Homoconjugation vs. Exciton Coupling in Chiral α,β-Unsaturated Bicyclo[3.3.1]nonane Dinitrile and Carboxylic Acids
Abstract
:1. Introduction
2. Results and Discussion

| Compound | Conformer a | ΔG, kcal/mol | Distribution,% | Angle (C=C-C=O), deg. (Symmetry) |
|---|---|---|---|---|
| 4 | trans-trans | 0.00 | 69.4 | 178.6 ( C2) |
| cis-trans | 0.53 | 28.0 | −0.3, 178.6 ( C1) | |
| cis-cis | 1.93 | 2.6 | −0.9 ( C2) | |
| 6 | trans-chair | 0.00 | 77.7 | 179.5 ( C1) |
| cis-chair | 0.78 | 20.4 | −0.6 ( C1) | |
| trans-boat | 2.26 | 1.6 | −179.4 ( C1) | |
| cis-boat | 3.14 | 0.3 | 0 ( C1) |
 ) in MeCN, diacid (+)-4 (
) in MeCN, diacid (+)-4 (  ) and ketoacid(+)-6 (solid) in EtOH.
) and ketoacid(+)-6 (solid) in EtOH.
 ) in MeCN, diacid (+)-4 (
) in MeCN, diacid (+)-4 (  ) and ketoacid(+)-6 (solid) in EtOH.
) and ketoacid(+)-6 (solid) in EtOH.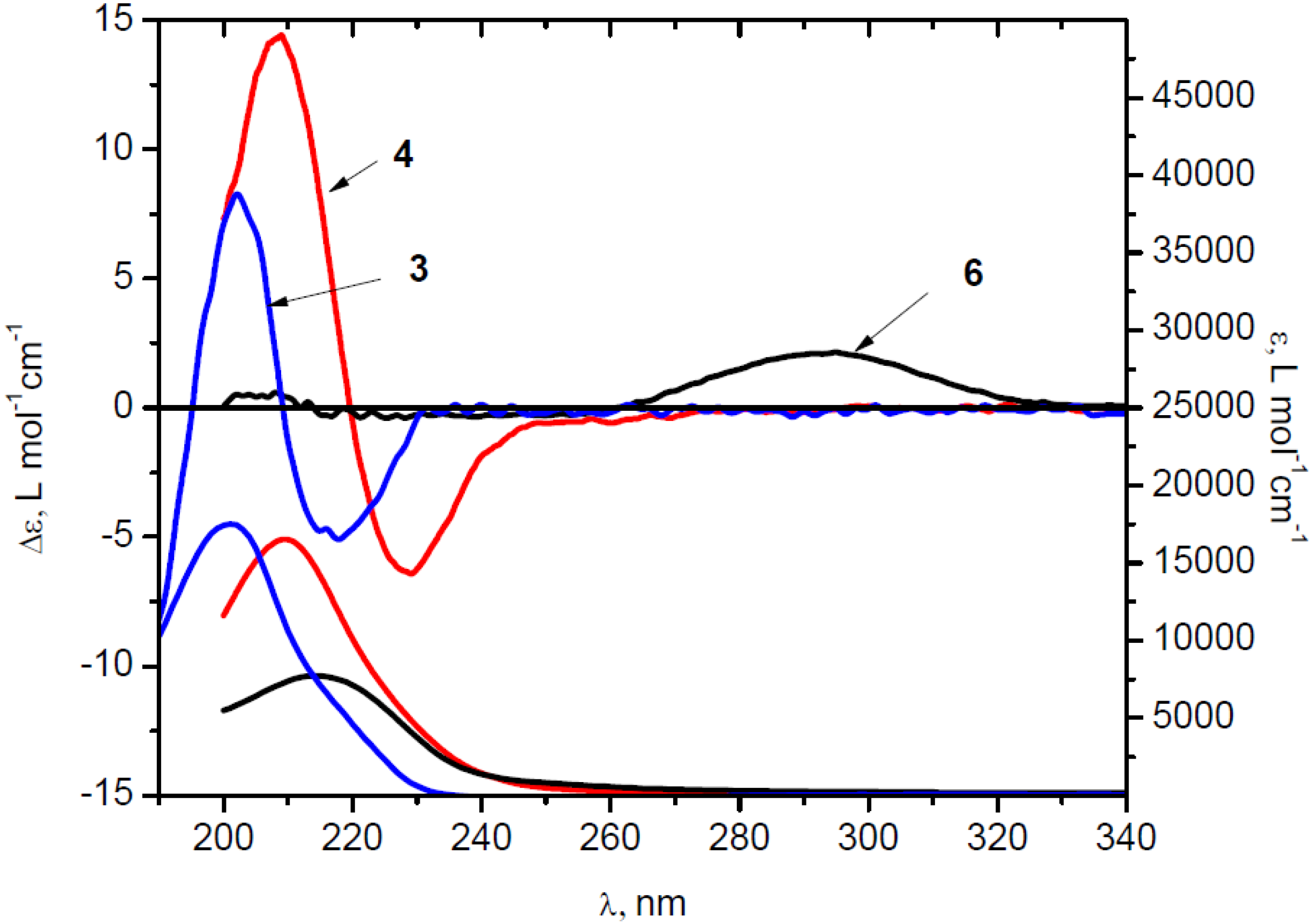
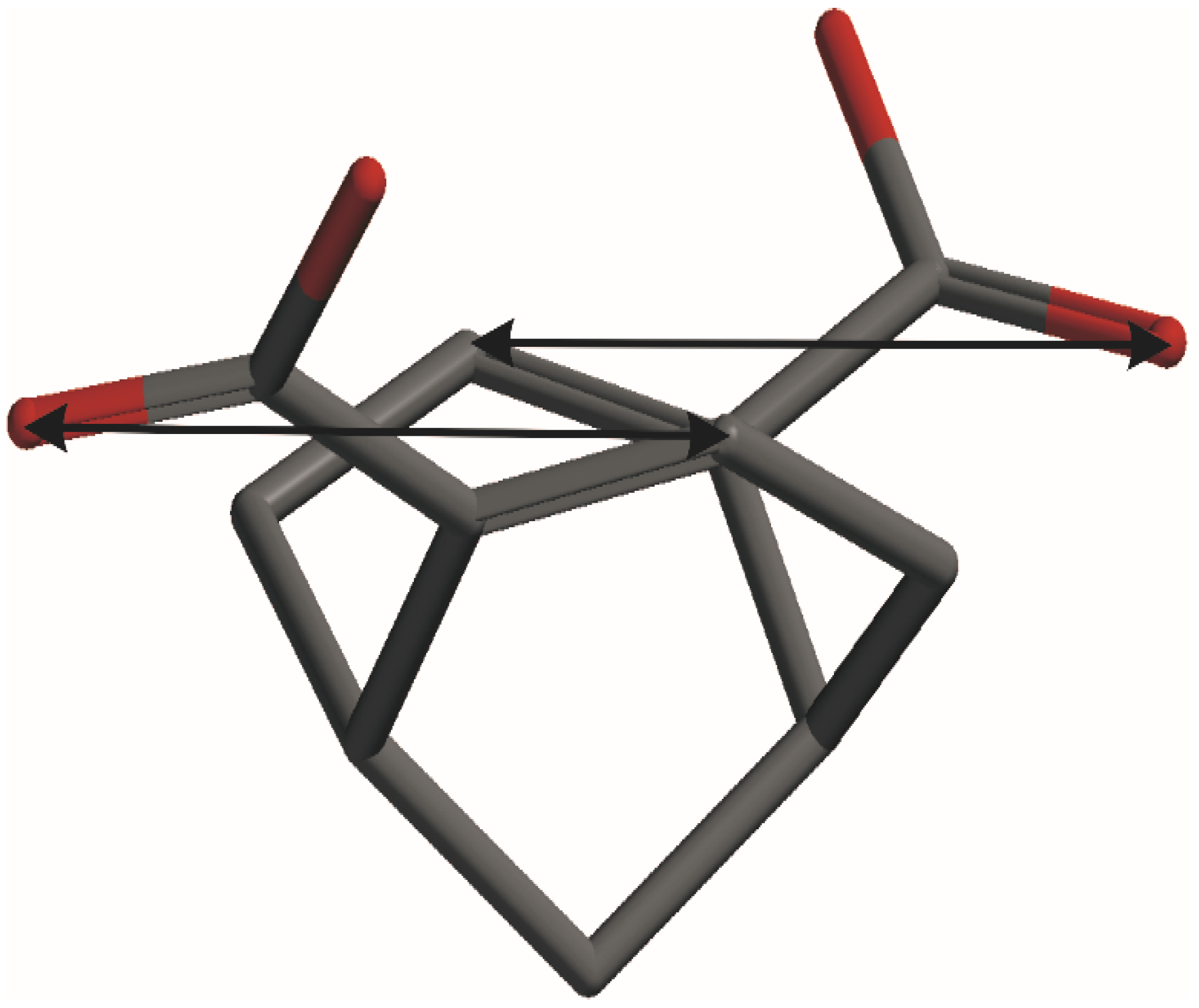
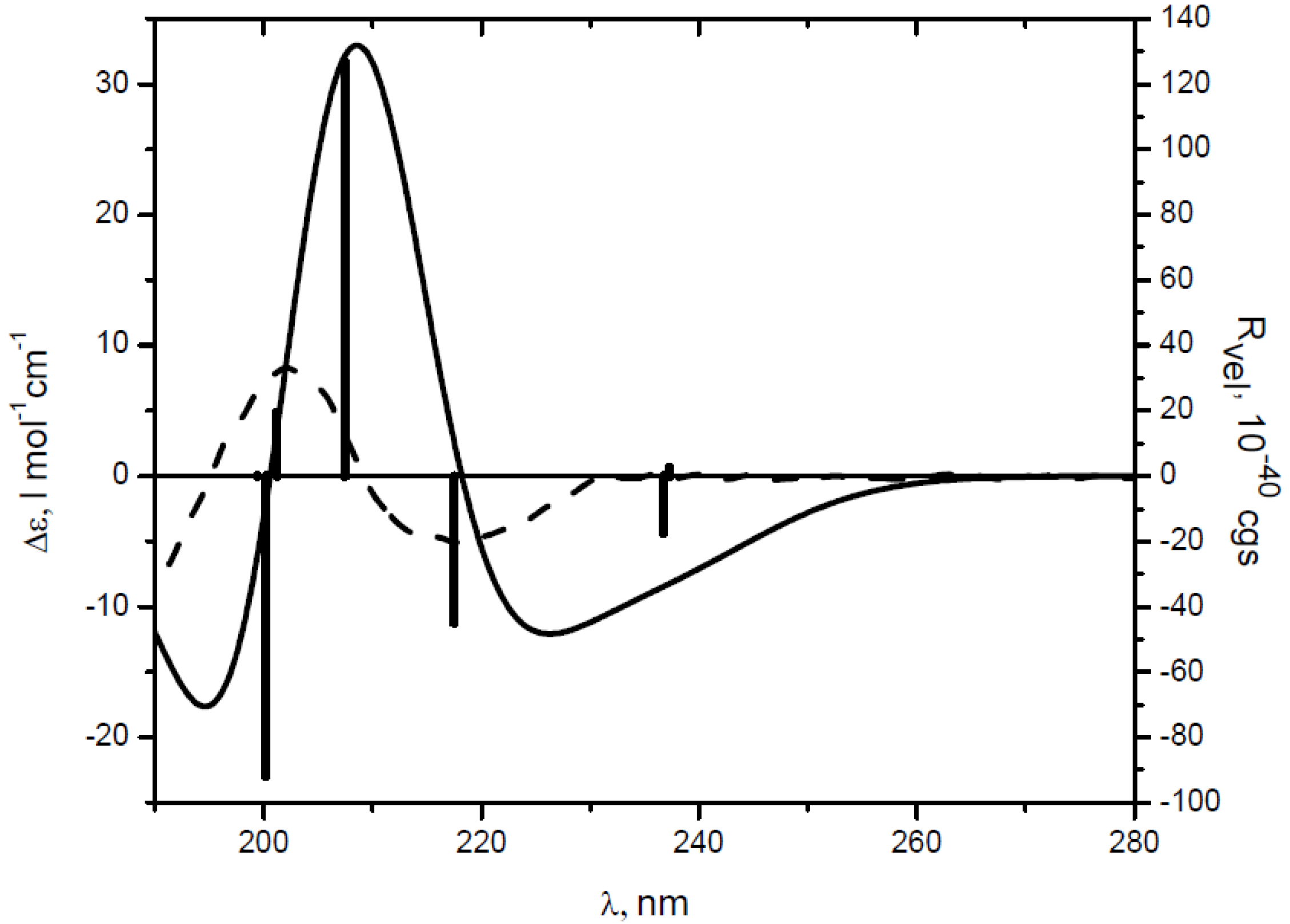
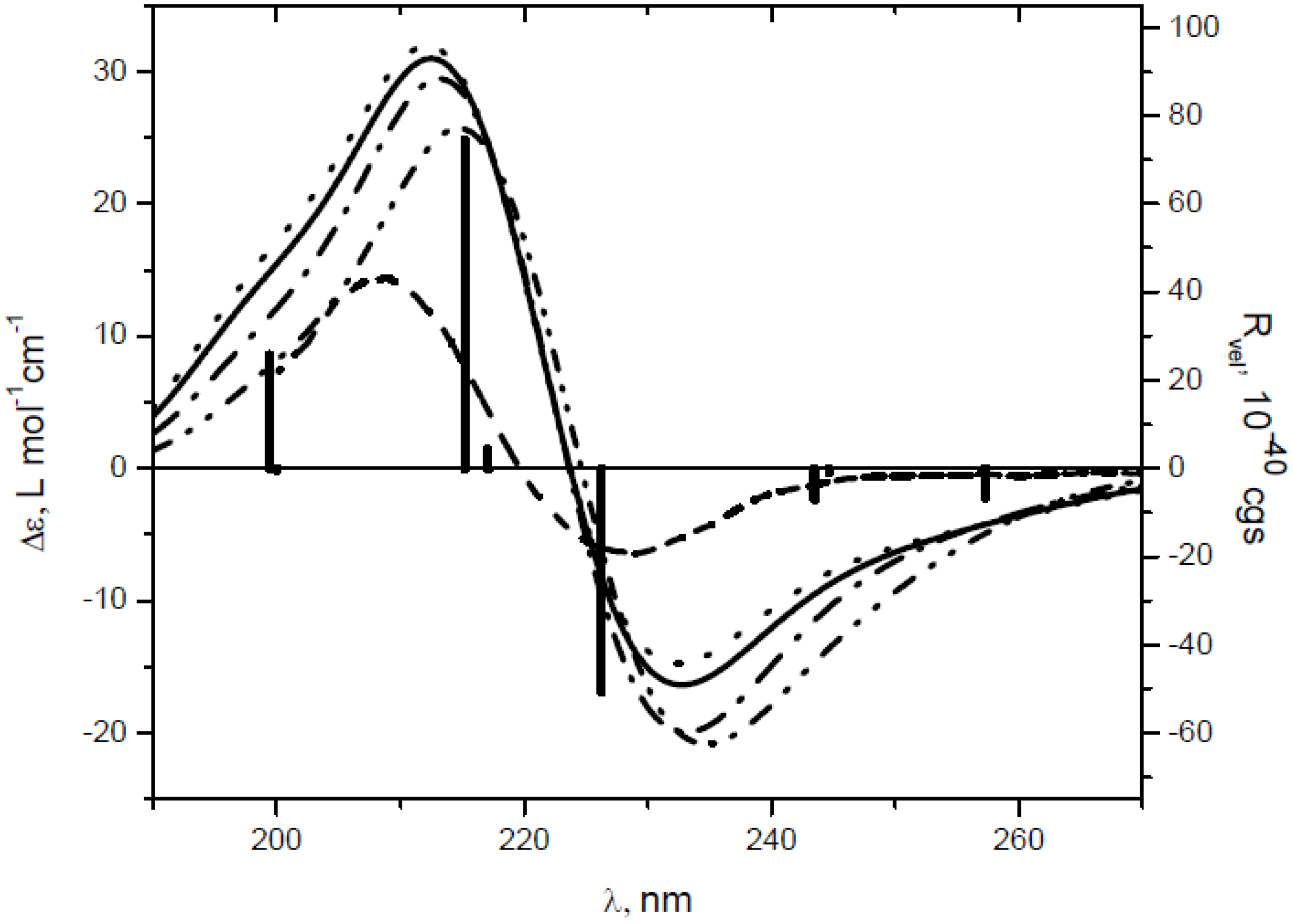
| Comp. | λ, nm (Δε) a | Transition | λ, nm | fb | Rvel c | Contributions d |
|---|---|---|---|---|---|---|
| 3 | 218 (−5.16) | 3 | 217.5 | 0.0015 | −44.91 | 45–47 (61%), 44–46 (34%) |
| 202 (8.46) | 4 | 207.5 | 0.501 | 126.42 | 44–47 (48%), 45–46 (33%) | |
| 4 | 229 (−6.41) | 5 | 226.2 | 0.0019 | −50.62 | 54–57 (66%), 55–56 (29%), |
| 209 (14.4) | 8 | 215.2 | 0.530 | 74.32 | 55–57 (45%), 54–56 (45%) |

3. Experimental Section
3.1. General Information
3.2. Theoretical Calculations
3.3. Preparation of Compounds 3–6
