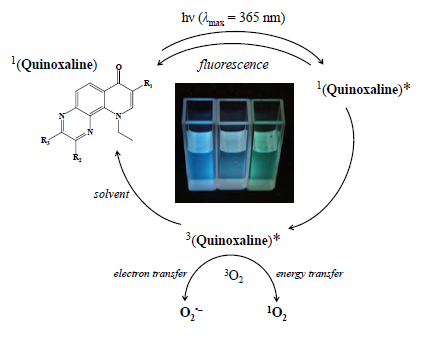
Fused-Ring Derivatives of Quinoxalines: Spectroscopic Characterization and Photoinduced Processes Investigated by EPR Spin Trapping Technique
Abstract
:1. Introduction
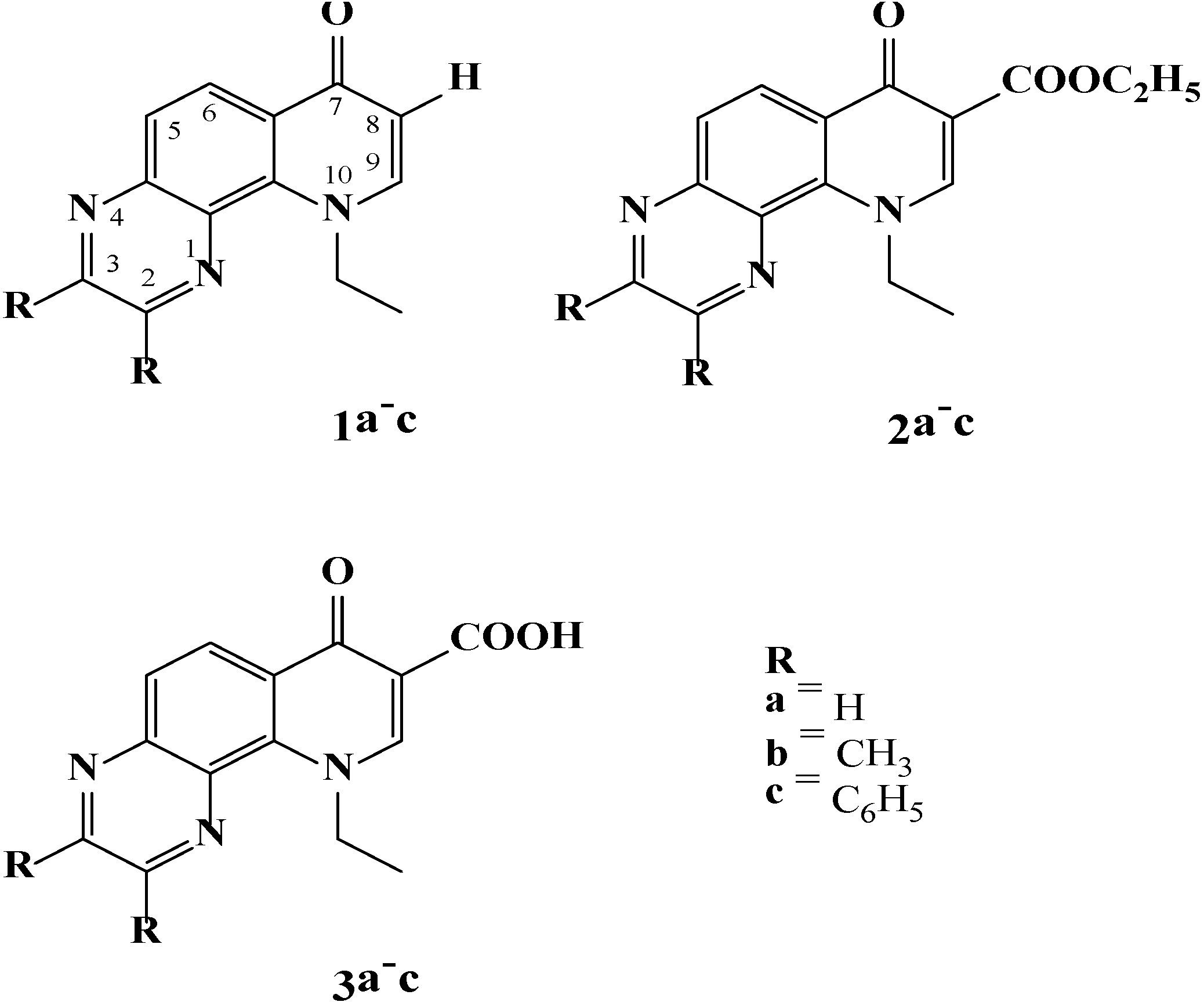
2. Results and Discussion
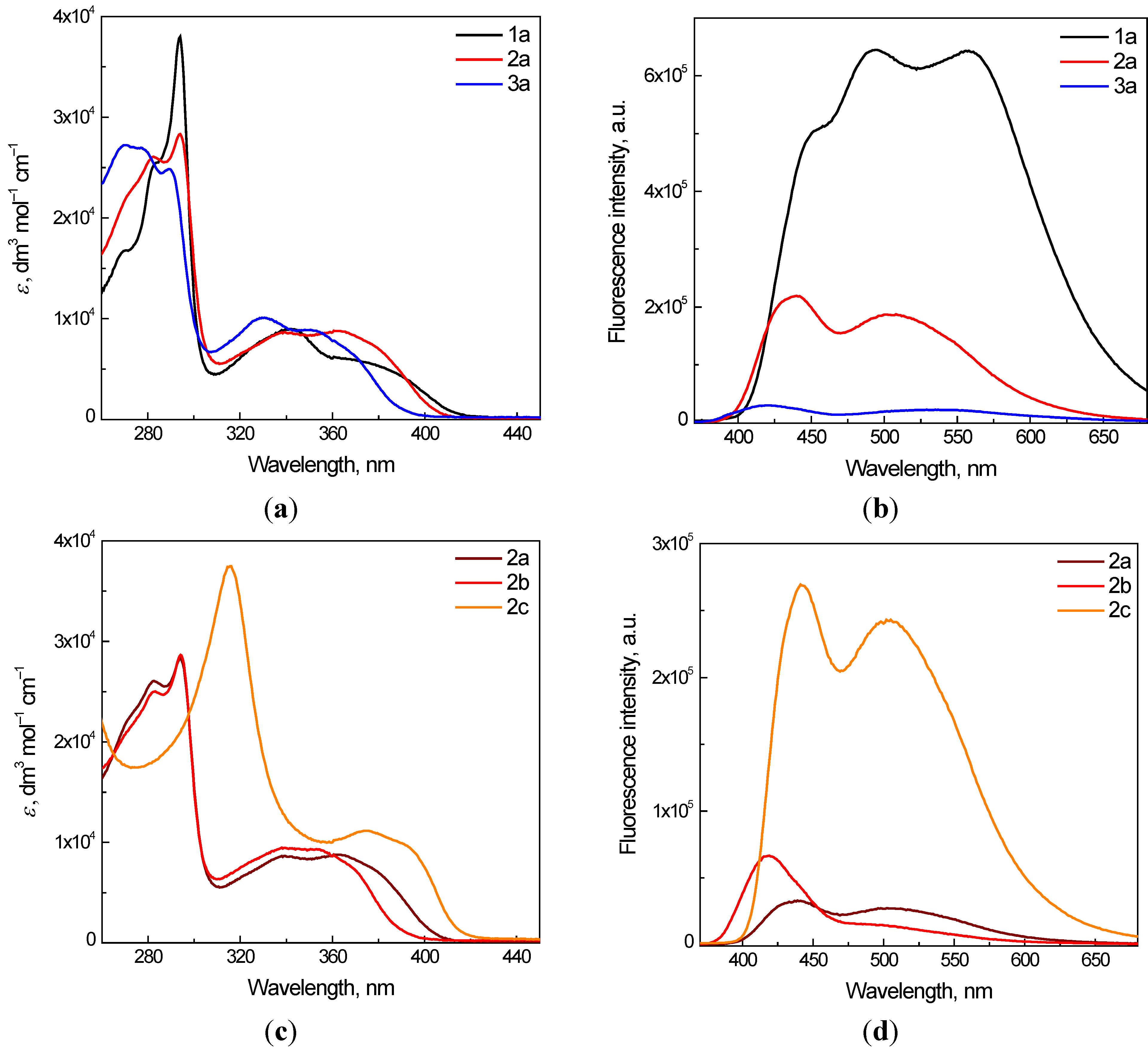
2.1. UV/vis and Fluorescence Spectroscopy
| Compd. | λmax (nm)/log ελ,max(dm3·mol−1·cm−1) | |
|---|---|---|
| DMSO | DMSO/Water (1:1 v:v) | |
| 1a | 380 sh/3.726, 342/3.955, 294/4.580 | 373 sh/3.540, 334/3.898, 290/4.414, 268/4.200 |
| 1b | 370 sh/3.663, 342/3.905, 295/4.541, 284/4.327, 270/4.195 | 367 sh/3.603, 332/3.960, 292/4.488, 281/4.349, 270/4.314 |
| 1c | 385 sh/3.887, 318/4.616 | 390 sh/3.778, 375/3.885, 317/4.531 |
| 2a | 376 sh/3.880, 361/3.945, 340/3.938, 294/4.452, 283/4.415 | 376 sh/3.742, 356/3.878, 333/3.915, 291/4.369, 279/4.365 |
| 2b | 370 sh/3.854, 354/3.965, 338/3.977, 294/4.457, 283/4.397 | 367 sh/3.773, 351/3.894, 332/3.923, 291/4.377, 279/4.347 |
| 2c | 395 sh/3.973, 375/4.048, 316/4.574 | 390 sh/3.970, 372/4.030, 314/4.510 |
| 3a | 368 sh/3.826, 353/3.943, 331/4.005, 290/4.393, 279/4.431, 270/4.435 | 368 sh/3.812, 351/3.949, 331/4.005, 291/4.391, 279/4.429, 270/4.435 |
| 3b | 372 sh/3.859, 352/4.012, 331/4.070, 290/4.458, 278/4.493, 271/4.496 | 367 sh/3.905, 351/4.015, 330/4.070, 290/4.456, 278/4.491, 271/4.496 |
| 3c | # 420 sh/3.437, 390/3.943, 370/3.960, 315/4.341 | & 400 sh/3.677, 386/3.872, 375/3.892, 316/4.388 |
2.2. FT-IR Spectroscopy
2.3. Photoinduced Processes of Quinoxaline Derivatives Monitored by EPR Spin Trapping Technique
| Spin-Adduct | Hyperfine Coupling Constants (mT) | g-Value | Reference | |
|---|---|---|---|---|
| aN | aH | |||
| DMSO | ||||
| •DMPO-O2− | 1.283 | 1.029, 0.136 | 2.0059 | [35,36] |
| •DMPO-OCH3 | 1.318 | 0.825, 0.187 | 2.0059 | [36,37] |
| •DMPO-OR | 1.426 | 1.249 | 2.0057 | [37] |
| •DMPO-CH3 | 1.472 | 2.114 | 2.0056 | [36] |
| •DMPOdegr | 1.533 | - | 2.0058 | [36] |
| •ND-CH3 | 1.422 | 1.300 (3H) | 2.0060 | [17,36] |
| •ND-CD3 | 1.410 | 0.193 (3D) | 2.0060 | [30] |
| •ND-CR1 | 1.341 | - | 2.0061 | [38,39] |
| •ND-(CH2)ar | 1.368 | 0.315 (2H) | 2.0061 | [36,38,39] |
| DMSO/H2O (1:1 v:v) | ||||
| •DMPO-O2−/OOH | 1.367 | 1.091, 0.132 | 2.0059 | [36] |
