Novel Cinnamic Acid Derivatives as Antioxidant and Anticancer Agents: Design, Synthesis and Modeling Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry

| No | Z | Y | Formula * | Rf | Clog P ** | RM # (±SD) | Mp °C | Yield% |
|---|---|---|---|---|---|---|---|---|
| 1i |  |  | C16H11NO2 | 0.74 a | 3.20 | −0.895 (0.004) e | 155–157 | 60 |
| 1ii |  | H | C10H7NO2 | 0.48 a | 1.61 | −0.972 (0.032) e | 258–260 | 67 |
| 2i |  |  | C17H13NO4 | 0.39 b | 3.58 | −0.706 (0.062) e | 163–165 | 77 |
| 2ii |  | H | C11H9NO4 | 0.52 b | 2.00 | −0.868 (0.019) e | 170–172 | 55 |
| 3i |  |  | C17H14O2 | 0.88 b | 3.84 | 0.987 (0.018) e | 145–147 | 12 |
| 3ii |  | H | C11H10O2 | 0.62 b | 2.25 | −0.893 (0.002) e | 163–165 | 9 |
| 4i |  |  | C17H13BrO2 | 0.85 c | 4.09 | 0.566 (0.001) e | 135–136 | 33 |
| 4ii |  | H | C11H9BrO2 | 0.89 d | 2.51 | −0.847 (0.044) e | 169–171 | 3 |
2.2. Physicochemical Studies
2.3. Biological Assays
| Compd. | LOX a IC50 (μM) (±SD) b | RA % 0.05 mM (±SD) b | RA % 0.1 mM (±SD) b | ||
|---|---|---|---|---|---|
| 20 min | 60 min | 20 min | 60 min | ||
| 1i | 60 ± 0.4 | 12 ± 0.3 | 14 ± 0.1 | 28 ± 0.6 | 20 ± 0.8 |
| 1ii | 27% ± 0.8 (0.01 mM) | 13 ± 0.3 | 9 ± 0.03 | 23 ± 0.5 | 21 ± 1.0 |
| 2i | 66.5 ± 1.2 | 10 ± 0.04 | 11 ± 0.02 | 23 ± 0.7 | 23 ± 0.9 |
| 2ii | 31% ± 0.7 (0.01 mM) | 11 ± 0.0.2 | 13 ± 0.04 | 29 ± 1.0 | 24 ± 0.6 |
| 3i | 74 ± 1.7 | 4 ± 0.02 | 3 ± 0.0 | 5 ± 0.0 | 6 ± 0.04 |
| 3ii | 24% ± 0.4 (0.01 mM) | 11 ± 0.06 | 7 ± 0.0 | 20 ± 0.2 | 16 ± 0.05 |
| 4i | 74 ± 1.8 | 7 ± 0.03 | 4 ± 0.01 | 8 ± 0.03 | 5 ± 0.0 |
| 4ii | 10 ± 0.05 | 17 ± 0.02 | 13 ± 0.05 | 30 ± 0.9 | 29 ± 0.6 |
| NDGA | 28 ± 0.4 | 85 ± 1.6 | 83 ± 1.1 | 81 ± 0.8 | 83 ± 0.7 |
| Compd. | HO (%) 0.1 mM (±SD) a | O2−··(%) 0.1 mM (±SD) a | ABTS+·(%) 0.1 mM (±SD) a | AAPH (%) 0.1 mM (±SD) a |
|---|---|---|---|---|
| 1i | no | 75 ± 1.0 | 26 ± 0.4 | 92 ± 1.2 |
| 1ii | no | 50 ± 0.5 | 47 ± 0.8 | 20 ± 0.1 |
| 2i | 33 ± 0.8 | no | 26 ± 0.5 | 89 ± 0.9 |
| 2ii | no | no | 35 ± 0.9 | 29 ± 0.7 |
| 3i | 81 ± 1.9 | no | 26 ± 0.8 | 84 ± 1.4 |
| 3ii | 81 ± 1.2 | no | 31 ± 1.1 | 30 ± 1.0 |
| 4i | no | no | 24 ± 0.5 | 88 ± 1.8 |
| 4ii | 100 ± 1.8 | no | 26 ± 0.4 | 63 ± 1.1 |
| Trolox | 73 ± 1.0 | - | 88 ± 1.7 | 63 ± 1.3 |
| Caffeic acid | - | 46 ± 0.5 | - | - |
| Ascorbic Acid | - | - | 96 ± 0.9 | - |
| Compd. | IC50 HT-29 (μM) | IC50 A-549 (μM) | IC50 OAW-42 (μM) | IC50 MDA-MB-231 (μM) | IC50 HeLa (μM) | IC50 MRC-5 (μM) |
|---|---|---|---|---|---|---|
| 1i | >240 | >240 | >240 | >240 | >240 | 222 ± 1.3 |