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Finch, N.; Taylor, W.I.; Emerson, T.R.; Klyne, W.; Swan, R.J. Optical rotatory dispersion curves of heteroyohimbine alkaloids. Tetrahedron 1966, 22, 1327–1333. [Google Scholar] [CrossRef]
- Campbell, M.M.; Sainsbury, M.; Searle, P.A. The biosynthesis and synthesis of shikimic acid, chorismic acid, and related compounds. Synthesis 1993, 179–193. [Google Scholar] [CrossRef]
- Pawlak, J.L.; Berchtold, G.A. Synthesis of disodium 3-[(1-carboxylatoethenyl)oxy]cyclohepta-1,6-diene-1-carboxylate: A seven-membered ring analog of chorismate. J. Org. Chem. 1988, 53, 4063–4069. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, A.; Ye, Z.G.; Lin, J.; Xu, L.Z.; Yang, S.L. New approach to the total synthesis of (−)-zeylenone from shikimic acid. Chem. Pharm. Bull. 2006, 54, 1459–1461. [Google Scholar] [CrossRef]
- Jiang, S.; Singh, G. Chemical synthesis of shikimic acid and its analogues. Tetrahedron 1998, 54, 4697–4753. [Google Scholar] [CrossRef]
- Eliel, E.L.; Wilen, S.H. Stereochemistry of Organic Compounds; Wiley: New York, NY, USA, 1994; pp. 991–1118. [Google Scholar]
- Karabencheva-Christova, T.G.; Carlsson, U.; Balali-Mood, K.; Black, G.W.; Christov, C.Z. Conformational Effects on the Circular Dichroism of Human Carbonic Anhydrase II: A Multilevel Computational Study. PLoS One 2013, 8, e56874. [Google Scholar]
- Kurtán, T.; Jia, R.; Li, Y.; Pescitelli, G.; Guo, Y.W. Absolute configuration of highly flexible natural products by the solid-state ECD/TDDFT method: Ximaolides and sinulaparvalides. Eur. J. Org. Chem. 2012, 6722–6728. [Google Scholar]
- Circular Dichroism: Principles and Applications, 2nd ed.; Berova, N.; Nakanishi, K.; Woody, R.W. (Eds.) John Wiley and Sons: New York, NY, USA, 2000.