| •DMPO-OCH3 | 1.406 | 0.969, 0.152 | 2.0059 | [36,37] |
| •DMPO-OH | 1.449 | 1.337 | 2.0059 | [40] |
| •DMPO-CH3 | 1.550 | 2.280 | 2.0056 | [36] |
| •DMPOdegr | 1.574 | - | 2.0058 | [17,36] |
| •DMPO-N3 | 1.427, 0.312 | 1.355 | 2.0059 | [36,41] |
| trans-•EMPO-O2−/OOH | 1.296 | 1.068 | 2.0058 | [42] |
| trans-•EMPO-OCH3 | 1.281 | 0.911 | 2.0059 | [43] |
| trans-•EMPO-OH | 1.291 | 1.179 | 2.0059 | [44] |
| •EMPO-CH3 | 1.433 | 2.088 | 2.0056 | [44] |
| •EMPO-N3 | 1.405, 0.224 | 1.242 | 2.0057 | [45] |

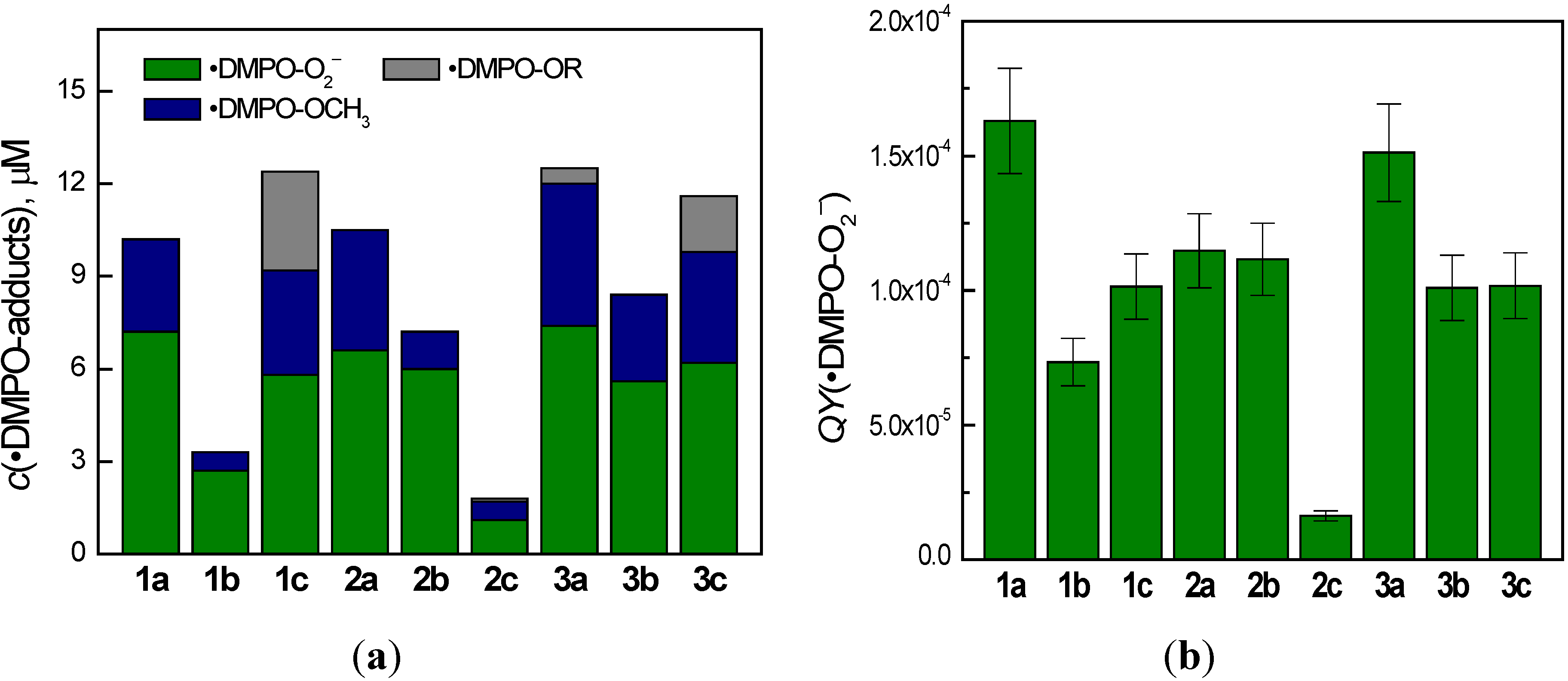


2.4. Photoinduced Processes of Quinoxalines in the Presence of Sterically Hindered Amines
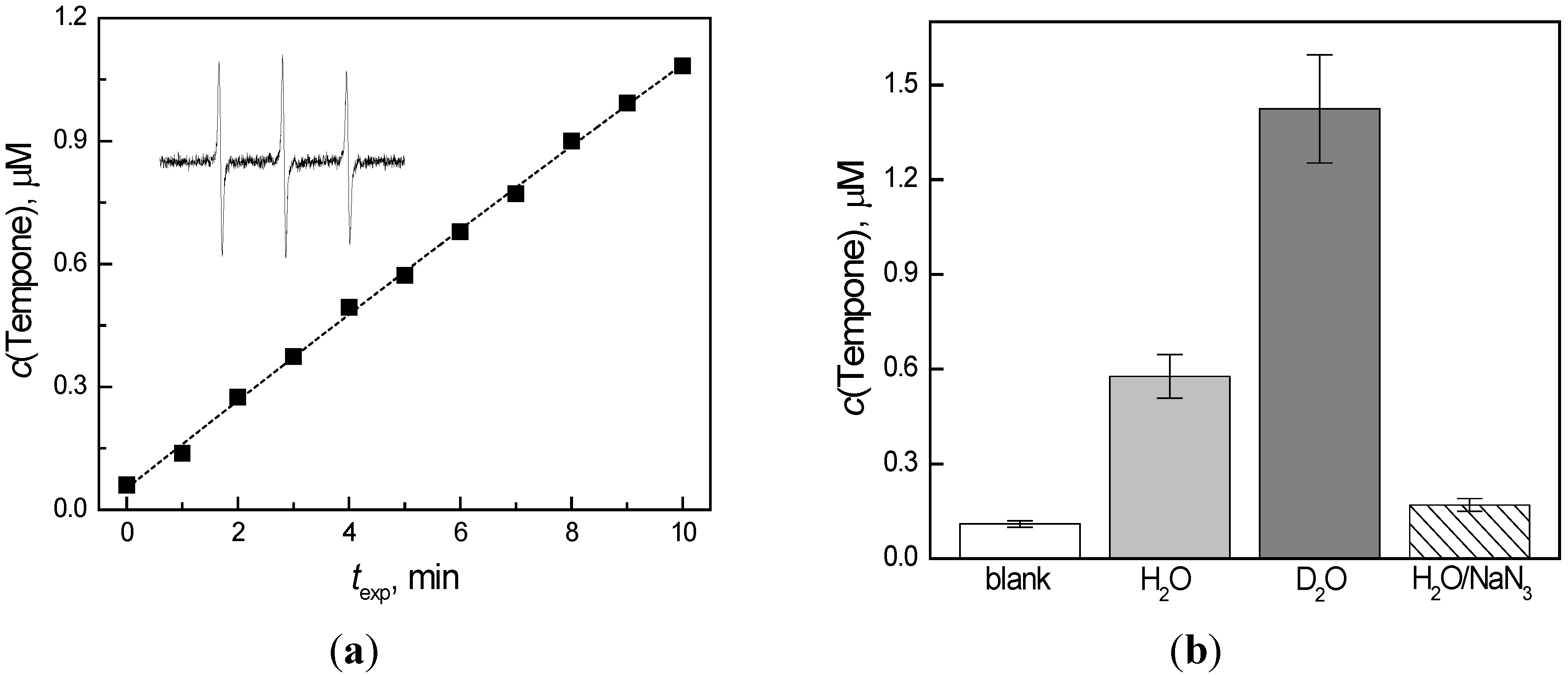
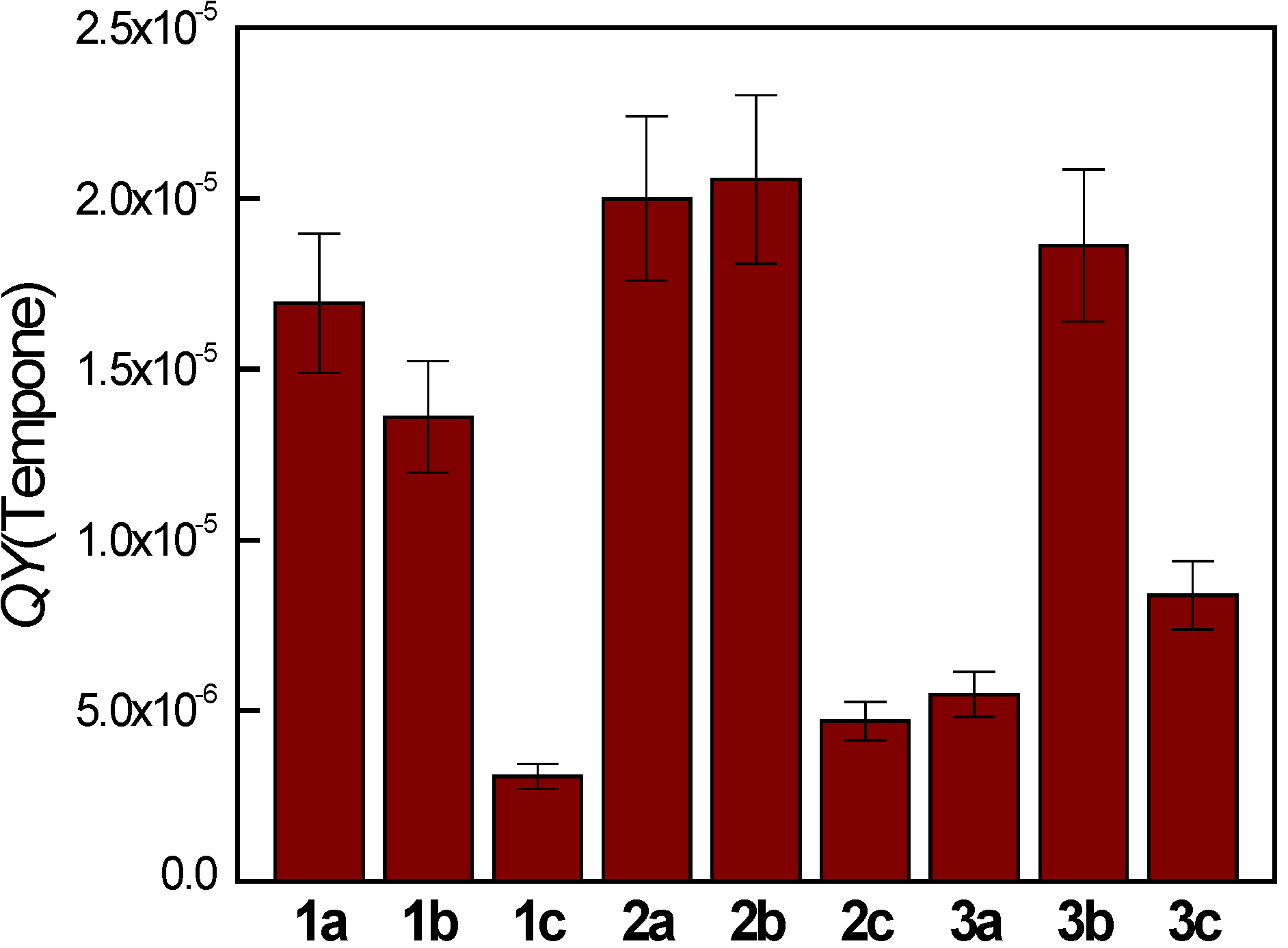
2.5. Quinoxaline Photoexcitation Monitored by UV/Vis Spectroscopy — Steady State Experiments

3. Experimental Section
4. Conclusions

Supplementary Material
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chowdhury, N.; Gangopadhyay, M.; Karthik, S.; Pradeep Singh, N.D.; Baidya, M.; Ghosh, S.K. Synthesis, photochemistry, DNA cleavage/binding and cytotoxic properties of fluorescent quinoxaline and quinoline hydroperoxides. J. Photochem. Photobiol. B Biol. 2014, 130, 188–198. [Google Scholar]
- Achelle, S.; Baudequin, C.; Plé, N. Luminescent materials incorporating pyrazine or quinoxaline moieties. Dyes Pigm. 2013, 98, 575–600. [Google Scholar]
- Li, S.; Li, A.; Yu, J.; Zhong, A.; Chen, S.; Tang, R.; Deng, X.; Qin, J.; Li, Q.; Li, Z. A new low-band gap polymer containing benzene-fused quinoxaline: Significantly enhanced performance caused by one additional benzene ring. Macromol. Rapid Comm. 2013, 34, 227–233. [Google Scholar]
- Chen, C.Y.; Hu, W.P.; Liu, M.C.; Yan, P.C.; Wang, J.J.; Chung, M.I. Efficient synthesis of quinoxalines with hypervalent iodine as a catalyst. Tetrahedron 2013, 69, 9735–9741. [Google Scholar]
- Burguete, A.; Pontiki, E.; Hadjipavlou-Litina, D.; Ancizu, S.; Villar, R.; Solano, B.; Moreno, E.; Torres, E.; Pérez, S.; Aldana, I.; et al. Synthesis and biological evaluation of new quinoxaline derivatives as antioxidant and anti-inflammatory agents. Chem. Biol. Drug Design 2011, 77, 255–267. [Google Scholar]