| 1ii | >>240 | >>240 | >>240 | >>240 | >>240 | >>240 |
| 2i | 141 ± 1.8 | 134 ± 1.6 | 160 ± 1.1 | 199 ± 1.9 | 117.5 ± 1.4 | 96.3 ± 1.1 |
| 2ii | >>240 | >>240 | >>240 | >>240 | 240 ± 1.6 | 240 ± 1.0 |
| 3i | >240 | >240 | >240 | >240 | >240 | >240 |
| 3ii | >>240 | >>240 | >>240 | >>240 | >>240 | 240 ± 1.8 |
| 4i | >240 | 210 ± 1.0 | 174 ± 1.8 | 250 ± 1.2 | 80 ± 0.9 | 92 ± 1.3 |
| 4ii | 54 ± 1.1 | 173.5 ± 1.4 | 63.5 ± 0.8 | 47.5 ± 0.7 | 30 ± 0.4 | 24 ± 0.6 |
2.4. Computational Studies
2.4.1. Computational Methods, Docking Simulations
2.4.2. Molecular Docking Studies on Lipoxygenase
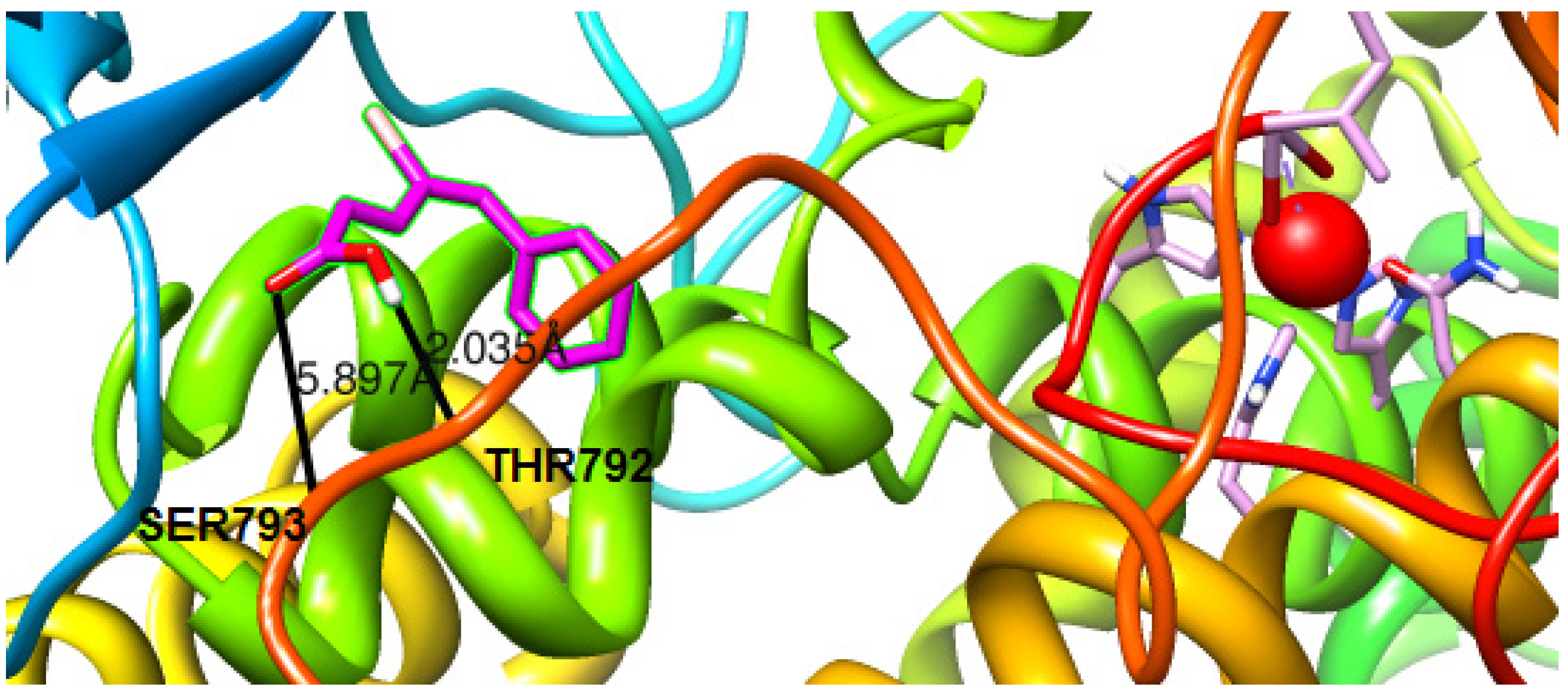
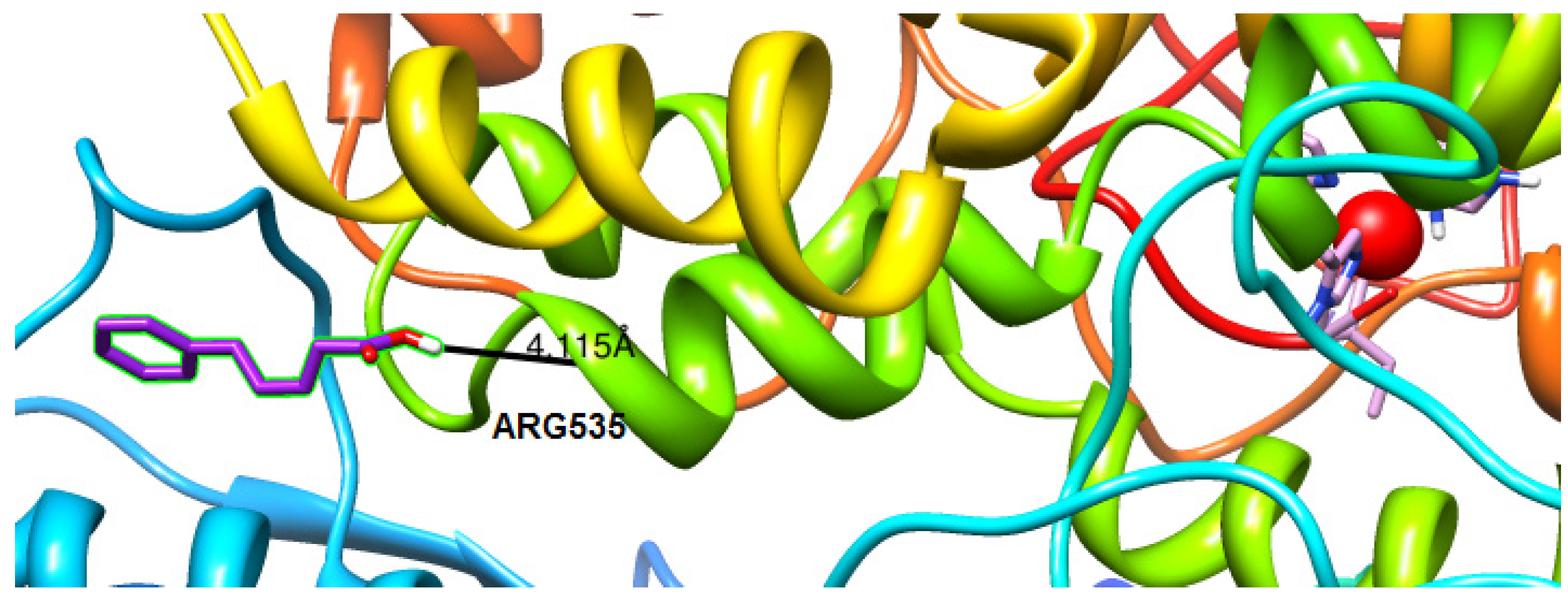
3. Experimental
3.1. Materials and Instruments
3.2. Chemistry General Procedure
3.2.1. Synthesis of Phenyl Substituted Cinnamic Acids 1i-4i [23,24,25]
3.2.2. General Procedure of the Synthesis of Cinnamic Acids 1ii–4ii [23,24,25]
3.3. Physicochemical Studies
3.3.1. Determination of RM Values
3.3.2. Estimation of Lipophilicity as Clog P
3.4. Biological Experiments
3.4.1. Experiments in vitro
3.4.1.1. Soybean Lipoxygenase Inhibition Study in vitro [23,24,25]
3.4.1.2. Interaction of the New Acrylic Acids with the Stable Radical 1,1-diphenyl-picrylhydrazyl (DPPH) [23,24,25]
3.4.1.3. Hydroxyl Radicals Scavenging Activity [23,24,25]
3.4.1.4. Superoxide Radical Scavenging Activity [23,24,25]
3.4.1.5. Inhibition of Linoleic Acid Peroxidation [25]
3.4.1.6. ABTS+-Decolorization Assay [25]
3.4.1.7. Cytotoxic Activity
Cell Lines and Culture Maintenance
Trypan Blue Exclusion
Cell Inoculation–Drug Exposure–SRB Cytotoxicity Assay
Calculation of Results
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
Abbreviations
| AA | Arachidonic Acid |
| AAPH | 2,2'-azobis (2-amidinopropane) hydrochloride |
| ACPYPE | AnteChamber PYthon Parser interface |
| clog P | Theoretically calculated lipophilicity |
| COX | Cyclooxygenase |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl radical |
| LDL | Low-density lipoprotein |
| LOX | Lipoxygenase |
| NBT | Nitroblue tetrazolium |
| ND | Neurodegenerative diseases |
| NDGA | Nordihydroguaretic acid |
| •OH | Hydroxyl radical |
| O2−· | Superoxide radical |
| ROS | Reactive oxygen species |
| RPTLC | Reverse-phase thin layer chromatography |
| TCA | Trichloroacetic acid |
References
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Karin, M. Inflammation and oncogenesis: A vicious connection. Curr. Opin. Genet. Dev. 2010, 20, 65–71. [Google Scholar] [CrossRef]