- Berova, N.; di Bari, L.; Pescitelli, G. Application of electronic circular dichroism in configurational and conformational analysis of organic compounds. Chem. Soc. Rev. 2007, 36, 914–931. [Google Scholar] [CrossRef]
- Orentas, E.; Bagdžiūnas, G.; Berg, U.; Žilinskas, A.; Butkus, E. Enantiospecific synthesis and chiroptical properties of bicyclic enones. Eur. J. Org. Chem. 2007, 25, 4251–4256. [Google Scholar]
- Gawronski, J.K. Conformations, Chiroptical and Related Spectral Properties of Enones. In The Chemistry of Enones; Patai, S., Rappoport, Z., Eds.; John Wiley and Sons: New York, NY, USA, 1989; pp. 55–105. [Google Scholar]
- Frelek, J.; Szczepek, W.J.; Weiss, H.P.; Reiss, G.J.; Frank, W.; Brechtel, J.; Schultheis, B.; Kuball, H.G. Chiroptical properties of the cisoid enone chromophore. J. Am. Chem. Soc. 1998, 120, 7010–7019. [Google Scholar] [CrossRef]
- Stephens, P.J.; McCann, D.M.; Butkus, E.; Stončius, S.; Cheeseman, J.R.; Frisch, M.J. Determination of absolute configuration using concerted ab initio DFT calculations of electronic circular dichroism and optical rotation: Bicyclo[3.3.1]nonane diones. J. Org. Chem. 2004, 69, 1948–1958. [Google Scholar]
- Berg, U.; Butkus, E. An analysis of the circular dichroism spectra of bicyclo[3.3.1]nonane diones. J. Chem. Res.(S) 1994, 356–357. [Google Scholar]
- Stončius, S.; Berg, U.; Butkus, E. Chiral bicyclic keto lactones: Determination of the absolute configuration by the study of chiroptical properties and chemical correlation. Tetrahedron Asymmetry 2004, 15, 2405–2413. [Google Scholar] [CrossRef]