- Chandra Shekhar, A.; Shanthan Rao, P.; Narsaiah, B.; Allanki, A.D.; Sijwali, P.S. Emergence of pyrido quinoxalines as new family of antimalarial agents. Eur. J. Med. Chem. 2014, 77, 280–287. [Google Scholar]
- Gil, A.; Pabón, A.; Galiano, S.; Burguete, A.; Pérez-Silanes, S.; Deharo, E.; Monge, A.; Aldana, I. Synthesis, biological evaluation and structure-activity relationships of new quinoxaline derivatives as anti-plasmodium falciparum agents. Molecules 2014, 19, 2166–2180. [Google Scholar]
- Moreno, E.; Ancizu, S.; Pérez-Silanes, S.; Torres, E.; Aldana, I.; Monge, A. Synthesis and antimycobacterial activity of new quinoxaline-2-carboxamide 1,4-di-N-oxide derivatives. Eur. J. Med. Chem. 2010, 45, 4418–4426. [Google Scholar]
- Patidar, A.K.; Jeyakandan, M.; Mobiya, A.K.; Selvam, G. Exploring potential of quinoxaline moiety. Int. J. PharmTech Res. 2011, 3, 386–392. [Google Scholar]
- Jantová, S.; Letašiová, S.; Brezová, V.; Čipák, L.; Lábaj, J. Photochemical and phototoxic activity of berberine on murine fibroblast NIH-3T3 and Ehrlich ascites carcinoma cells. J. Photochem. Photobiol. B Biol. 2006, 85, 163–176. [Google Scholar] [CrossRef]
- Motten, A.; Martinez, L.; Holt, N.; Sik, R.; Reszka, K.; Chignell, C.; Tonnesen, H.; Roberts, J. Photophysical studies on antimalarial drugs. Photochem. Photobiol. 1999, 69, 282–287. [Google Scholar]
- Sandvik, S.; Bilski, P.; Martinez, L.; Sik, R.; Chignell, C. Photophysics of fluorinated quinolones. Photochem. Photobiol. 1999, 69, 83S–83S. [Google Scholar]
- Martinez, L.; Sik, R.; Chignell, C. Fluoroquinolone antimicrobials: Singlet oxygen, superoxide and phototoxicity. Photochem. Photobiol. 1998, 67, 399–403. [Google Scholar]
- Martinez, L.; Chignell, C. Photocleavage of DNA by the fluoroquinolone antibacterials. J. Photochem. Photobiol. B Biol. 1998, 45, 51–59. [Google Scholar]
- Lhiaubet-Vallet, V.; Bosca, F.; Miranda, M. Photosensitized DNA damage: The case of fluoroquinolones. Photochem. Photobiol. 2009, 85, 861–868. [Google Scholar]
- De Guidi, G.; Bracchitta, G.; Catalfo, A. Photosensitization reactions of fluoroquinolones and their biological consequences. Photochem. Photobiol. 2011, 87, 1214–1229. [Google Scholar]