- Hussain, S.P.; Hofseth, L.J.; Harris, C.C. Radical causes of cancer. Nat. Rev. Cancer 2003, 3, 276–285. [Google Scholar] [CrossRef]
- Wang, M.T.; Honn, K.V.; Nie, D. Cyclooxygenases, prostanoids, and tumor progression. Cancer Metastasis Rev. 2007, 26, 525–534. [Google Scholar] [CrossRef]
- Niki, E. Do antioxidants impair signaling by reactive oxygen species and lipid oxidation products? FEBS Lett. 2012, 586, 3767–3770. [Google Scholar] [CrossRef]
- Brash, A.R. Lipoxygenases: Occurrence, functions, catalysis, and acquisition of substrate. J. Biol. Chem. 1999, 274, 23679–23682. [Google Scholar] [CrossRef]
- Norel, X.; Brink, C. The quest for new cysteinyl-leukotriene and lipoxin receptors: Recent clues. Pharmacol. Ther. 2004, 103, 81–94. [Google Scholar] [CrossRef]
- Vera-Ramirez, L.; Sanchez-Rovira, P.; Ramirez-Tortosa, M.C.; Ramirez-Tortosa, C.L.; Granados-Principal, S.; Lorente, J.A.; Quiles, J.L. Free radicals in breast carcinogenesis, breast cancer progression and cancer stem cells. Biological bases to develop oxidative-based therapies. Crit. Rev. Oncol. Hematol. 2011, 80, 347–368. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radi. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Chung, H.S.; Shin, J.C. Characterization of antioxidant alkaloids and phenolic acids from anthocyanin-pigmented rice (Oryza sativa cv. Heugjinjubyeo). Food Chem. 2007, 104, 1670–1677. [Google Scholar] [CrossRef]
- Bezerra, D.P.; Castro, F.O.; Alves, A.P.N.N.; Pessoa, C.; Moraes, M.O.; Silveira, E.R.; Lima, M.A.S.; Elmiro, F.J.M.; Costa-Lotufo, L.V. In vivo growth-inhibition of Sarcoma 180 by piplartine and piperine, two alkaloid amides from Piper. Braz. J. Med. Biol. Res. 2006, 39, 801–807. [Google Scholar] [CrossRef]
- Naz, S.; Ahmad, S.; Ajaz Rasool, S.; Asad Sayeed, S.; Siddiqi, R. Antibacterial activity directed isolation of compounds from Onosma hispidum. Microbiol. Res. 2006, 161, 43–48. [Google Scholar] [CrossRef]
- Carvalho, S.A.; da Silva, E.F.; de Souza, M.V.N.; Lourenço, M.C.S.; Vicente, F.R. Synthesis and antimycobacterial evaluation of new trans-cinnamic acid hydrazide derivatives. Bioorg. Med. Chem. Lett. 2008, 18, 538–541. [Google Scholar] [CrossRef]
- Sova, M. Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini Rev. Med. Chem. 2012, 12, 749–767. [Google Scholar] [CrossRef]