- Quast, H.; Görlach, Y.; Stawitz, J.; Peters, E.-M.; Peters, K.; Schnering, H.G.V. Synthese und struktur von 2,6-dicyanbicyclo[3.3.1]nona-2,6-dienen und 2,6-dicyanbarbaralanen. Chem. Berichte 1984, 117, 2745–2760. [Google Scholar] [CrossRef]
- Saravanan, P.; Anand, R.V.; Singh, V.K. Cu(OTf)2 catalyzed trimethylsilyl cyanide addition to carbonyl compounds. Tetrahedron Lett. 1998, 39, 3823–3824. [Google Scholar] [CrossRef]
- Vitnik, V.D.; Ivanović, M.D.; Vitnik, Ž.J; Đorđević, J.B; Žižak, Ž.D; Juranić, I.O. One-step conversion of ketones to conjugated acids using bromoform. Synth.Commun. 2009, 39, 1457–1471. [Google Scholar] [CrossRef]
- Quast, H.; Becker, C.; Witzel, M.; Peters, E.-M.; Peters, K.; von Schnering, H.G. Syntheses and structures of 2,6-substituted barbaralanes. Liebigs Ann. Chem. 1996, 985–997. [Google Scholar]
- Kasha, M.; Rawls, H.R.; El-Bayoumi, M.A. The exciton model in molecular spectroscopy. Pure Appl. Chem. 1965, 11, 371–392. [Google Scholar]
- Person, R.V.; Monde, K.; Humpf, H.-U.; Berova, N.; Nakanishi, K. A new approach in exciton-coupled circular dichroism (ECCD)-insertion of an auxiliary stereogenic center. Chirality 1995, 7, 128–135. [Google Scholar] [CrossRef]
- Boiadjiev, S.E.; Lightner, D.A. Exciton chirality. (A) Origins of and (B) applications from strongly fluorescent dipyrrinone chromophores. Monatsch. Chem. 2005, 136, 489–508. [Google Scholar] [CrossRef]
- Vlahov, I.R.; Bazin, H.G.; Linhardt, R.J. Circular dichroism and absolute conformation of Δ4-uronate derivatives: A semi-empirical rule for inherent chiral α-alkoxy acrylates. Chem. Commun. 1998, 1819–1820. [Google Scholar] [CrossRef]