- Barbieriková, Z.; Bella, M.; Kučerák, J.; Milata, V.; Jantová, S.; Dvoranová, D.; Veselá, M.; Staško, A.; Brezová, V. Photoinduced superoxide radical anion and singlet oxygen generation in the presence of novel selenadiazoloquinolones (an EPR study). Photochem. Photobiol. 2011, 87, 32–44. [Google Scholar]
- Jantová, S.; Koňariková, K.; Letašiová, S.; Paulovičová, E.; Milata, V.; Brezová, V. Photochemical and phototoxic properties of ethyl 1,4-dihydro-8-nitro-4-oxoquinoline-3-carboxylate, a new quinoline derivative. J. Photochem. Photobiol. B Biol. 2011, 102, 77–91. [Google Scholar]
- Kroon, R.; Gehlhaar, R.; Steckler, T.T.; Henriksson, P.; Müller, C.; Bergqvist, J.; Hadipour, A.; Heremans, P.; Andersson, M.R. New quinoxaline and pyridopyrazine-based polymers for solution-processable photovoltaics. Sol. Energy Mater. Sol. Cells 2012, 105, 280–286. [Google Scholar]
- Gers, C.F.; Nordmann, J.; Kumru, C.; Frank, W.; Müller, T.J.J. Solvatochromic fluorescent 2-substituted 3-ethynyl quinoxalines: Four-component synthesis, photophysical properties, and electronic structure. J. Org. Chem. 2014, 79, 3296–3310. [Google Scholar] [CrossRef]
- Abu-Sheaib, E.S.; Zahra, J.A.; El-Abadelah, M.M.; Boese, R. Heterocycles [h]-fused onto 4-oxoquinoline-3-carboxylic acid, part VI [1]. Synthesis and X-ray structure of model indolo[3,2-b]- and [2,3-b]pyrido[2,3-f]quinoxaline-3-carboxylic esters. Monatsh. Chem. 2008, 139, 1061–1066. [Google Scholar] [CrossRef]
- Abu-Sheaib, E.S.; Zahra, J.A.; El-Abadelah, M.M.; Voelter, W. Heterocycles [h]-fused onto 4-oxoquinoline-3-carboxylic acid, V [1]. Synthesis and antibacterial activity of some new 2,3-disubstituted 7-oxo-7,10-dihydropyrido[2,3-f]quinoxaline-8-carboxylic acids and esters. Z. Naturforsch. B 2008, 63, 555–563. [Google Scholar]
- Saloň, J.; Milata, V.; Prónayová, N.; Leško, J. Utilisation of 6-amino-2,3-dimethylquinoxaline for the synthesis of tricyclic pyridoquinoxalines via Gould-Jacobs reaction. Collect. Czechoslov. Chem. Commun. 2001, 66, 1691–1697. [Google Scholar]
- Saloň, J.; Milata, V.; Chudík, M.; Prónayová, N.; Leško, J.; Seman, M.; Belicová, A. Synthesis, properties, and reactions of 5-substituted derivatives of 2,3-diphenylquinoxaline. Monatsh. Chem. 2004, 135, 283–291. [Google Scholar]