- Bernini, R.; Mincione, E.; Barontini, M.; Provenzano, G.; Setti, L. Obtaining 4-vinylphenols by decarboxylation of natural 4-hydroxycinnamic acids under microwave irradiation. Tetrahedron 2007, 63, 9663–9667. [Google Scholar] [CrossRef]
- Zang, L.Y.; Cosma, G.; Gardner, H.; Shi, X.; Castranova, V.; Vallyathan, V. Effect of Antioxidant Protection by P-coumaric acid on Low-density Lipoprotein Cholesterol Oxidation. Am. J. Physiol. Cell Physiol. 2000, 279, C954–C960. [Google Scholar]
- Roleira, F.M.; Siquet, C.; Orru, E.; Garrido, E.M.; Garrido, J.; Milhazes, N.; Podda, G.; Paiva-Martins, F.; Reis, S.; Carvalho, R.A.; et al. Lipophilic phenolic antioxidants: Correlation between antioxidant profile, partition coefficients and redox properties. Bioorg. Med. Chem. 2010, 18, 5816–5825. [Google Scholar] [CrossRef]
- Han, D.C.; Lee, M.Y.; Shin, K.D.; Jeon, S.B.; Kim, J.M.; Son, K.H.; Kim, H.C.; Kim, H.M.; Kwon, B.M. 2'-benzoyloxycinnamaldehyde induces apoptosis in human carcinoma via reactive oxygen species. J. Biol. Chem. 2004, 279, 6911–6920. [Google Scholar]
- Bandgar, B.P.; Gawande, S.S.; Bodade, R.G.; Totre, J.V.; Khobragade, C.N. Synthesis and biological evaluation of simple methoxylated chalcones as anticancer, anti-inflammatory and antioxidant agents. Bioorg. Med. Chem. 2010, 18, 1364–1370. [Google Scholar]
- Cheng, J.H.; Huang, A.M.; Hour, T.C.; Yang, S.C.; Pu, Y.S.; Lin, C.N. Antioxidant xanthone derivatives induce cell cycle arrest and apoptosis and enhance cell death induced by cisplatin in NTUB1 cells associated with ROS. Eur. J. Med. Chem. 2011, 46, 1222–1231. [Google Scholar] [CrossRef]
- Kanagalakshmi, K.; Premanathan, M.; Priyanka, R.; Hemalatha, B.; Vanangamudi, A. Synthesis, anticancer and antioxidant activities of 7-methoxyisoflavanone and 2,3-diarylchromanones. Eur. J. Med. Chem. 2010, 45, 2447–2452. [Google Scholar] [CrossRef]
- Karali, N.; Guzel, O.; Ozsoy, N.; Ozbey, S.; Salman, A. Synthesis of new spiroindolinones incorporating a benzothiazole moiety as antioxidant agents. Eur. J. Med. Chem. 2010, 45, 1068–1077. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D. Antioxidant and anti-inflammatory activity of aryl-acetic and hydroxamic acids as novel lipoxygenase inhibitors. Med. Chem. 2006, 2, 251–264. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D. Synthesis and pharmacochemical evaluation of novel aryl-acetic acid inhibitors of lipoxygenase, antioxidants, and anti-inflammatory agents. Bioorg. Med. Chem. 2007, 15, 5819–5827. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D.; Geromichalos, G.; Papageorgiou, A. Anticancer activity and quantitative-structure activity relationship (QSAR) studies of a series of antioxidant/anti-inflammatory aryl-acetic and hydroxamic acids. Chem. Biol. Drug Des. 2009, 74, 266–275. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D.; Litinas, K.; Nicolotti, O.; Carotti, A. Design, synthesis and pharmacobiological evaluation of novel acrylic acid derivatives acting as lipoxygenase and cyclooxygenase-1 inhibitors with antioxidant and anti-inflammatory activities. Eur. J. Med. Chem. 2011, 46, 191–200. [Google Scholar] [CrossRef]
- Davies, W.; Holmes, B.M.; Kefford, J.F. ω-Bromo-o-cyanostyrenes and related compounds. J. Chem. Soc. 1939. [Google Scholar] [CrossRef]
- Bokaldere, R.M.; Duffy, J.E.S.; Finn, P.W.; Harris, C.J.; Kalvinsh, I.; Lolya, D.; Loza, E.; Maria, R.; Semenikhina, V.; Starchenkov, I.; et al. Carbamic Acid Compounds Comprising an Ether Linkage as HDAC Inhibitors. WO2002026703 A1, 2002. [Google Scholar]
- Venkatasamy, R.; Faas, L.; Young, A.R.; Raman, A.; Hider, R.C. Effects of piperine analogues on stimulation of melanocyte proliferation and melanocyte differentiation. Bioorg. Med. Chem. 2004, 12, 1905–1920. [Google Scholar] [CrossRef]
- Chapman, O.L.; Adams, W.R. Photochemical transformations. XXII. Photoisomerization of substituted acrylic acids and acrylamides to β-lactones and β-lactams. J. Am. Chem. Soc. 1968, 90, 2333–2342. [Google Scholar] [CrossRef]
- Kumar, S.; Sapra, S.; Kumar, R.; Gupta, M.K.; Koul, S.; Kour, T.; Saxena, A.K.; Suri, O.P.; Dhar, K.L. Synthesis of combretastatin analogs: Evaluation of in vitro anticancer activity and molecular docking studies. Med. Chem. Res. 2012, 21, 3720–3729. [Google Scholar] [CrossRef]
- Buckles, R.E.; Bellis, M.P.; Coder, W.D. Amine-catalyzed Condensations of Benzaldehydes with Phenylacetic Acids1. J. Am. Chem. Soc. 1951, 73, 4972–4974. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.J. Exploring QSAR Fundamentals and applications in Chemistry and Biology; Heller, S.R., Ed.; ACS Professional Reference Book: Washington, DC, USA, 1995; volume I, pp. 279–280. [Google Scholar]
- BioByte Home Page. Available online: http://www.biobyte.com/ (accessed on 1 June 2012).
- Niki, E. Antioxidant capacity: Which capacity and how to assess it? J. Berry Res. 2011, 1, 169–176. [Google Scholar]
- Taraporewala, I.B.; Kauffman, J.M. Synthesis and structure-activity relationships of anti-inflammatory 9,10-dihydro-9-oxo-2-acridine-alkanoic acids and 4-(2-carboxyphenyl)aminobenzenealkanoic acids. J. Pharm. Sci. 1990, 79, 173–178. [Google Scholar] [CrossRef]