- Hoffmann, R. Interaction of orbitals through space and through bonds. Acc. Chem. Res. 1971, 4, 1–9. [Google Scholar] [CrossRef]
- Hoffmann, R.; Heilbronner, E.; Gleiter, R. Interaction of nonconjugated double bonds. J. Am. Chem. Soc. 1970, 92, 706–707. [Google Scholar] [CrossRef]
- Lightner, D.A.; Gawronski, J.K.; Bouman, T.D. Electronic structure of symmetric homoconjugated dienes. Circular dichroism of (1S)-2-deuterio- and 2-methylnorbornadiene and (1S)-2-deuterio- and 2-methylbicyclo[2.2.2]octadiene. J. Am. Chem. Soc. 1980, 102, 5749–5754. [Google Scholar] [CrossRef]
- Lightner, D.A.; Gawronski, J.K.; Hansen, A.E.; Bouman, T.D. Charge-transfer band of 7-norbornenone. Circular dichroism of (1R)-2-deuteriobicyclo[2.2.1]hept-2-en-7-one and (1R)-2-methylbicyclo[2.2.1]hept-2-en-7-one. J. Am. Chem. Soc. 1981, 103, 4291–4296. [Google Scholar]
- Lightner, D.A.; Paquette, L.A.; Chayangkoon, P.; Lin, H.S.; Peterson, J.R. Synthesis and chiroptical properties of (1S)-[2,5-2H2]bicyclo[2.2.2]octa-2,5,7-triene (2,5-dideuteriobarrelene). J. Org. Chem. 1988, 53, 1969–1973. [Google Scholar] [CrossRef]
- Doerner, T.; Gleiter, R.; Robbins, T.A.; Chayangkoon, P.; Lightner, D.A. Homoconjugation and transannular orbital interactions detected by photoelectron and carbon-13 NMR spectroscopy. Bicyclo[3.3.1]nona-3,7-diene-2,6-dione and bicyclo[3.3.1]nonane-2,6-dione. J. Am. Chem. Soc. 1992, 114, 3235–3241. [Google Scholar] [CrossRef]
- Robbins, T.A.; Toan, V.V.; Givens, J.W., III; Lightner, D.A. Transannular orbital interaction between ketone and olefin chromophores detected by circular dichroism and carbon-13 NMR spectroscopy. Dimethanonaphthalenones and trimethanoanthracenones. J. Am. Chem. Soc. 1992, 114, 10799–10810. [Google Scholar] [CrossRef]