- Wang, K.; Shi, W.; Jia, J.; Chen, S.; Ma, H. Characterization of 2-phenylbenzo[g]quinoxaline derivatives as viscosity-sensitive fluorescent probes. Talanta 2009, 77, 1795–1799. [Google Scholar] [CrossRef]
- Samadi-Maybodi, A.; Akhoondi, R. A highly efficient turn-on fluorescent sensor for determination of water in organic solvents. J. Fluoresc. 2012, 22, 1217–1222. [Google Scholar]
- Valeur, B. Molecular Fluorescence: Principles and Applications; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]
- Bevilaqua, T.; Goncalves, T.; Venturini, C.; Machado, V. Solute-solvent and solvent-solvent interactions in the preferential solvation of 4-[4-(dimethylamino)styryl]-1-methylpyridinium iodide in 24 binary solvent mixtures. Spectrochim. Acta Part A 2006, 65, 535–542. [Google Scholar]
- Denisov, G.S.; Golubev, N.S.; Schreiber, V.M.; Shajakhmedov, S.S.; Shurukhina, A.V. Effect of intermolecular hydrogen bonding and proton transfer on fluorescence of salicylic acid. J. Mol. Struct. 1997, 436–437, 153–160. [Google Scholar] [CrossRef]
- Barbieriková, Z.; Bella, M.; Sekeráková, L.; Lietava, J.; Bobeničová, M.; Dvoranová, D.; Milata, V.; Sádecká, J.; Topoľská, D.; Heizer, T.; et al. Spectroscopic characterization, photoinduced processes and cytotoxic properties of substituted N-ethyl selenadiazoloquinolones. J. Phys. Org. Chem. 2013, 26, 565–574. [Google Scholar]
- Barbieriková, Z.; Bella, M.; Lietava, J.; Dvoranová, D.; Staško, A.; Füzik, T.; Milata, V.; Jantová, S.; Brezová, V. Spectroscopic characterization and photoinduced processes of 4-oxoquinoline derivatives. J. Photochem. Photobiol. A Chem. 2011, 224, 123–134. [Google Scholar]
- Rimarčík, J.; Punyain, K.; Lukeš, V.; Klein, E.; Dvoranová, D.; Kelterer, A.M.; Milata, V.; Lietava, J.; Brezová, V. Theoretical and spectroscopic study of ethyl 1,4-dihydro-4-oxoquinoline-3- carboxylate and its 6-fluoro and 8-nitro derivatives in neutral and radical anion forms. J. Mol. Struct. 2011, 994, 61–69. [Google Scholar]
- Reszka, K.; Bilski, P.; Sik, R.; Chignell, C. Photosensitized generation of superoxide radical in aprotic-solvents—An EPR and spin-trapping study. Free Radic. Res. Comm. 1993, 19, S33–S44. [Google Scholar]