- Muller, K. 5-Lipoxygenase and 12-lipoxygenase: Attractive targets for the development of novel antipsoriatic drugs. Arch. Pharm. (Weinheim) 1994, 327, 3–19. [Google Scholar] [CrossRef]
- Kemal, C.; Louis-Flamberg, P.; Krupinski-Olsen, R.; Shorter, A.L. Reductive inactivation of soybean lipoxygenase 1 by catechols: A possible mechanism for regulation of lipoxygenase activity. Biochemistry 1987, 26, 7064–7072. [Google Scholar] [CrossRef]
- Van der Zee, J.; Eling, T.E.; Mason, R.P. Formation of free-radical metabolites in the reaction between soybean lipoxygenase and its inhibitors. An ESR study. Biochemistry 1989, 28, 8363–8367. [Google Scholar] [CrossRef]
- Pidgeon, G.P.; Lysaght, J.; Krishnamoorthy, S.; Reynolds, J.V.; O’Byrne, K.; Nie, D.; Honn, K.V. Lipoxygenase metabolism: Roles in tumor progression and survival. Cancer Metastasis Rev. 2007, 26, 503–524. [Google Scholar] [CrossRef]
- Karmali, R.A. Eicosanoids in breast cancer. Eur. J. Cancer Clin. Oncol. 1987, 23, 5–7. [Google Scholar] [CrossRef]
- Kort, W.J.; Bijma, A.M.; van Dam, J.J.; van der Ham, A.C.; Hekking, J.M.; van der Ingh, H.F.; Meijer, W.S.; van Wilgenburg, M.G.; Zijlstra, F.J. Eicosanoids in breast cancer patients before and after mastectomy. Prostaglandins Leukot Essent Fatty Acids 1992, 45, 319–327. [Google Scholar] [CrossRef]
- Nigam, S.; Becker, R.; Rosendahl, U.; Hammerstein, J.; Benedetto, C.; Barbero, M.; Slater, T.F. The concentrations of 6-keto-PGF1 alpha and TXB2 in plasma samples from patients with benign and malignant tumours of the breast. Prostaglandins 1985, 29, 513–528. [Google Scholar]
- Moody, T.W.; Leyton, J.; Martinez, A.; Hong, S.; Malkinson, A.; Mulshine, J.L. Lipoxygenase inhibitors prevent lung carcinogenesis and inhibit non-small cell lung cancer growth. Exp. Lung Res. 1998, 24, 617–628. [Google Scholar]
- Rioux, N.; Castonguay, A. Inhibitors of lipoxygenase: A new class of cancer chemopreventive agents. Carcinogenesis 1998, 19, 1393–1400. [Google Scholar] [CrossRef]
- Bortuzzo, C.; Hanif, R.; Kashfi, K.; Staiano-Coico, L.; Shiff, S.J.; Rigas, B. The effect of leukotrienes B and selected HETEs on the proliferation of colon cancer cells. Biochim. Biophys. Acta 1996, 1300, 240–246. [Google Scholar] [CrossRef]
- CambridgeSoft, USA, ChemDraw Ultra_v.12, 1986–2009. Available online: http://www.cambridgesoft.com/software/chemDraw/ (accessed on 26 February 2013).
