; Sawada, N. The mercaptopyruvate pathway in cysteine catabolism: A physiologic role and related disease of the multifunctional 3-mercaptopyruvate sulfurtransferase. Curr. Med. Chem. 2006, 13, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Devalier-Klutchko, C.; Flavin, M. Role of Bacterial cystathionine β-cleavage enzyme in disulfide decomposition. Biochim. Biophys. Acta 1965, 99, 371–375. [Google Scholar]
- Flavin, M.; Slaughter, C. γ-Cystathionase (Neurospora). Methods Enzymol. 1962, 5, 433–439. [Google Scholar]
- Malloy, M.H.; Rassin, D.K.; Gaull, G.E. Plasma cyst(e)ine in homocyst(e)inemia. Am. J. Clin. Nutr. 1981, 34, 2619–2621. [Google Scholar] [PubMed]
- Wilcken, D.E.L.; Gupta, V.J. Cysteine–homocysteine mixed disulphide: Differing plasma concentrations in normal men and women. Clin. Sci. 1979, 57, 211–215. [Google Scholar] [PubMed]
- Schneider, J.A.; Bradley, K.H.; and Seegmiller, J.E. Identification and measurement of cysteine-homocysteine mixed disulfide in plasma. J. Lab. Clin. Med. 1968, 71, 122–125. [Google Scholar] [PubMed]
- Wróbel, M.; Lewandowska, I.; Bronowicka-Adamska, P.; Paszewski, A. The level of sulfane sulfur in the fungus Aspergillus nidulans wild type and mutant strains. Amino Acids 2009, 37, 565–571. [Google Scholar]
- Toohey, J.I. Vitamin B12 and methionine synthesis: A critical review: Is nature’s most beautiful cofactor misunderstood? Biofactors 2006, 26, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Thomas, T.J. Polyamines in cell growth and cell death: Molecular mechanisms and therapeutic applications. Cell. Mol. Life Sci. 2001, 58, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Gaull, G.; Sturman, J.A.; Räihä, N.C. Development of mammalian sulfur metabolism: Absence of cystathionase in human fetal tissues. Pediatr. Res. 1972, 6, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Levonin, A.; Lapatto, R.; Saksela, M.; Raivio, K.O. Human cystathionine γ-lyase: Developmental and in vitro expression of two isoforms. Biochem. J. 2000, 347, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Colín-González, A.L.; Santana, R.A.; Silva-Islas, C.A.; Chánez-Cárdenas, M.E.; Santamaría, A.; Maldonado, P.D. The antioxidant mechanisms underlying the aged garlic extract- and S-allylcysteine-induced protection. Oxid. Med. Cell. Longev. 2012, 2012, 907162. [Google Scholar]
- Sun, W.H.; Liu, F.; Chen, Y.; Zhu, Y.C. Hydrogen sulfide decreases the levels of ROS by inhibiting mitochondrial complex IV and increasing SOD Activities in cardiomyocytes under ischemia/reperfusion. Biochem. Biophys. Res. Commun. 2012, 421, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Iciek, M.; Kwiecień, I.; Chwatko, G.; Sokołowska-Jeżewicz, M.; Kowalczyk-Pachel, D.; Rokita, H. The effects of garlic-derived sulfur compounds on cell proliferation, caspase 3 activity, thiol levels and anaerobic sulfur metabolism in human hepatoblastoma HepG2 cells. Cell. Biochem. Funct. 2012, 30, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Fontecave, M. Iron-sulfur clusters: Ever-expanding roles. Nat. Chem. Biol. 2006, 2, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Frey, P.A.; Hegeman, A.D.; Ruzicka, F.J. The radical SAM superfamily. Crit. Rev. Biochem. Mol. Biol. 2008, 43, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Hänzelmann, P.; Schindelin, H. Crystal structure of the S-adenosylmethionine-dependent enzyme MoaA and its implications for molybdenum cofactor deficiency in humans. Proc. Nat. Acad. Sci. USA 2004, 101, 12870–12875. [Google Scholar]
- Massey, V.; Williams, C.H.; Palmer, G. The presence of S0-containing impurities in commercial samples of oxidized glutathione and their catalytic effect in the reduction of cytochrome c. Biochem. Biophys. Res. Commun. 1971, 42, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Prütz, W.A. Reduction of resazurin by glutathione activated by sulfanes and selenite. J. Chem. Soc. 1994, 14, 1639–1640. [Google Scholar]
- Bloem, E.; Haneklaus, S.; Schnug, E. Significance of sulfur compounds in the protection of plants against pests and diseases. J. Plant Nutr. 2005, 28, 763–784. [Google Scholar] [CrossRef]