- Herscu-Kluska, R.; Masarwa, A.; Saphier, M.; Cohen, H.; Meyerstein, D. Mechanism of the reaction of radicals with peroxides and dimethyl sulfoxide in aqueous solution. Chem. Eur. J. 2008, 14, 5880–5889. [Google Scholar]
- Pieta, P.; Petr, A.; Kutner, W.; Dunsch, L. In situ ESR spectroscopic evidence of the spin-trapped superoxide radical, O2•−, electrochemically generated in DMSO at room temperature. Electrochim. Acta 2008, 53, 3412–3415. [Google Scholar]
- Buettner, G.R. Spin trapping: ESR parameters of spin adducts. Free Radic. Biol. Med. 1987, 3, 259–303. [Google Scholar]
- Dikalov, S.; Mason, R. Reassignment of organic peroxyl radical adducts. Free Radic. Biol. Med. 1999, 27, 864–872. [Google Scholar]
- Konaka, R.; Terabe, S.; Mizuta, T.; Sakata, S. Spin trapping by use of nitrosodurene and its derivatives. Can. J. Chem. 1982, 60, 1532–1542. [Google Scholar]
- Terabe, S.; Kuruma, K.; Konaka, R. Spin trapping by use of nitroso-compounds. Part VI. Nitrosodurene and other nitrosobenzene derivatives. J. Chem. Soc. Perkin Trans. 2 1973, 1252–1258. [Google Scholar] [CrossRef]
- Zalibera, M.; Rapta, P.; Staško, A.; Brindzová, L.; Brezová, V. Thermal generation of stable SO4̇•− spin trap adducts with super-hyperfine structure in their EPR spectra: An alternative EPR spin trapping assay for radical scavenging capacity determination in dimethylsulphoxide. Free Radic. Res. 2009, 43, 457–469. [Google Scholar]
- Barbieriková, Z.; Mihalíková, M.; Brezová, V. Photoinduced oxidation of sterically hindered amines in acetonitrile solutions and titania suspensions (an EPR study). Photochem. Photobiol. 2012, 88, 1442–1454. [Google Scholar]
- Olive, G.; Mercier, A.; le Moigne, F.; Rockenbauer, A.; Tordo, P. 2-Ethoxycarbonyl-2-methyl-3,4-dihydro-2H-pyrrole-1-oxide: Evaluation of the spin trapping properties. Free Radic. Biol. Med. 2000, 28, 403–408. [Google Scholar]
- Stolze, K.; Udilova, N.; Nohl, H. Spin adducts of superoxide, alkoxyl, and lipid-derived radicals with EMPO and its derivatives. Biol. Chem. 2002, 383, 813–820. [Google Scholar]
- Stolze, K.; Rohr-Udilova, N.; Rosenau, T.; Hofinger, A.; Kolarich, D.; Nohl, H. Spin trapping of C- and O-centered radicals with methyl-, ethyl-, pentyl-, and phenyl-substituted EMPO derivatives. Bioorg. Med. Chem. 2006, 14, 3368–3376. [Google Scholar] [CrossRef]