- Di Bari, L.; Guillarme, S.; Hermitage, S.; Jay, D.A.; Pescitelli, G.; Whiting, A. Absolute stereochemistry assignment of N-phosphorylimine-derived aza-Diels-Alder adducts with TDDFT CD calculations. Chirality 2005, 17, 323–331. [Google Scholar] [CrossRef]
- Salvadori, P.; Rosini, C.; di Bari, L. Conformation and Chiroptical Properties of Dienes and Polyenes. In The Chemistry of Dienes and Polyenes; Rappoport, Z., Ed.; John Wiley and Sons: New York, NY, USA, 1997; Volume 1, pp. 111–147. [Google Scholar]
- Charney, E.; Ziffer, H.; Weiss, U. Optical activity of non-planar conjugated dienes-II transoid dienes. Tetrahedron 1965, 21, 3121–3126. [Google Scholar] [CrossRef]
- Rauk, A.; Peoples, H.A. The electronic structure and optical activity of conjugated dienes: 1,3-butadiene and α- and β-phellandrene. J. Comp. Chem. 1980, 1, 240–256. [Google Scholar] [CrossRef]
- Wagniere, G.; Hug, W. Polarization and the sign of the long-wavelength Cotton-effects in chromophores of symmetry C2. Tetrahedron Lett. 1970, 11, 4765–4768. [Google Scholar] [CrossRef]
- Hug, W.; Wagniere, G. The optical activity of chromophores of symmetry C2. Tetrahedron 1972, 28, 1241–1248. [Google Scholar] [CrossRef]
- Buss, V.; Kolster, K. Electronic structure calculations on helicenes. Concerning the chirality of helically twisted aromatic systems. Chem. Phys. 1996, 203, 309–316. [Google Scholar] [CrossRef]