- Cooper, R.M.; Williams, J.S. Elemental sulphur as an induced antifungal substance in plant defense. J. Exp. Bot. 2004, 55, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Nwachukwu, I.D.; Slusarenko, A.J.; Gruhlke, M.C. Sulfur and sulfur compounds in plant defense. Nat. Prod. Commun. 2012, 7, 395–400. [Google Scholar] [PubMed]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in health and disease. Antioxid. Redox Signal. 2011, 14, 1338–1367. [Google Scholar]
- Stadtman, T.C. Selenoproteins—Tracing the role of a trace element in protein function. PLoS Biol. 2005, 3, e421. [Google Scholar] [CrossRef] [PubMed]
- Ganther, H.E. Selenotrisulfides. Formation by the reaction of thiols with selenious acid. Biochemsitry 1968, 7, 2898–2905. [Google Scholar]
- Ogasawara, Y.; Lacourciere, G.; Stadtman, T.C. Formation of a selenium-substituted rhodanese by reaction with selenite and glutathione: Possible role of a protein pereselenide in a selenium delivery system. Proc. Nat. Acad. Sci. USA 2001, 98, 9494–9498. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F.; Hill, K.E.; Motley, A.K. Selenoprotein metabolism and function: Evidence for more than one function for selenoprotein P. J. Nutr. 2003, 133, 1517S–1520S. [Google Scholar] [PubMed]
- MacFarquhar, J.K.; Broussard, D.L.; Melstrom, P.; Hutchinson, R.; Wolkin, A.; Martin, C.; Burk, R.F.; Dunn, J.R.; Green, A.L.; Hammond, R.; et al. Acute selenium toxicity associated with a dietary supplement. Arch. Intern. Med. 2010, 170, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Aldosary, B.M.; Sutter, M.E.; Schwartz, M.; Morgan, B.W. Case series of selenium toxicity from a nutritional supplement. Clin. Toxicol. 2012, 50, 57–64. [Google Scholar] [CrossRef]
- Yang, G.Q.; Wang, S.Z.; Zhou, R.H.; Sun, S.Z. Endemic seleniun intoxication of humans in china. Am. J. Clin. Nutr. 1983, 37, 872–881. [Google Scholar] [PubMed]
- Desta, B.; Maldonado, G.; Reid, H.; Puschner, B.; Maxwell, J.; Agasan, A.; Humphreys, L.; Holt, T. Acute selenium toxicosis in polo ponies. J. Vet. Diagn. Investig. 2011, 23, 623–628. [Google Scholar] [CrossRef]
- Brozmanová, J.; Mániková, D.; Vlčková, V.; Chovanec, M. Selenium: A double-edged sword for defense and offence in cancer. Arch. Toxicol. 2010, 84, 919–938. [Google Scholar]
- Song, G.; Zhang, Z.; Wen, L.; Chen, C.; Shi, Q.; Zhang, Y.; Ni, J.; Liu, Q. Selenomethionine ameliorates cognitive decline, reduces tau hyperphosphorylation, and reverses synaptic deficit in the triple transgenic mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2014, 41, 85–99. [Google Scholar]
- Bellinger, F.P.; He, Q.P.; Bellinger, M.T.; Lin, Y.; Raman, A.V.; White, L.R.; Berry, M.J. Association of selenoprotein P with Alzheimer’s pathology in human cortex. J. Alzheimer’s Dis. 2008, 15, 465–472. [Google Scholar]
- Levander, O.A.; Moris, V.C.; Higgs, D.J. Selenium as a catalyst for the reduction of cytochrome c by glutathione. Biochemistry 1973, 12, 4591–4595. [Google Scholar] [CrossRef] [PubMed]
- Rhead, W.J.; Schrauzer, G.N. The selenium catalyzed reduction of methylene blue by thiols. Bioinorg. Chem. 1974, 3, 225–242. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Toohey, J.I.; Cooper, A.J.L. Thiosulfoxide (Sulfane) Sulfur: New Chemistry and New Regulatory Roles in Biology. Molecules 2014, 19, 12789-12813. https://doi.org/10.3390/molecules190812789
Toohey JI, Cooper AJL. Thiosulfoxide (Sulfane) Sulfur: New Chemistry and New Regulatory Roles in Biology. Molecules. 2014; 19(8):12789-12813. https://doi.org/10.3390/molecules190812789
Chicago/Turabian StyleToohey, John I., and Arthur J. L. Cooper. 2014. "Thiosulfoxide (Sulfane) Sulfur: New Chemistry and New Regulatory Roles in Biology" Molecules 19, no. 8: 12789-12813. https://doi.org/10.3390/molecules190812789
APA StyleToohey, J. I., & Cooper, A. J. L. (2014). Thiosulfoxide (Sulfane) Sulfur: New Chemistry and New Regulatory Roles in Biology. Molecules, 19(8), 12789-12813. https://doi.org/10.3390/molecules190812789