- Stolze, K.; Rohr-Udilova, N.; Rosenau, T.; Hofinger, A.; Nohl, H. Free radical trapping properties of several ethyl-substituted derivatives of 5-ethoxycarbonyl-5-methyl-1-pyrroline N-oxide (EMPO). Bioorg. Med. Chem. 2007, 15, 2827–2836. [Google Scholar] [CrossRef]
- Sawyer, D.T.; Valentine, J.S. How super is superoxide? Accounts Chem. Res. 1981, 14, 393–400. [Google Scholar] [CrossRef]
- Brezová, V.; Staško, A.; Biskupič, S.; Blažková, A.; Havlínová, B. Kinetics of hydroxyl radical spin trapping in photoactivated homogeneous (H2O2) and heterogeneous (TiO2, O2) aqueous systems. J. Phys. Chem. 1994, 98, 8977–8984. [Google Scholar]
- Abellan, M.; Dillert, R.; Gimenez, J.; Bahnemann, D. Evaluation of two types of TiO2-based catalysts by photodegradation of DMSO in aqueous suspension. J. Photochem. Photobiol. A Chem. 2009, 202, 164–171. [Google Scholar]
- Mitroka, S.; Zimmeck, S.; Troya, D.; Tanko, J. How solvent modulates hydroxyl radical reactivity in hydrogen atom abstractions. J. Am. Chem. Soc. 2010, 132, 2907–2913. [Google Scholar]
- Halliwel, B.; Gutteridge, J. Free Radicals in Biology and Medicine, 3rd ed.; Oxford University Press: Oxford, UK, 1999; p. 386. [Google Scholar]
- Nakamura, K.; Ishiyama, K.; Ikai, H.; Kanno, T.; Sasaki, K.; Niwano, Y.; Kohno, M. Reevaluation of analytical methods for photogenerated singlet oxygen. J. Clin. Biochem. Nutr. 2011, 49, 87–95. [Google Scholar]
- Wu, H.; Song, Q.; Ran, G.; Lu, X.; Xu, B. Recent developments in the detection of singlet oxygen with molecular spectroscopic methods. TrAC Trend. Anal. Chem. 2011, 30, 133–141. [Google Scholar]
- Jenny, T.A.; Turro, N.J. Solvent and deuterium isotope effects on the lifetime of singlet oxygen determined by direct emission spectroscopy at 1.27 μM. Tetrahedron Lett. 1982, 23, 2923–2926. [Google Scholar] [CrossRef]
- Wilkinson, F.; Helman, W.; Ross, A. Rate constants for the decay and reactions of the lowest electronically excited singlet-state of molecular-oxygen in solution-An expanded and revised compilation. J. Phys. Chem. Ref. Data 1995, 24, 663–1021. [Google Scholar]
- Li, M.; Cline, C.; Koker, E.; Carmichael, H.; Chignell, C.; Bilski, P. Quenching of singlet molecular oxygen (1O2) by azide anion in solvent mixtures. Photochem. Photobiol. 2001, 74, 760–764. [Google Scholar]