- Harada, N.; Uda, H.; Nozoe, T.; Okamoto, Y.; Wakabayashi, H.; Ishikawa, S. Absolute stereostructure of novel chiral troponoid spiro compounds as determined by theoretical calculation of CD spectra. J. Am. Chem. Soc. 1987, 109, 1661–1665. [Google Scholar]
- Wallentin, C.J.; Orentas, E.; Butkus, E.; Warnmark, K. Baker’s yeast for sweet dough enables large-scale synthesis of enantiomerically pure bicyclo[3.3.1]nonane-2,6-dione. Synthesis 2009, 864–867. [Google Scholar]
- SPARTAN’10 for Windows, Version 1.0.1.; Wavefunction, Inc.: 1840 Von Karman Avenue, Suite 370, Irvine, CA, USA, 2010.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.02, Gaussian, Inc.: Wallingford, CT, USA, 2009.
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: Quantifying the comparison of calculated and experimental electronic circular dichroism spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 3, 4, 6 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagdžiūnas, G.; Butkus, E.; Stončius, S. Homoconjugation vs. Exciton Coupling in Chiral α,β-Unsaturated Bicyclo[3.3.1]nonane Dinitrile and Carboxylic Acids. Molecules 2014, 19, 9893-9906. https://doi.org/10.3390/molecules19079893
Bagdžiūnas G, Butkus E, Stončius S. Homoconjugation vs. Exciton Coupling in Chiral α,β-Unsaturated Bicyclo[3.3.1]nonane Dinitrile and Carboxylic Acids. Molecules. 2014; 19(7):9893-9906. https://doi.org/10.3390/molecules19079893
Chicago/Turabian StyleBagdžiūnas, Gintautas, Eugenijus Butkus, and Sigitas Stončius. 2014. "Homoconjugation vs. Exciton Coupling in Chiral α,β-Unsaturated Bicyclo[3.3.1]nonane Dinitrile and Carboxylic Acids" Molecules 19, no. 7: 9893-9906. https://doi.org/10.3390/molecules19079893