- OpenBabel, version 2.2.3, 2006. Available online: http://sourceforge.net/projects/openbabel (accessed on 10 February 2010).
- Available online: http://www.cgl.ucsf.edu/chimera (accessed on 10 February 2010).
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar]
- Hess, B.; Kutzner, C.; van der Spoel, D.; Lindahl, E. GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 2008, 4, 435–447. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic atom type and bond type perception in molecular mechanical calculations. J. Mol. Graph. Model 2006, 25, 247–260. [Google Scholar] [CrossRef]
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.L.; Dror, R.O.; Shaw, D.E. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins 2010, 78, 1950–1958. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar]
- Autodock Vina, version 1.1.1. The Scripps Research Institute: San Diego, CA, USA, 2010.
- PyRx-Python Prescription, v.0.5.The Scripps Research Institute, 2008–2010. Available online: http://pyrx.scripps.edu/ (accessed on 10 February 2010).
- Minor, W.; Steczko, J.; Stec, B.; Otwinowski, Z.; Bolin, J.T.; Walter, R.; Axelrod, B. Crystal structure of soybean lipoxygenase L-1 at 1.4 A resolution. Biochemistry 1996, 35, 10687–10701. [Google Scholar]
- Berman, H.; Henrick, K.; Nakamura, H. Announcing the worldwide Protein Data Bank. Nat. Struct. Mol. Biol. 2003, 10, 980–980. [Google Scholar]
- Life technologies. Available online: http://www.lifetechnologies.com (accessed on 10 February 2010).
- Gorman, A.; McCarthy, J.; Finucane, D.; Reville, W.; Cotter, T. Techniques in Apoptosis. A User’s Guide; Cotter, T.G., Martin, S.J., Eds.; Portland Press Ltd: London, UK, 1996; pp. 6–7. [Google Scholar]
- Costar-Corning. Available online: http://www.corning.com/lifesciences (accessed on 10 February 2010).
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Papazisis, K.T.; Geromichalos, G.D.; Dimitriadis, K.A.; Kortsaris, A.H. Optimization of the sulforhodamine B colorimetric assay. J. Immunol. Methods 1997, 208, 151–158. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pontiki, E.; Hadjipavlou-Litina, D.; Litinas, K.; Geromichalos, G. Novel Cinnamic Acid Derivatives as Antioxidant and Anticancer Agents: Design, Synthesis and Modeling Studies. Molecules 2014, 19, 9655-9674. https://doi.org/10.3390/molecules19079655
Pontiki E, Hadjipavlou-Litina D, Litinas K, Geromichalos G. Novel Cinnamic Acid Derivatives as Antioxidant and Anticancer Agents: Design, Synthesis and Modeling Studies. Molecules. 2014; 19(7):9655-9674. https://doi.org/10.3390/molecules19079655
Chicago/Turabian StylePontiki, Eleni, Dimitra Hadjipavlou-Litina, Konstantinos Litinas, and George Geromichalos. 2014. "Novel Cinnamic Acid Derivatives as Antioxidant and Anticancer Agents: Design, Synthesis and Modeling Studies" Molecules 19, no. 7: 9655-9674. https://doi.org/10.3390/molecules19079655