- Bella, M.; Milata, V. Application of 9-ethyl[1,2,5]selenadiazolo[3,4-h]quinolones in the synthesis of tricyclicazoloquinolones. Tetrahedron 2014, 70, 4814–4819. [Google Scholar] [CrossRef]
- Alberti, A.; Macciantelli, D. Spin trapping. In Electron Paramagnetic Resonance: A Practitioner’s Toolkit; Brustolon, M., Giamelo, E., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 287–323. [Google Scholar]
- Duling, D.R. Simulation of multiple isotropic spin-trap EPR spectra. In J. Magn. Reson. B.; 1994; 104, pp. 105–110. Available online: http://www.niehs.nih.gov/research/resources/software/tox-pharm/tools/ (accessed on 11 August 2014). [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision A.1; Gaussian, Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cancès, E. The IEF version of the PCM solvation method: An overview of a new method addressed to study molecular solutes at the QM ab initio level. J. Mol. Struct. Theochem. 1999, 464, 211–226. [Google Scholar] [CrossRef]
- Furche, F.; Ahlrichs, R. Adiabatic time-dependent density functional methods for excited state properties. J. Chem. Phys. 2002, 117, 7433–7447. [Google Scholar]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Barbieriková, Z.; Dvoranová, D.; Bella, M.; Milata, V.; Czímerová, A.; Brezová, V. Fused-Ring Derivatives of Quinoxalines: Spectroscopic Characterization and Photoinduced Processes Investigated by EPR Spin Trapping Technique. Molecules 2014, 19, 12078-12098. https://doi.org/10.3390/molecules190812078
Barbieriková Z, Dvoranová D, Bella M, Milata V, Czímerová A, Brezová V. Fused-Ring Derivatives of Quinoxalines: Spectroscopic Characterization and Photoinduced Processes Investigated by EPR Spin Trapping Technique. Molecules. 2014; 19(8):12078-12098. https://doi.org/10.3390/molecules190812078
Chicago/Turabian StyleBarbieriková, Zuzana, Dana Dvoranová, Maroš Bella, Viktor Milata, Adriana Czímerová, and Vlasta Brezová. 2014. "Fused-Ring Derivatives of Quinoxalines: Spectroscopic Characterization and Photoinduced Processes Investigated by EPR Spin Trapping Technique" Molecules 19, no. 8: 12078-12098. https://doi.org/10.3390/molecules190812078